Sex-Related Differences in Hip Kinematics During General Movements in Early Infancy: A Biomechanical Cross-Sectional Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design/Ethics
2.2. Participants
2.3. Procedure and Video Processing
2.4. Statistical Analysis
3. Results
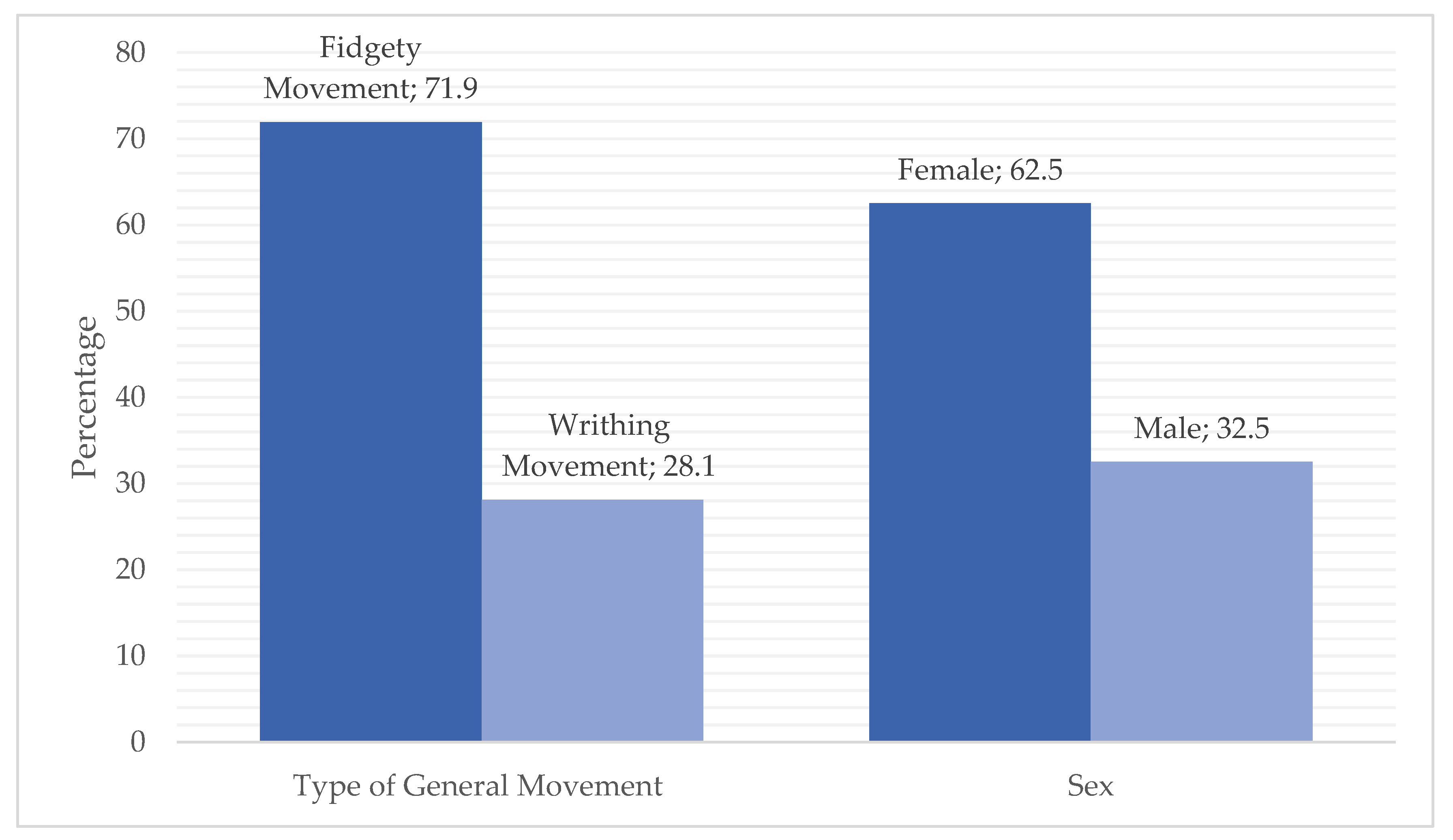
3.1. Frequency Statistics
3.2. Interrater Reliability
3.3. Laterality and Overall RoM Patterns
3.4. Correlation Analyses
3.5. Effects of Sex and General Movement Type on Hip Abduction Range of Motion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GM | General Movements |
| FM | Fidgety movements |
| WM | Writhing movements |
| ASIS | Anterior superior iliac spine |
| ABD | Abduction |
| RoM | Range of motion |
References
- Wu, Y.C.; van Rijssen, I.M.; Buurman, M.T.; Dijkstra, L.J.; Hamer, E.G.; Hadders-Algra, M. Temporal and spatial localisation of general movement complexity and variation—Why Gestalt assessment requires experience. Acta Paediatr. Int. J. Paediatr. 2021, 110, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Balta, D.; Kuo, H.; Wang, J.; Porco, I.G.; Morozova, O.; Schladen, M.M.; Cereatti, A.; Lum, P.S.; Della Croce, U. Characterization of Infants’ General Movements Using a Commercial RGB-Depth Sensor and a Deep Neural Network Tracking Processing Tool: An Exploratory Study. Sensors 2022, 22, 7426. [Google Scholar] [CrossRef]
- Einspieler, C.; Bos, A.F.; Spittle, A.J.; Bertoncelli, N.; Burger, M.; Peyton, C.; Toldo, M.; Utsch, F.; Zhang, D.; Marschik, P.B. The General Movement Optimality Score-Revised (GMOS-R) with Socioeconomically Stratified Percentile Ranks. J. Clin. Med. 2024, 13, 2260. [Google Scholar] [CrossRef]
- Chirigos, A.J.; Ostrander, B.; Burton, V.J.; Mirecki, M.; Maitre, N.L. Prechtl’s General Movements Assessment at writhing age guides MRI use in clinical implementation network. Pediatr. Res. 2024, 95, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Letzkus, L.; Pulido, J.V.; Adeyemo, A.; Baek, S.; Zanelli, S. Machine learning approaches to evaluate infants’ general movements in the writhing stage—A pilot study. Sci. Rep. 2024, 14, 4522. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Prechtl, H.F.R. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, C.Y.P.; Einspieler, C.; Genovesi, F.F.; Ibidi, S.M.; Hasue, R.H. The general movement checklist: A guide to the assessment of general movements during preterm and term age. J. Pediatr. 2021, 97, 445–452. [Google Scholar] [CrossRef]
- Barnes, F.; Graham, L.; Loganathan, P.; Nair, V. General Movement Assessment Predicts Neuro-Developmental Outcome in Very Low Birth Weight Infants at Two Years—A Five-Year Observational Study. Indian. J. Pediatr. 2021, 88, 28–33. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Tacke, U.; Pietz, J.; Rupp, A.; Philippi, H. Predictive value of the General Movements Assessment and Standardized Infant NeuroDevelopmental Assessment in infants at high risk of neurodevelopmental disorders. Dev. Med. Child. Neurol. 2024, 66, 1361–1368. [Google Scholar] [CrossRef]
