Transformative Potential of Induced Pluripotent Stem Cells in Congenital Heart Disease Research and Treatment
Abstract
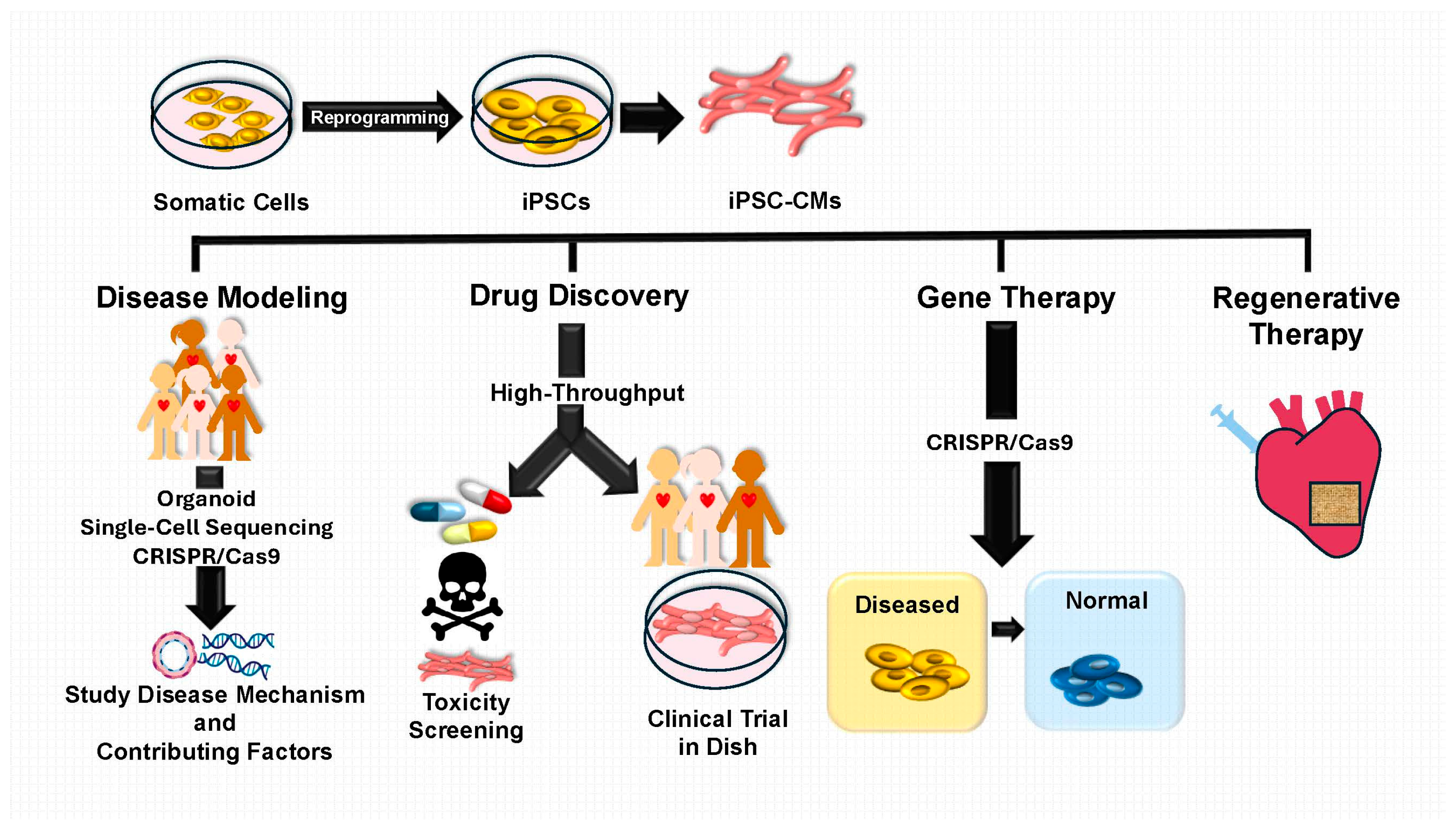
1. Introduction
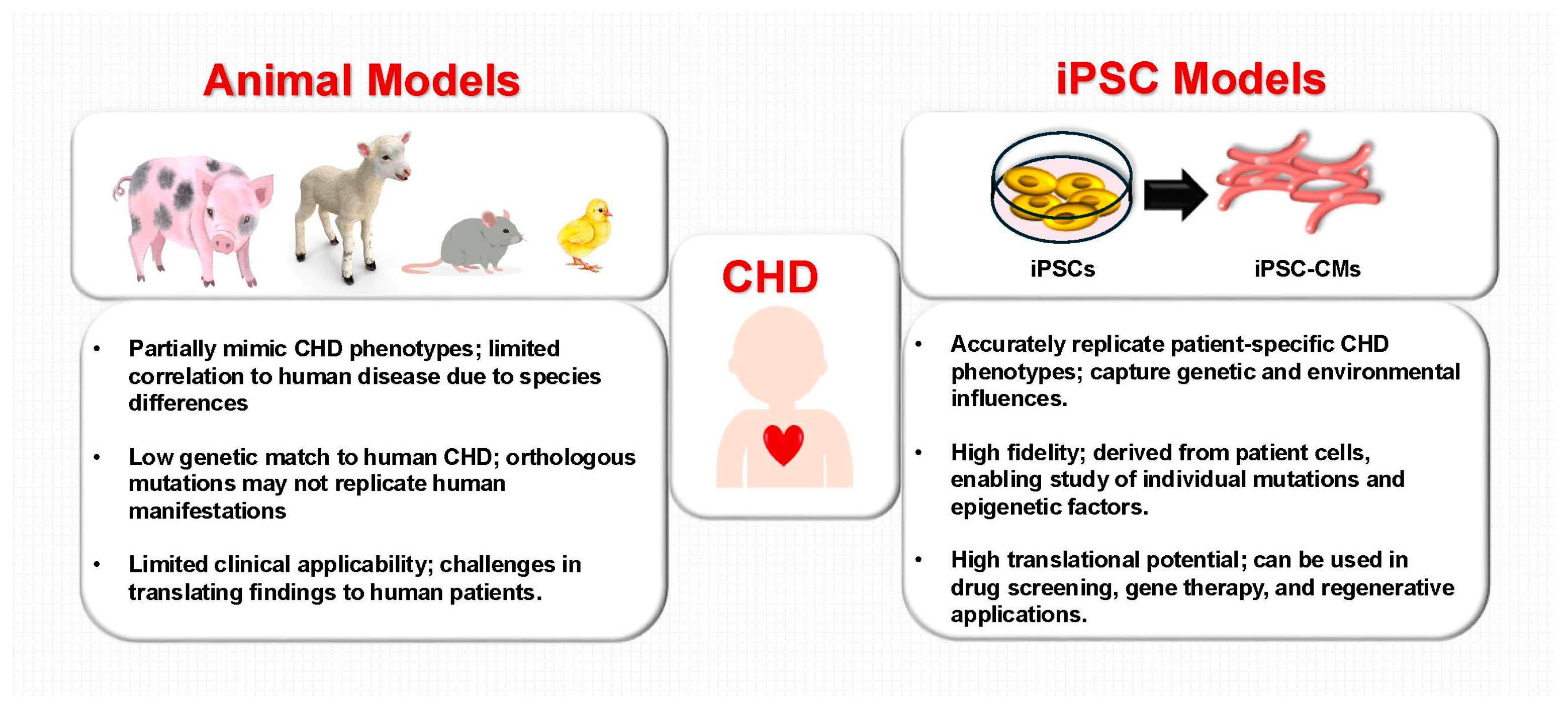
2. iPSC-Based Modeling of CHD
2.1. Advances in iPSC-Based Disease Modeling
2.2. Patient-Specific iPSC Models for CHD
2.3. iPSC-Derived Cardiac Organoids
2.4. Single-Cell Technologies in iPSC-Based CHD Research
| CHD Subtype | iPSC-Based Model | Key Findings | Genetic Pathways |
|---|---|---|---|
| HLHS | iPSC-CMs, cardiac organoids, endothelial cells | Altered calcium handling, mitochondrial dysfunction [69,70], dysregulated histone acetylation patterns that impaired differentiation [71,72,73], endothelial-mesenchymal transition defects [23] | NKX2-5, NOTCH1 [22,23,40], Myh6 [19] |
| TOF | Patient-derived iPSC-CMs, CRISPR-engineered TOF models | Dysregulated collagen expression [17], contractile defects, abnormal right ventricular development, disrupted metabolic pathways (butanoate metabolism) [74,75]. | GATA4, TBX1, JAG1 [17] |
| BAV | iPSC-derived endothelial and smooth muscle cells | Abnormal valvulogenesis, endothelial dysfunction, early calcification | GATA4 [76,77], NOTCH1 [36] |
| SVAS | iPSC-derived vascular smooth muscle cells | Elastin deficiency leading to vascular abnormalities and stenosis [37] | ELN [37] |
| Cardiac Septal Defects ASD, VSD, AVSD | Patient-specific iPSC-CMs, 3D cardiac tissue models | Defective septal development, impaired myocardial proliferation, disrupted signaling pathways [38] | TBX5, GATA4 [78], NKX2-5 [38,78,79,80] |
| BTHS | iPSC-CMs with TAZ mutations | Mitochondrial dysfunction, impaired cardiolipin remodeling, excessive ROS generation [39] | TAZ, PPAR pathways [39] |
| LQTS | iPSC-CMs carrying patient-specific KCNQ1, KCNH2 mutations | Prolonged action potential duration, abnormal ion channel activity | KCNQ1, KCNH2, SCN5A [81,82,83] |
| LVNC | iPSC-CMs, Fibroblasts | Decreased ventricular development, deep trabeculae, metabolic maturation defects [84] | Mkl2, Myh7, Nkx2-5 [84] |
| HOS | iPSC-CMs | Epigenetic alterations affecting cardiac developmental genes [85] | TBX5 [86] |
| OFT malformations | iPSC-CMs, organoid [54,55,56] | Decreased transcription levels in cardiomyocytes | GATA6 [87] |
3. Molecular Insights and Precision Medicine Using iPSCs
3.1. Genetic and Epigenetic Contributions in iPSC Models
3.2. Patient-Specific Therapies Enabled by iPSCs
3.2.1. Drug Testing and Screening with iPSC-CMs
3.2.2. Gene Therapy and Genome Editing Using iPSC Models
3.2.3. Clinical Trials and Regenerative Applications of iPSC-Based Therapies
4. iPSC-Based Drug Discovery and Testing for CHD
4.1. High-Throughput Screening Platforms Using iPSC-CMs
4.2. Functional Restoration Studies in iPSC Models
4.3. Technical Advances in iPSC-CM Differentiation and Scale-Up
4.4. Drug Repurposing Using iPSC-Derived Models
5. Limitations and Challenges of iPSCs in CHD Research
5.1. Immaturity of iPSC-CMs
5.2. Large-Scale Feasibility and Reproducibility
5.3. Tumorigenicity and Genomic Instability
5.4. Immunogenicity and Compatibility Issues
5.5. Cell Engraftment and Integration Challenges
5.6. Cost, Expertise, and Ethical Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| APC | Automated patch clamp |
| ASDs | Atrial septal defects |
| AVSD | Atrioventricular septal defect |
| BAV | Bicuspid aortic valve |
| BTHS | Barth syndrome |
| CAVD | Calcific aortic valve disease |
| cGMP | Current good manufacturing practices |
| CHD | Congenital heart disease |
| CiPA | Comprehensive in vitro Proarrhythmia Assay |
| CPC | Cardiac progenitor cell |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| DEGs | Differentially expressed genes |
| FDA | U.S. Food and Drug Administration |
| HLHS | Hypoplastic left heart syndrome |
| HOS | Holt–Oram syndrome |
| iPSCs | Induced pluripotent stem cells |
| iPSC-CMs | iPSC-derived cardiomyocytes |
| ISCI | International Stem Cell Initiative |
| LQTS | Long QT syndrome |
| LVNC | Left ventricular non-compaction |
| MEAs | Microelectrode arrays |
| miRNAs | MicroRNAs |
| MPS | Microphysiological systems |
| NIH | National Institutes of Health |
| OFT | Outflow tract |
| PAIVS | Pulmonary atresia with intact ventricular septum |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| scRNA-seq | Single-cell RNA sequencing |
| STE | Speckle tracking echocardiography |
| SVAS | Supravalvular aortic stenosis |
| SVD | Single ventricle defects |
| TOF | Tetralogy of Fallot |
| VSD | Ventricular septal defect |
References
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.J.; Mulder, B.J. Changing landscape of congenital heart disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.M.; Walton, M.A.; Carter, P.M. The major causes of death in children and adolescents in the united states. N. Engl. J. Med. 2018, 379, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.; Baker, D.; Chin, M.; Cinquegrani, M.; Feldman, A.; Francis, G. Heart failure society of america acc/aha guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary. A report of the american college of cardiology/american heart association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure): Developed in collaboration with the international society for heart and lung transplantation; endorsed by the heart failure society of america. Circulation 2001, 104, 2996–3007. [Google Scholar]