- Meinecke, L.; Breitbach-Faller, N.; Bartz, C.; Damen, R.; Rau, G.; Disselhorst-Klug, C. Movement analysis in the early detection of newborns at risk for developing spasticity due to infantile cerebral palsy. Hum. Mov. Sci. 2006, 25, 125–144. [Google Scholar] [CrossRef]
- Alexander, C.; Amery, N.; Salt, A.; Morgan, C.; Spittle, A.; Ware, R.S.; Elliott, C.; Valentine, J. Inter-rater reliability and agreement of the General Movement Assessment and Motor Optimality Score-Revised in a large population-based sample. Early Hum. Dev. 2024, 193, 106019. [Google Scholar] [CrossRef] [PubMed]
- Jardine, L.A.; Mausling, R.M.; Caldararo, D.; Colditz, P.W.; Davies, M.W. Accelerometer measures in extremely preterm and or extremely low birth weight infants and association with abnormal general movements assessments at 28- and 32-weeks postmenstrual age. Early Hum. Dev. 2022, 174, 105685. [Google Scholar] [CrossRef] [PubMed]
- Tacchino, C.; Impagliazzo, M.; Maggi, E.; Bertamino, M.; Blanchi, I.; Campone, F.; Durand, P.; Fato, M.; Giannoni, P.; Iandolo, R.; et al. Spontaneous movements in the newborns: A tool of quantitative video analysis of preterm babies. Comput. Methods Programs Biomed. 2021, 199, 105838. [Google Scholar] [CrossRef]
- Solopova, I.A.; Selionov, V.A.; Dolinskaya, I.Y.; Keshishian, E.S. General Movements as a Factor Reflecting the Normal or Impaired Motor Development in Infants. Hum. Physiol. 2020, 46, 432–442. [Google Scholar] [CrossRef]
- Sizer, P.S.; James, C.R. Considerations of Sex Differences in Musculoskeletal Anatomy BT—The Active Female: Health Issues Throughout the Lifespan; Robert-McComb, J.J., Norman, R., Zumwalt, M., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 25–54. [Google Scholar] [CrossRef]
- Leong, A. Sexual dimorphism of the pelvic architecture: A struggling response to destructive and parsimonious forces by natural & mate selection. McGill J. Med. 2020, 9, 61–66. [Google Scholar] [CrossRef]
- Castagnini, F.; Bordini, B.; Cosentino, M.; Di Liddo, M.; Tella, G.; Masetti, C.; Traina, F. Age and Sex Influence the Use of Modular Femoral Components in Total Hip Arthroplasty Performed for Primary Osteoarthritis. J. Clin. Med. 2023, 12, 984. [Google Scholar] [CrossRef]
- Taylor-Haas, J.A.; Long, J.T.; Garcia, M.C.; Rauh, M.J.; Paterno, M.V.; Brindle, R.A.; Bazett-Jones, D.M.; Ford, K.R. The influence of maturation and sex on pelvis and hip kinematics in youth distance runners. J. Sci. Med. Sport. 2022, 25, 272–278. [Google Scholar] [CrossRef]
- Kiapour, A.; Joukar, A.; Elgafy, H.; Erbulut, D.U.; Agarwal, A.K.; Goel, V.K. Biomechanics of the Sacroiliac Joint: Anatomy, Function, Biomechanics, Sexual Dimorphism, and Causes of Pain. Int. J. Spine Surg. 2020, 14, S3–S13. [Google Scholar] [CrossRef]
- Desseauve, D.; Fradet, L.; Gachon, B.; Cherni, Y.; Lacouture, P.; Pierre, F. Biomechanical comparison of squatting and “optimal” supine birth positions. J. Biomech. 2020, 105, 109783. [Google Scholar] [CrossRef]