- Jha, B.S.; Farnoodian, M.; Bharti, K. Regulatory considerations for developing a phase i investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl. Med. 2021, 10, 198–208. [Google Scholar] [CrossRef]
- Balafkan, N.; Mostafavi, S.; Schubert, M.; Siller, R.; Liang, K.X.; Sullivan, G.; Bindoff, L.A. A method for differentiating human induced pluripotent stem cells toward functional cardiomyocytes in 96-well microplates. Sci. Rep. 2020, 10, 18498. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Robertson, J.A. Human embryonic stem cell research: Ethical and legal issues. Nat. Rev. Genet. 2001, 2, 74–78. [Google Scholar] [CrossRef]
- Wert, G.D.; Mummery, C. Human embryonic stem cells: Research, ethics and policy. Hum. Reprod. 2003, 18, 672–682. [Google Scholar] [CrossRef]
- Lu, J.; Kong, X.; Luo, C.; Kathy Li, K. Application of epigenome-modifying small molecules in induced pluripotent stem cells. Med. Res. Rev. 2013, 33, 790–822. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Chung, W.K. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb. Perspect. Med. 2014, 4, a013953. [Google Scholar] [CrossRef]
- Buja, L.M.; Butany, J. Cardiovascular Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–26. [Google Scholar]
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental risk factors for congenital heart disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037234. [Google Scholar] [CrossRef]
- Grunert, M.; Appelt, S.; Schönhals, S.; Mika, K.; Cui, H.; Cooper, A.; Cyganek, L.; Guan, K.; Sperling, S.R. Induced pluripotent stem cells of patients with tetralogy of fallot reveal transcriptional alterations in cardiomyocyte differentiation. Sci. Rep. 2020, 10, 10921. [Google Scholar] [CrossRef]
- Kitani, T.; Tian, L.; Zhang, T.; Itzhaki, I.; Zhang, J.Z.; Ma, N.; Liu, C.; Rhee, J.-W.; Romfh, A.W.; Lui, G.K. Rna sequencing analysis of induced pluripotent stem cell-derived cardiomyocytes from congenital heart disease patients. Circ. Res. 2020, 126, 923–925. [Google Scholar] [CrossRef]
- Kim, M.-S.; Fleres, B.; Lovett, J.; Anfinson, M.; Samudrala, S.S.K.; Kelly, L.J.; Teigen, L.E.; Cavanaugh, M.; Marquez, M.; Geurts, A.M. Contractility of induced pluripotent stem cell-cardiomyocytes with an myh6 head domain variant associated with hypoplastic left heart syndrome. Front. Cell Dev. Biol. 2020, 8, 440. [Google Scholar] [CrossRef]
- Xu, X.; Zou, R.; Liu, X.; Su, Q. Alternative splicing signatures of congenital heart disease and induced pluripotent stem cell-derived cardiomyocytes from congenital heart disease patients. Medicine 2022, 101, e30123. [Google Scholar] [CrossRef]
- Xu, X.; Jin, K.; Bais, A.S.; Zhu, W.; Yagi, H.; Feinstein, T.N.; Nguyen, P.K.; Criscione, J.D.; Liu, X.; Beutner, G. Uncompensated mitochondrial oxidative stress underlies heart failure in an ipsc-derived model of congenital heart disease. Cell Stem Cell 2022, 29, 840–855. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Yu, M.; Lee, D.; Alharti, S.; Hellen, N.; Ahmad Shaik, N.; Banaganapalli, B.; Sheikh Ali Mohamoud, H.; Elango, R. Induced pluripotent stem cell modelling of hlhs underlines the contribution of dysfunctional notch signalling to impaired cardiogenesis. Hum. Mol. Genet. 2017, 26, 3031–3045. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Tian, L.; Martin, M.; Paige, S.L.; Galdos, F.X.; Li, J.; Klein, A.; Zhang, H.; Ma, N.; Wei, Y. Intrinsic endocardial defects contribute to hypoplastic left heart syndrome. Cell Stem Cell 2020, 27, 574–589.e8. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, V.; Simmons, C.; Gong, T.; Sharma, A.; Burridge, P.W.; Patlolla, B.; Lee, A.S. Screening drug-induced arrhythmia using human induced pluripotent stem cell–derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 2013, 128, S3–S13. [Google Scholar] [CrossRef]
- Margulis, M.; Sorota, S. Additive effects of combined application of multiple herg blockers. J. Cardiovasc. Pharmacol. 2008, 51, 549–552. [Google Scholar] [CrossRef]
- Mantakaki, A.; Fakoya, A.O.J.; Sharifpanah, F. Recent advances and challenges on application of tissue engineering for treatment of congenital heart disease. PeerJ 2018, 6, e5805. [Google Scholar] [CrossRef]
- Majumdar, U.; Yasuhara, J.; Garg, V. In vivo and in vitro genetic models of congenital heart disease. Cold Spring Harb. Perspect. Biol. 2021, 13, a036764. [Google Scholar] [CrossRef]
- Sanchez-Freire, V.; Lee, A.S.; Hu, S.; Abilez, O.J.; Liang, P.; Lan, F.; Huber, B.C.; Ong, S.-G.; Hong, W.X.; Huang, M. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J. Am. Coll. Cardiol. 2014, 64, 436–448. [Google Scholar] [CrossRef]
- Hrstka, S.C.; Li, X.; Nelson, T.J.; Group, W.P.G.P. Notch1-dependent nitric oxide signaling deficiency in hypoplastic left heart syndrome revealed through patient-specific phenotypes detected in bioengineered cardiogenesis. Stem Cells 2017, 35, 1106–1119. [Google Scholar] [CrossRef]
- Hall, B.; Alonzo, M.; Texter, K.; Garg, V.; Zhao, M.T. Probing single ventricle heart defects with patient-derived induced pluripotent stem cells and emerging technologies. Birth Defects Res. 2022, 114, 959–971. [Google Scholar] [CrossRef]
- Adhicary, S.; Ye, S.; Lin, H.; Texter, K.; Garg, V.; Zhao, M.-T. Establishment of nchi009-a, an ipsc line from a patient with hypoplastic left heart syndrome (hlhs) carrying a heterozygous notch1 mutation. Stem Cell Res. 2023, 66, 103013. [Google Scholar] [CrossRef] [PubMed]