- Liu, J.; Lewton, K.L.; Colletti, P.M.; Powers, C.M. Hip Adduction during Running: Influence of Sex, Hip Abductor Strength and Activation, and Pelvis and Femur Morphology. Med. Sci. Sports Exerc. 2021, 53, 2346–2353. [Google Scholar] [CrossRef]
- Xie, P.P.; István, B.; Liang, M. Sex-specific differences in biomechanics among runners: A systematic review with meta-analysis. Front. Physiol. 2022, 13, 994076. [Google Scholar] [CrossRef] [PubMed]
- Øberg, G.K.; Jacobsen, B.K.; Jørgensen, L. Predictive value of general movement assessment for cerebral palsy in routine clinical practice. Phys. Ther. 2015, 95, 1489–1495. [Google Scholar] [CrossRef]
- Braga, S.R.; Júnior, A.R.; Akkari, M.; Sassioto Silveira Figueiredo, M.J.P.; Waisberg, G.; Santili, C. Developmental Dysplasia of the Hip—Part 1. Rev. Bras. Ortop. 2022, 58, E839–E846. [Google Scholar] [CrossRef]
- Lim, Y.; Chambers, T.; Walck, C.; Siddicky, S.; Mannen, E.; Huayamave, V. Challenges in Kinetic-Kinematic Driven Musculoskeletal Subject-Specific Infant Modeling. Math. Comput. Appl. 2022, 27, 1. [Google Scholar] [CrossRef]
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Norkin, C.; White, J. Manual de Goniometría: Evaluación de la Movilidad Articular; Paidotribo: Barcelona, Spain, 2019; Volume 592. [Google Scholar]
- Mondal, R.; Nandy, A.; Datta, D.; Majumdar, R.; Hazra, A.; Das, S.K. Newborn joint mechanics. J. Matern. Fetal Neonatal. Med. 2022, 35, 7259–7266. [Google Scholar] [CrossRef] [PubMed]
- Valentin, T.; Uhl, K.; Einspieler, C. The effectiveness of training in Prechtl’s method on the qualitative assessment of general movements. Early Hum. Dev. 2005, 81, 623–627. [Google Scholar] [CrossRef]
- Gao, Q.; Yao, S.; Tian, Y.; Zhang, C.; Zhao, T.; Wu, D.; Yu, G.; Lu, H. Automating General Movements Assessment with quantitative deep learning to facilitate early screening of cerebral palsy. Nat. Commun. 2023, 14, 1–11. [Google Scholar] [CrossRef]
- Pires, C.D.S.; Marba, S.T.M.; Caldas, J.P.D.S.; Stopiglia, M.D.C.S. Predictive value of the general movements assessment in preterm infants: A meta-analysis. Rev. Paul. Pediatria 2020, 38, e2018286. [Google Scholar] [CrossRef]
- Beunen, G.; Malina, R.M. Growth and biologic maturation: Relevance to athletic performance. Young Athl. 2008, 1, 3–17. [Google Scholar] [CrossRef]
- Boswell, L.; Adde, L.; Fjørtoft, T.; Pascal, A.; Russow, A.; Støen, R.; Thomas, N.; Broeck, C.V.D.; de Regnier, R.-A. Development of Movement and Postural Patterns in Full-Term Infants Who Are at Low Risk in Belgium, India, Norway, and the United States. Phys. Ther. 2024, 104, pzae081. [Google Scholar] [CrossRef] [PubMed]
- Utsch, F.; Silva, L.B.; da Cunha Júnior, A.L.; Alves, E.P.; Diniz Silva, C.R.; Vilaça, D.M.F.; Antunes, A.A.M. The role of fidgety movements and early motor repertoire in predicting mobility outcomes in infants with myelomeningocele. Eur. J. Paediatr. Neurol. 2024, 51, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Kiyama, R.; Nakai, Y.; Kawada, M.; Miyazaki, T.; Takeshita, Y.; Makizako, H. Sex differences in age-related differences in joint motion during gait in community-dwelling middle-age and older individuals. Gait Posture 2023, 103, 153–158. [Google Scholar] [CrossRef]