- Page, D.J.; Miossec, M.J.; Williams, S.G.; Monaghan, R.M.; Fotiou, E.; Cordell, H.J.; Sutcliffe, L.; Topf, A.; Bourgey, M.; Bourque, G. Whole exome sequencing reveals the major genetic contributors to nonsyndromic tetralogy of fallot. Circ. Res. 2019, 124, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.; Alonzo, M.; Ye, S.; Lin, H.; Hernandez-Rosario, L.; McBride, K.L.; Texter, K.; Garg, V.; Zhao, M.-T. Generation of an induced pluripotent stem cell line nchi003-a from a 11-year-old male with pulmonary atresia with intact ventricular septum (pa-ivs). Stem Cell Res. 2022, 64, 102893. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.; Alonzo, M.; Ye, S.; Yu, Y.; Contreras, J.; Hayden, J.; Garg, V.; Zhao, M.-T. Characterization of an induced pluripotent stem cell line (nchi013-a) from a 5-year-old male with pulmonary atresia with intact ventricular septum and a biventricular repair. Stem Cell Res. 2024, 80, 103526. [Google Scholar] [CrossRef]
- Theodoris, C.V.; Li, M.; White, M.P.; Liu, L.; He, D.; Pollard, K.S.; Bruneau, B.G.; Srivastava, D. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of notch1 haploinsufficiency. Cell 2015, 160, 1072–1086. [Google Scholar] [CrossRef]
- Ge, X.; Ren, Y.; Bartulos, O.; Lee, M.Y.; Yue, Z.; Kim, K.-Y.; Li, W.; Amos, P.J.; Bozkulak, E.C.; Iyer, A. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation 2012, 126, 1695–1704. [Google Scholar] [CrossRef]
- Ang, Y.-S.; Rivas, R.N.; Ribeiro, A.J.; Srivas, R.; Rivera, J.; Stone, N.R.; Pratt, K.; Mohamed, T.M.; Fu, J.-D.; Spencer, C.I. Disease model of gata4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell 2016, 167, 1734–1749. [Google Scholar] [CrossRef]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L. Modeling the mitochondrial cardiomyopathy of barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Jaffré, F. HiPSCs as a unique platform to model cardiogenesis in notch1-associated hlhs: HiPSCs to model complex congenital heart defects. Circ. Res. 2023, 132, 205–207. [Google Scholar] [CrossRef]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Pereira-Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D. Generation and maturation of human ipsc-derived 3d organotypic cardiac microtissues in long-term culture. Sci. Rep. 2022, 12, 17409. [Google Scholar] [CrossRef]
- Simunovic, M.; Brivanlou, A.H. Embryoids, organoids and gastruloids: New approaches to understanding embryogenesis. Development 2017, 144, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Sumbal, J.; Chiche, A.; Charifou, E.; Koledova, Z.; Li, H. Primary mammary organoid model of lactation and involution. Front. Cell Dev. Biol. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Shimonosono, M.; Morimoto, M.; Hirose, W.; Tomita, Y.; Matsuura, N.; Flashner, S.; Ebadi, M.S.; Okayasu, E.H.; Lee, C.Y.; Britton, W.R. Modeling epithelial homeostasis and perturbation in three-dimensional human esophageal organoids. Biomolecules 2024, 14, 1126. [Google Scholar] [CrossRef] [PubMed]
- Volmert, B.; Kiselev, A.; Juhong, A.; Wang, F.; Riggs, A.; Kostina, A.; O’Hern, C.; Muniyandi, P.; Wasserman, A.; Huang, A. A patterned human primitive heart organoid model generated by pluripotent stem cell self-organization. Nat. Commun. 2023, 14, 8245. [Google Scholar] [CrossRef]
- Ho, B.X.; Pang, J.K.S.; Chen, Y.; Loh, Y.-H.; An, O.; Yang, H.H.; Seshachalam, V.P.; Koh, J.L.; Chan, W.-K.; Ng, S.Y. Robust generation of human-chambered cardiac organoids from pluripotent stem cells for improved modelling of cardiovascular diseases. Stem Cell Res. Ther. 2022, 13, 529. [Google Scholar] [CrossRef]
- Hoang, P.; Kowalczewski, A.; Sun, S.; Winston, T.S.; Archilla, A.M.; Lemus, S.M.; Ercan-Sencicek, A.G.; Gupta, A.R.; Liu, W.; Kontaridis, M.I. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Rep. 2021, 16, 1228–1244. [Google Scholar] [CrossRef]
- Cashman, T.J.; Josowitz, R.; Johnson, B.V.; Gelb, B.D.; Costa, K.D. Human engineered cardiac tissues created using induced pluripotent stem cells reveal functional characteristics of braf-mediated hypertrophic cardiomyopathy. PLoS ONE 2016, 11, e0146697. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Siatra, P.; Vatsellas, G.; Chatzianastasiou, A.; Balafas, E.; Manolakou, T.; Papapetropoulos, A.; Agapaki, A.; Mouchtouri, E.-T.; Ruchaya, P.J.; Korovesi, A.G. Return of the tbx5; lineage-tracing reveals ventricular cardiomyocyte-like precursors in the injured adult mammalian heart. NPJ Regen. Med. 2023, 8, 13. [Google Scholar] [CrossRef]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Haendeler, S.; Caballero, A.T.; Novatchkova, M.; Netzer, M.A.; Ginistrelli, L.C.; Juncosa, E.M.; Bhattacharya, T. Multi-chamber cardioids unravel human heart development and cardiac defects. Cell 2023, 186, 5587–5605.e27. [Google Scholar] [CrossRef]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T. In vitro generation of functional murine heart organoids via fgf4 and extracellular matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.X.; Liu, X.Y.; Hou, J.Y.; Ni, S.H.; Wang, J.; Zhao, C.M.; Zhang, W.; Kong, Y.; Huang, R.T. Tbx1 loss-of-function mutation contributes to congenital conotruncal defects. Exp. Ther. Med. 2018, 15, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Calmont, A.; Ivins, S.; Van Bueren, K.L.; Papangeli, I.; Kyriakopoulou, V.; Andrews, W.D.; Martin, J.F.; Moon, A.M.; Illingworth, E.A.; Basson, M.A. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating gbx2 expression in the pharyngeal ectoderm. Development 2009, 136, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, F.; Morishima, M.; Taddei, I.; Lindsay, E.A.; Baldini, A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum. Mol. Genet. 2002, 11, 915–922. [Google Scholar] [CrossRef]
- Phillips, H.M.; Stothard, C.A.; Shaikh Qureshi, W.M.; Kousa, A.I.; Briones-Leon, J.A.; Khasawneh, R.R.; O’Loughlin, C.; Sanders, R.; Mazzotta, S.; Dodds, R. Pax9 is required for cardiovascular development and interacts with tbx1 in the pharyngeal endoderm to control 4th pharyngeal arch artery morphogenesis. Development 2019, 146, dev177618. [Google Scholar] [CrossRef]
- Khalil, A.; Tanos, R.; El-Hachem, N.; Kurban, M.; Bouvagnet, P.; Bitar, F.; Nemer, G. A hand to tbx5 explains the link between thalidomide and cardiac diseases. Sci. Rep. 2017, 7, 1416. [Google Scholar] [CrossRef]
- Yang, J.; Lei, W.; Xiao, Y.; Tan, S.; Yang, J.; Lin, Y.; Yang, Z.; Zhao, D.; Zhang, C.; Shen, Z. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif. 2024, 57, e13631. [Google Scholar] [CrossRef]
- Hiroi, Y.; Kudoh, S.; Monzen, K.; Ikeda, Y.; Yazaki, Y.; Nagai, R.; Komuro, I. Tbx5 associates with nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat. Genet. 2001, 28, 276–280. [Google Scholar] [CrossRef]
- Friedman, C.E.; Nguyen, Q.; Lukowski, S.W.; Helfer, A.; Chiu, H.S.; Miklas, J.; Levy, S.; Suo, S.; Han, J.-D.J.; Osteil, P. Single-cell transcriptomic analysis of cardiac differentiation from human pscs reveals hopx-dependent cardiomyocyte maturation. Cell Stem Cell 2018, 23, 586–598.e8. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Welch, J.D.; Ma, H.; Zhou, Y.; Vaseghi, H.R.; Yu, S.; Wall, J.B.; Alimohamadi, S.; Zheng, M. Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 2017, 551, 100–104. [Google Scholar] [CrossRef]
- Cheng, S.; Brenière-Letuffe, D.; Ahola, V.; Wong, A.O.; Keung, H.Y.; Gurung, B.; Zheng, Z.; Costa, K.D.; Lieu, D.K.; Keung, W. Single-cell rna sequencing reveals maturation trajectory in human pluripotent stem cell-derived cardiomyocytes in engineered tissues. iScience 2023, 26, 106302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Lukowski, S.W.; Chiu, H.S.; Friedman, C.E.; Senabouth, A.; Crowhurst, L.; Bruxmer, T.J.; Christ, A.N.; Palpant, N.J.; Powell, J.E. Determining cell fate specification and genetic contribution to cardiac disease risk in hipsc-derived cardiomyocytes at single cell resolution. BioRxiv 2017, 229336. [Google Scholar]
- Lam, Y.Y.; Keung, W.; Chan, C.H.; Geng, L.; Wong, N.; Brenière-Letuffe, D.; Li, R.A.; Cheung, Y.F. Single-cell transcriptomics of engineered cardiac tissues from patient-specific induced pluripotent stem cell–derived cardiomyocytes reveals abnormal developmental trajectory and intrinsic contractile defects in hypoplastic right heart syndrome. J. Am. Heart Assoc. 2020, 9, e016528. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xu, Q.; Du, Y.; Tang, C.; Cui, H.; Xia, X.; Zheng, R.; Sun, Y.; Shang, H. Single-cell spatial transcriptomics in cardiovascular development, disease, and medicine. Genes Dis. 2024, 11, 101163. [Google Scholar] [CrossRef]
- Wan, X.; Xiao, J.; Tam, S.S.T.; Cai, M.; Sugimura, R.; Wang, Y.; Wan, X.; Lin, Z.; Wu, A.R.; Yang, C. Integrating spatial and single-cell transcriptomics data using deep generative models with spatialscope. Nat. Commun. 2023, 14, 7848. [Google Scholar] [CrossRef]
- Palmer, J.A.; Rosenthal, N.; Teichmann, S.A.; Litvinukova, M. Revisiting cardiac biology in the era of single cell and spatial omics. Circ. Res. 2024, 134, 1681–1702. [Google Scholar] [CrossRef]
- Roth, R.; Kim, S.; Kim, J.; Rhee, S. Single-cell and spatial transcriptomics approaches of cardiovascular development and disease. BMB Rep. 2020, 53, 393–399. [Google Scholar] [CrossRef]
- Jiang, Y.; Habibollah, S.; Tilgner, K.; Collin, J.; Barta, T.; Al-Aama, J.Y.; Tesarov, L.; Hussain, R.; Trafford, A.W.; Kirkwood, G. An induced pluripotent stem cell model of hypoplastic left heart syndrome (hlhs) reveals multiple expression and functional differences in hlhs-derived cardiac myocytes. Stem Cells Transl. Med. 2014, 3, 416–423. [Google Scholar] [CrossRef]
- Paige, S.L.; Galdos, F.X.; Lee, S.; Chin, E.T.; Ranjbarvaziri, S.; Feyen, D.A.; Darsha, A.K.; Xu, S.; Ryan, J.A.; Beck, A.L. Patient-specific induced pluripotent stem cells implicate intrinsic impaired contractility in hypoplastic left heart syndrome. Circulation 2020, 142, 1605–1608. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yoshida, M.; Tarui, S.; Hirata, M.; Nagai, Y.; Kasahara, S.; Naruse, K.; Ito, H.; Sano, S.; Oh, H. Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of nkx2-5, hand1, and notch1 in hypoplastic left heart syndrome. PLoS ONE 2014, 9, e102796. [Google Scholar] [CrossRef]