- Bailey, C.A.; Porta, M.; Pilloni, G.; Arippa, F.; Côté, J.N.; Pau, M. Does variability in motor output at individual joints predict stride time variability in gait? Influences of age, sex, and plane of motion. J. Biomech. 2020, 99, 109574. [Google Scholar] [CrossRef] [PubMed]
- Aartolahti, E.; Lönnroos, E.; Hartikainen, S.; Häkkinen, A. Long-term strength and balance training in prevention of decline in muscle strength and mobility in older adults. Aging Clin. Exp. Res. 2020, 32, 59–66. [Google Scholar] [CrossRef]
- Hunter, S.K.; Angadi, S.S.; Bhargava, A.; Harper, J.; Hirschberg, A.L.; Levine, B.D.; Moreau, K.L.; Nokoff, N.J.; Stachenfeld, N.S.; Bermon, S. The Biological Basis of Sex Differences in Athletic Performance: Consensus Statement for the American College of Sports Medicine. Transl. J. Am. Coll. Sports Med. 2023, 8, 1–33. [Google Scholar] [CrossRef]
- Kwong, A.K.L.; Fitzgerald, T.L.; Doyle, L.W.; Cheong, J.L.Y.; Spittle, A.J. Predictive validity of spontaneous early infant movement for later cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2018, 60, 480–489. [Google Scholar] [CrossRef]

| Variable Pair | r | p-Value | |
|---|---|---|---|
| Sex | Right Hip ABD RoM | 0.069 | 0.707 |
| Left Hip ABD RoM | −0.074 | 0.688 | |
| GM Type | Right Hip ABD | −0.073 | 0.693 |
| Left Hip ABD | 0.053 | 0.772 | |
| Factor | Right Hip F | Right Hip p | Right Hip η2 | Left Hip F | Left Hip p | Left Hip η2 |
|---|---|---|---|---|---|---|
| Sex | 1.23 | 0.277 | 0.013 | 0.26 | 0.613 | 0.003 |
| GM Type | 2.25 | 0.145 | 0.024 | 0.05 | 0.828 | 0.001 |
| Sex × GM Type | 0.01 | 0.924 | 0.0002 | 0.15 | 0.703 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Santy, L.F.; Gutiérrez, B.M.T.; Iza, C.M.C.; Pérez, J.P.H. Sex-Related Differences in Hip Kinematics During General Movements in Early Infancy: A Biomechanical Cross-Sectional Study. Children 2025, 12, 651. https://doi.org/10.3390/children12050651
Flores-Santy LF, Gutiérrez BMT, Iza CMC, Pérez JPH. Sex-Related Differences in Hip Kinematics During General Movements in Early Infancy: A Biomechanical Cross-Sectional Study. Children. 2025; 12(5):651. https://doi.org/10.3390/children12050651
Chicago/Turabian StyleFlores-Santy, Lucía Fernanda, Barbara Martina Trujillo Gutiérrez, Cristina Mileny Campaña Iza, and Juan Pablo Hervás Pérez. 2025. "Sex-Related Differences in Hip Kinematics During General Movements in Early Infancy: A Biomechanical Cross-Sectional Study" Children 12, no. 5: 651. https://doi.org/10.3390/children12050651
APA StyleFlores-Santy, L. F., Gutiérrez, B. M. T., Iza, C. M. C., & Pérez, J. P. H. (2025). Sex-Related Differences in Hip Kinematics During General Movements in Early Infancy: A Biomechanical Cross-Sectional Study. Children, 12(5), 651. https://doi.org/10.3390/children12050651