- Zhu, J.-y.; van de Leemput, J.; Han, Z. The roles of histone lysine methyltransferases in heart development and disease. J. Cardiovasc. Dev. Dis. 2023, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Azarcon, P.; Hickenlooper, S.; Bia, R.; Horiuchi, E.; Szulik, M.W.; Franklin, S. The role of demethylases in cardiac development and disease. J. Mol. Cell. Cardiol. 2021, 158, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kong, S.; Song, S.; Dong, H.; Zhang, Z.; Fan, T. Metabolic variation dictates cardiac pathogenesis in patients with tetralogy of fallot. Front. Pediatr. 2022, 9, 819195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, M.; Zhou, Z.; Tu, J.; Zhang, R.; Huang, Y.; Zhang, Y.; Cui, H.; Zhuang, J.; Chen, J. Integrative metabolomics dictate distinctive signature profiles in patients with tetralogy of fallot. Pediatr. Res. 2025, 97, 785–797. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, W.; Jiao, J.; Nielsen, J.B.; Mathis, M.R.; Heydarpour, M.; Lettre, G.; Folkersen, L.; Prakash, S.; Schurmann, C. Protein-altering and regulatory genetic variants near gata4 implicated in bicuspid aortic valve. Nat. Commun. 2017, 8, 15481. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Feng, H.; Zhou, W.; Qiu, P.; Zhou, D.; Yang, D.; Zhang, J.; Willer, C.; Chen, Y.E. Bicuspid aortic valve–associated regulatory regions reveal gata4 regulation and function during human-induced pluripotent stem cell–based endothelial-mesenchymal transition—Brief report. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 312–322. [Google Scholar] [CrossRef]
- Ye, L.; Yu, Y.; Zhao, Z.-A.; Zhao, D.; Ni, X.; Wang, Y.; Fang, X.; Yu, M.; Wang, Y.; Tang, J.-M. Patient-specific ipsc-derived cardiomyocytes reveal abnormal regulation of fgf16 in a familial atrial septal defect. Cardiovasc. Res. 2022, 118, 859–871. [Google Scholar] [CrossRef]
- Sharma, A.; Wasson, L.K.; Willcox, J.A.; Morton, S.U.; Gorham, J.M.; DeLaughter, D.M.; Neyazi, M.; Schmid, M.; Agarwal, R.; Jang, M.Y. Gata6 mutations in hipscs inform mechanisms for maldevelopment of the heart, pancreas, and diaphragm. eLife 2020, 9, e53278. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Xiang, M.; Olson, P.; Guzzetta, A.; Zhang, K.; Moskowitz, I.P.; Xie, L. Gata4 potentiates second heart field proliferation and hedgehog signaling for cardiac septation. Proc. Natl. Acad. Sci. USA 2017, 114, E1422–E1431. [Google Scholar] [CrossRef]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Winterstern, A.; Feldman, O.; Gepstein, A.; Arbel, G.; Hammerman, H. Modelling the long qt syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–229. [Google Scholar] [CrossRef]
- Liang, P.; Sallam, K.; Wu, H.; Li, Y.; Itzhaki, I.; Garg, P.; Zhang, Y.; Termglichan, V.; Lan, F.; Gu, M. Patient-specific and genome-edited induced pluripotent stem cell–derived cardiomyocytes elucidate single-cell phenotype of brugada syndrome. J. Am. Coll. Cardiol. 2016, 68, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- McKeithan, W.L.; Savchenko, A.; Yu, M.S.; Cerignoli, F.; Bruyneel, A.A.; Price, J.H.; Colas, A.R.; Miller, E.W.; Cashman, J.R.; Mercola, M. An automated platform for assessment of congenital and drug-induced arrhythmia with hipsc-derived cardiomyocytes. Front. Physiol. 2017, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Gifford, C.A.; Ranade, S.S.; Samarakoon, R.; Salunga, H.T.; De Soysa, T.Y.; Huang, Y.; Zhou, P.; Elfenbein, A.; Wyman, S.K.; Bui, Y.K. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science 2019, 364, 865–870. [Google Scholar] [CrossRef]
- van Ouwerkerk, A.F.; Bosada, F.M.; van Duijvenboden, K.; Houweling, A.C.; Scholman, K.T.; Wakker, V.; Allaart, C.P.; Uhm, J.-S.; Mathijssen, I.B.; Baartscheer, T. Patient-specific tbx5-g125r variant induces profound transcriptional deregulation and atrial dysfunction. Circulation 2022, 145, 606–619. [Google Scholar] [CrossRef]
- Lahm, H.; Dzilic, E.; Neb, I.; Doppler, S.A.; Schneider, S.; Lange, R.; Krane, M.; Dreßen, M. Correction of a deleterious tbx5 mutation in an induced pluripotent stem cell line (dhmi004-a-1) using a completely plasmid-free crispr/cas 9 approach. Stem Cell Res. 2023, 70, 103126. [Google Scholar] [CrossRef]
- Jiang, Y.; Tarzami, S.; Burch, J.B.; Evans, T. Common role for each of the cgata-4/5/6 genes in the regulation of cardiac morphogenesis. Dev. Genet. 1998, 22, 263–277. [Google Scholar] [CrossRef]
- Franco, E.D.; Shaw-Smith, C.; Flanagan, S.E.; Shepherd, M.; Hattersley, A.T.; Ellard, S. GATA6 mutations cause a broad phenotypic spectrum of diabetes from pancreatic agenesis to adult-onset diabetes without exocrine insufficiency. Diabetes 2013, 62, 993–997. [Google Scholar] [CrossRef]
- Richards, A.A.; Garg, V. Genetics of congenital heart disease. Curr. Cardiol. Rev. 2010, 6, 91–97. [Google Scholar] [CrossRef]
- Miles, M.L.; Cowan, N.; Jackson, G. A nonsense gata6 mutation explains history of congenital heart defects and 10 years of poorly-controlled diabetes lacking dka in a non-obese 30 year-old incidentally found to have pancreatic hypoplasia. AACE Clin. Case Rep. 2020, 6, e123–e126. [Google Scholar] [CrossRef]
- Maitra, M.; Koenig, S.N.; Srivastava, D.; Garg, V. Identification of gata6 sequence variants in patients with congenital heart defects. Pediatr. Res. 2010, 68, 281–285. [Google Scholar] [CrossRef]
- Pugnaloni, F.; Martini, L.; De Rose, D.U.; Landolfo, F.; Giliberti, P.; Ruta, R.; Novelli, A.; Rapini, N.; Barbetti, F.; Toscano, A. A new variant in the GATA6 gene associated with tracheoesophageal fistula, pulmonary vein stenosis and neonatal diabetes. Horm. Res. Paediatr. 2024, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef]
- Zaidi, S.; Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 2017, 120, 923–940. [Google Scholar] [CrossRef]
- Sevim Bayrak, C.; Zhang, P.; Tristani-Firouzi, M.; Gelb, B.D.; Itan, Y. De novo variants in exomes of congenital heart disease patients identify risk genes and pathways. Genome Med. 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Chai, S.; Wan, X.; Ramirez-Navarro, A.; Tesar, P.J.; Kaufman, E.S.; Ficker, E.; George, A.L.; Deschênes, I. Physiological genomics identifies genetic modifiers of long qt syndrome type 2 severity. J. Clin. Investig. 2018, 128, 1043–1056. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; He, D.; Cheng, Y.; Li, Y.; Qi, D.; Li, B.; Zheng, F. Identification of DNA methylation-regulated genes as potential biomarkers for coronary heart disease via machine learning in the framingham heart study. Clin. Epigenetics 2022, 14, 122. [Google Scholar] [CrossRef]
- Chhatwal, K.; Smith, J.J.; Bola, H.; Zahid, A.; Venkatakrishnan, A.; Brand, T. Uncovering the genetic basis of congenital heart disease: Recent advancements and implications for clinical management. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 464–480. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Moukette, B.; Hayasaka, T.; Haskell, A.K.; Mah, J.; Sepúlveda, M.N.; Tang, Y.; Kim, I.-m. Noncoding rnas as key regulators for cardiac development and cardiovascular diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 166. [Google Scholar] [CrossRef]
- Diez-Cuñado, M.; Wei, K.; Bushway, P.J.; Maurya, M.R.; Perera, R.; Subramaniam, S.; Ruiz-Lozano, P.; Mercola, M. Mirnas that induce human cardiomyocyte proliferation converge on the hippo pathway. Cell Rep. 2018, 23, 2168–2174. [Google Scholar] [CrossRef]
- Ashiq, S.; Sabar, M.F. The role of genetics in the understanding of complex congenital heart diseases. Pak. Heart J. 2022, 54, 383–384. [Google Scholar] [CrossRef]
- Blinova, K.; Dang, Q.; Millard, D.; Smith, G.; Pierson, J.; Guo, L.; Brock, M.; Lu, H.; Kraushaar, U.; Zeng, H. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 2018, 24, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Blinova, K.; Stohlman, J.; Vicente, J.; Chan, D.; Johannesen, L.; Hortigon-Vinagre, M.P.; Zamora, V.; Smith, G.; Crumb, W.J.; Pang, L. Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 2017, 155, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kitani, T.; Ong, S.-G.; Lam, C.K.; Rhee, J.-W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Lan, F.; Lee, A.S.; Gong, T.; Sanchez-Freire, V.; Wang, Y.; Diecke, S.; Sallam, K.; Knowles, J.W.; Wang, P.J. Drug screening using a library of human induced pluripotent stem cell–derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 2013, 127, 1677–1691. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, X.; Wu, C.; He, X.; Huang, Z.; Li, Y.; Liao, L.; Xiang, J.; Li, M.; Wu, L. Intracellular calcium dysregulation in heart and brain diseases: Insights from induced pluripotent stem cell studies. J. Neuropathol. Exp. Neurol. 2024, 83, 993–1002. [Google Scholar] [CrossRef]
- Yu, Y.; Deschenes, I.; Zhao, M.-T. Precision medicine for long qt syndrome: Patient-specific ipscs take the lead. Expert Rev. Mol. Med. 2023, 25, e5. [Google Scholar] [CrossRef]
- Lin, H.; McBride, K.L.; Garg, V.; Zhao, M.-T. Decoding genetics of congenital heart disease using patient-derived induced pluripotent stem cells (ipscs). Front. Cell Dev. Biol. 2021, 9, 630069. [Google Scholar] [CrossRef]
- Garg, P.; Oikonomopoulos, A.; Chen, H.; Li, Y.; Lam, C.K.; Sallam, K.; Perez, M.; Lux, R.L.; Sanguinetti, M.C.; Wu, J.C. Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J. Am. Coll. Cardiol. 2018, 72, 62–75. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Hu, Y.-F.; Hsieh, P.C.-H. The role of large animal models in cardiac regeneration research using human pluripotent stem cell-derived cardiomyocytes. Curr. Cardiol. Rep. 2023, 25, 325–331. [Google Scholar] [CrossRef]
- Vo, Q.D.; Saito, Y.; Nakamura, K.; Iida, T.; Yuasa, S. Induced pluripotent stem cell-derived cardiomyocytes therapy for ischemic heart disease in animal model: A meta-analysis. Int. J. Mol. Sci. 2024, 25, 987. [Google Scholar] [CrossRef]
- Liu, D.; Chen, M.; Mendoza, B.; Cheng, H.; Hu, R.; Li, L.; Trinh, C.T.; Tuskan, G.A.; Yang, X. Crispr/cas9-mediated targeted mutagenesis for functional genomics research of crassulacean acid metabolism plants. J. Exp. Bot. 2019, 70, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.T. Crispr/cas9 targeted mutagenesis for functional genetics in maize. Plants 2021, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Bai, R.; Wu, F.; Lu, W.-J.; Zhang, J. Generation of a tbx5 homozygous knockout embryonic stem cell line (wae009-a-45) by crispr/cas9 genome editing. Stem Cell Res. 2021, 51, 102156. [Google Scholar] [CrossRef] [PubMed]
- Hockemeyer, D.; Jaenisch, R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 2016, 18, 573–586. [Google Scholar] [CrossRef]
- Deacon, D.C.; Happe, C.L.; Chen, C.; Tedeschi, N.; Manso, A.M.; Li, T.; Dalton, N.D.; Peng, Q.; Farah, E.N.; Gu, Y. Combinatorial interactions of genetic variants in human cardiomyopathy. Nat. Biomed. Eng. 2019, 3, 147–157. [Google Scholar] [CrossRef]
- Fear, V.S.; Forbes, C.A.; Shaw, N.C.; Farley, K.O.; Mantegna, J.L.; Htun, J.P.; Syn, G.; Viola, H.; Cserne Szappanos, H.; Hool, L. Gene editing and cardiac disease modelling for the interpretation of genetic variants of uncertain significance in congenital heart disease. Stem Cell. Res. Ther. 2023, 14, 345. [Google Scholar] [CrossRef]
- Carvalho, T. Stem cell–derived heart cells injected into first patient. Nat. Med. 2023, 29, 1030–1031. [Google Scholar] [CrossRef]
- HeartWorks, I. “Autologous Induced Pluripotent Stem Cells of Cardiac Lineage for Congenital Heart Disease.” World Health Organization International Clinical Trials Registry Platform. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05647213 (accessed on 23 January 2025).
- U.S. FOOD & DRUG. “Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products (hct/ps) Small Entity Compliance Guide.” Silver Spring, MD: Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services. 2022. Available online: https://www.fda.gov/media/70689/download (accessed on 23 January 2025).
- Martin, U. Therapeutic application of pluripotent stem cells: Challenges and risks. Front. Med. 2017, 4, 229. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Del Álamo, J.C.; Lemons, D.; Serrano, R.; Savchenko, A.; Cerignoli, F.; Bodmer, R.; Mercola, M. High throughput physiological screening of ipsc-derived cardiomyocytes for drug development. Biochim. Biophys. Acta, Mol. Cell Res. 2016, 1863, 1717–1727. [Google Scholar] [CrossRef]
- Danker, T.; Möller, C. Early identification of herg liability in drug discovery programs by automated patch clamp. Front. Pharmacol. 2014, 5, 203. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Kimler, K.; Boucher, S.; Asprer, J.; Sylakowski, K.; Lakshmipathy, U.; Kuninger, D.; Piper, D. A robust platform for generation and high throughput functional analysis of human ipsc-derived cardiomyocytes. In Proceedings of the Drug Discovery (ACC 2016), Liverpool, UK, 13–14 October 2016. [Google Scholar]
- Feyen, D.A.; Perea-Gil, I.; Maas, R.G.; Harakalova, M.; Gavidia, A.A.; Arthur Ataam, J.; Wu, T.-H.; Vink, A.; Pei, J.; Vadgama, N. Unfolded protein response as a compensatory mechanism and potential therapeutic target in pln r14del cardiomyopathy. Circulation 2021, 144, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P. Human ipsc-based cardiac microphysiological system for drug screening applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef] [PubMed]
- Fermini, B.; Hancox, J.C.; Abi-Gerges, N.; Bridgland-Taylor, M.; Chaudhary, K.W.; Colatsky, T.; Correll, K.; Crumb, W.; Damiano, B.; Erdemli, G. A new perspective in the field of cardiac safety testing through the comprehensive in vitro proarrhythmia assay paradigm. J. Biomol. Screen. 2016, 21, 1–11. [Google Scholar] [CrossRef]
- Qu, Y.; Gao, B.; Fang, M.; Vargas, H.M. Human embryonic stem cell derived cardiac myocytes detect herg-mediated repolarization effects, but not nav1. 5 induced depolarization delay. J. Pharmacol. Toxicol. Methods 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Bruyneel, A.A.; Muser, T.; Parthasarathy, V.; Feyen, D.; Mercola, M. Phenotypic screening of ipsc-derived cardiomyocytes for cardiotoxicity testing and therapeutic target discovery. In Cardiovascular Regenerative Medicine: Tissue Engineering and Clinical Applications; Serpooshan, V., Wu, S.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 19–34. [Google Scholar]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Kempf, H.; Olmer, R.; Kropp, C.; Rückert, M.; Jara-Avaca, M.; Robles-Diaz, D.; Franke, A.; Elliott, D.A.; Wojciechowski, D.; Fischer, M. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014, 3, 1132–1146. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-chip: A fast track for engineered human tissues in drug development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, H.; Dong, X.; Zhang, X.; Wang, C.; Li, X.; Zhang, X.; Na, J.; Zhou, J.; Wang, C. Single-cell atlas of multilineage cardiac organoids derived from human induced pluripotent stem cells. Life Med. 2022, 1, 179–195. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Y.; Sun, Y.; Han, R.; Jiang, H. Current advances in human-induced pluripotent stem cell-based models and therapeutic approaches for congenital heart disease. Mol. Cell. Biochem. 2025, 480, 159–172. [Google Scholar] [CrossRef]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tønnessen, T.; Kryshtal, D.O. Thyroid and glucocorticoid hormones promote functional t-tubule development in human-induced pluripotent stem cell–derived cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; Van Helden, R.W.; Garcia, A.K.; Mircea, M.; Kostidis, S.; Davis, R.P. Human-ipsc-derived cardiac stromal cells enhance maturation in 3d cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 2020, 26, 862–879. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Ruiz, S.; Yi, F.; Herrerias, A.; Batchelder, E.M.; Belmonte, J.C.I. Rapid and highly efficient generation of induced pluripotent stem cells from human umbilical vein endothelial cells. PLoS ONE 2011, 6, e19743. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Bellin, M.; Casini, S.; Davis, R.P.; D’aniello, C.; Haas, J.; Ward-van Oostwaard, D.; Tertoolen, L.G.; Jung, C.B.; Elliott, D.A.; Welling, A. Isogenic human pluripotent stem cell pairs reveal the role of a kcnh2 mutation in long-qt syndrome. EMBO J. 2013, 32, 3161–3175. [Google Scholar] [CrossRef]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Soares, F.A.; Sheldon, M.; Rao, M.; Mummery, C.; Vallier, L. International coordination of large-scale human induced pluripotent stem cell initiatives: Wellcome trust and isscr workshops white paper. Stem Cell Rep. 2014, 3, 931–939. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kurtz, A.; Yuan, B.-Z.; Zeng, F.; Lomax, G.; Loring, J.F.; Crook, J.; Ju, J.H.; Clarke, L.; Inamdar, M.S. Report of the international stem cell banking initiative workshop activity: Current hurdles and progress in seed-stock banking of human pluripotent stem cells. Stem Cells Transl. Med. 2017, 6, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Colatsky, T.; Fermini, B.; Gintant, G.; Pierson, J.B.; Sager, P.; Sekino, Y.; Strauss, D.G.; Stockbridge, N. The comprehensive in vitro proarrhythmia assay (cipa) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 2016, 81, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Katori, R.; Sasamoto, Y.; Taniwaki, Y.; Takayanagi, H.; Tsujikawa, M.; Sekiguchi, K.; Quantock, A.J.; Nishida, K. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human ips cells. Nat. Protoc. 2017, 12, 683–696. [Google Scholar] [CrossRef]
- Tavernier, G.; Wolfrum, K.; Demeester, J.; De Smedt, S.C.; Adjaye, J.; Rejman, J. Activation of pluripotency-associated genes in mouse embryonic fibroblasts by non-viral transfection with in vitro-derived mrnas encoding oct4, sox2, klf4 and cmyc. Biomaterials 2012, 33, 412–417. [Google Scholar] [CrossRef]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Wang, L.; Ma, X.; Pu, J.; Lin, L.; Deng, Q.; Li, Y.; Wang, W.; Jin, Y. A fast chemical reprogramming system promotes cell identity transition through a diapause-like state. Nat. Cell Biol. 2023, 25, 1146–1156. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.-N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef]
- Hendry, S.L., II; van der Bogt, K.E.; Sheikh, A.Y.; Arai, T.; Dylla, S.J.; Drukker, M.; McConnell, M.V.; Kutschka, I.; Hoyt, G.; Cao, F. Multimodal evaluation of in vivo magnetic resonance imaging of myocardial restoration by mouse embryonic stem cells. J. Thorac. Cardiovasc. Surg. 2008, 136, 1028–1037. [Google Scholar] [CrossRef]
- Hung, T.-C.; Suzuki, Y.; Urashima, T.; Caffarelli, A.; Hoyt, G.; Sheikh, A.Y.; Yeung, A.C.; Weissman, I.; Robbins, R.C.; Bulte, J.M. Multimodality evaluation of the viability of stem cells delivered into different zones of myocardial infarction. Circ. Cardiovasc. Imaging 2008, 1, 6–13. [Google Scholar] [CrossRef]
- Terrovitis, J.; Stuber, M.; Youssef, A.; Preece, S.; Leppo, M.; Kizana, E.; Schär, M.; Gerstenblith, G.; Weiss, R.G.; Marbán, E. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 2008, 117, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Sanganalmath, S.K.; Bolli, R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Leslie, S.; Martin, N.G.; Peschanski, M.; Rao, M.; Taylor, C.J.; Trounson, A.; Turner, D.; Yamanaka, S.; Wilmut, I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 2013, 13, 382–384. [Google Scholar] [CrossRef] [PubMed]
- King, N.M.; Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell. Res. Ther. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Yap, K.K. Inequality issues in stem cell medicine. Stem Cells Transl. Med. 2016, 5, 265–266. [Google Scholar] [CrossRef]
- Baylis, F.; Robert, J.S. The inevitability of genetic enhancement technologies. Bioethics 2004, 18, 1–26. [Google Scholar] [CrossRef]
- Sugarman, J. Human stem cell ethics: Beyond the embryo. Cell Stem Cell 2008, 2, 529–533. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Sun, J.P.; Zuo, R.; Shen, Y.; Zhao, M.; Zhao, W.; Luo, Z. Cardiac function evaluated by two-dimensional speckle tracking imaging in fetuses with congenital heart disease of ventricular afterload increase. J. Matern. Fetal Neonatal Med. 2023, 36, 2214663. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Bruno, A.; Nicolosi, G.L.; Bianchi, S.; Lombardo, M.; Muti, P. Echocardiographic assessment of biventricular mechanics of fetuses and infants of gestational diabetic mothers: A systematic review and meta-analysis. Children 2024, 11, 1451. [Google Scholar] [CrossRef]
- Miao, Q.; Shim, W.; Tee, N.; Lim, S.Y.; Chung, Y.Y.; Ja, K.M.M.; Ooi, T.H.; Tan, G.; Kong, G.; Wei, H. Ipsc-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium. J. Cell. Mol. Med. 2014, 18, 1644–1654. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashali, M.A.; Deschênes, I.; Saad, N.S. Transformative Potential of Induced Pluripotent Stem Cells in Congenital Heart Disease Research and Treatment. Children 2025, 12, 669. https://doi.org/10.3390/children12060669
Mashali MA, Deschênes I, Saad NS. Transformative Potential of Induced Pluripotent Stem Cells in Congenital Heart Disease Research and Treatment. Children. 2025; 12(6):669. https://doi.org/10.3390/children12060669
Chicago/Turabian StyleMashali, Mohammed A., Isabelle Deschênes, and Nancy S. Saad. 2025. "Transformative Potential of Induced Pluripotent Stem Cells in Congenital Heart Disease Research and Treatment" Children 12, no. 6: 669. https://doi.org/10.3390/children12060669
APA StyleMashali, M. A., Deschênes, I., & Saad, N. S. (2025). Transformative Potential of Induced Pluripotent Stem Cells in Congenital Heart Disease Research and Treatment. Children, 12(6), 669. https://doi.org/10.3390/children12060669






