Evaluation of Anthropometric Measurements of 17,693 Newborns: Have Percentile Cut-Off Values Changed?
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCormick, M.C. The contribution of low birth weight to infant mortality and childhood morbidity. N. Engl. J. Med. 1985, 312, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Tint, M.-T.; Fortier, M.V.; Aris, I.M.; Shek, L.P.-C.; Tan, K.H.; Chan, S.-Y.; Gluckman, P.D.; Chong, Y.-S.; Godfrey, K.M.; et al. Which anthropometric measures best reflect neonatal adiposity? Int. J. Obes. 2018, 42, 501–506. [Google Scholar] [CrossRef]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in young adulthood for very-low-birth-weight infants. N. Engl. J. Med. 2002, 346, 149. [Google Scholar] [CrossRef]
- Joglekar, C.V.; Fall, C.H.D.; Deshpande, V.U.; Joshi, N.; Bhalerao, A.; Solat, V.; Deokar, T.M.; Chougule, S.D.; Leary, S.D.; Osmond, C.; et al. Newborn size, infant and childhood growth, and body composition and cardiovascular disease risk factors at the age of 6 years: The Pune Maternal Nutrition Study. Int. J. Obes. 2007, 31, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Köksal, N.; Özkan, H.; Gider, C.; Kiliçbay, I.; Kiliçbay, F.; Can, S.; Öcal, M. Long term Follow-up of Babies with Low Birth Weight (SGA) According to Gestational Age—Review. J. Curr. Pediatr. 2004, 2, 73–79. [Google Scholar]
- Fenton, T.R.; Kim, J.H. A systematic review and metaanalysis to revise the Fenton growth chart for premature infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21(st) Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef]
- World Health Organization. Nutrition for Health. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development; World Health Organization: Geneva, Switzerland, 2009. Available online: https://apps.who.int/iris/handle/10665/44026 (accessed on 1 February 2025).
- Bertino, E.; Di Nicola, P.; Varalda, A.; Occhi, L.; Giuliani, F.; Coscia, A. Neonatal growth charts. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 67–69. [Google Scholar] [CrossRef]
- Kramer, M.S.; Platt, R.W.; Wen, S.W.; Josetph, K.S.; Allen, A.; Abrahamowicz, M.; Blondel, B.; Breart, G. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001, 108, E35. [Google Scholar] [CrossRef]
- Ovali, F. Intrauterine growth curves for Turkish infants born between 25 and 42 weeks of gestation. J. Trop. Pediatr. 2003, 49, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Telatar, B.; Comert, S.; Vitrinel, A.; Erginoz, E. Anthropometric measurements of term neonates from a state hospital in Turkey. East. Mediterr. Health J. 2009, 15, 1412–1419. [Google Scholar] [PubMed]
- Atıcı, A.; Kanık, A.; Çelik, Y.; Helvacı, İ.; Turkish Neonatology Society Study Group. Intrauterine Growth References for Turkish Infants. Turk. Arch. Pediatr. 2024, 59, 270–276. [Google Scholar] [PubMed]
- Kurtoğlu, S.; Hatipoğlu, N.; Mazıcıoğlu, M.M.; eAkın, M.A.; Çoban, D.; Gökoğlu, S.; Baştuğ, O. Body weight, length and head circumference at birth in a cohort of Turkish newborns. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 132–139. [Google Scholar] [CrossRef]
- Salihoglu, O.; Karatekin, G.; Uslu, S.; Can, E.; Baksu, B.; Nuhoglu, A. New intrauterine growth percentiles: A hospital-based study in Istanbul, Turkey. J. Pak. Med. Assoc. 2012, 62, 1070–1074. [Google Scholar] [PubMed]
- Bertino, E.; Di Nicola, P.; Giuliani, F.; Coscia, A.; Varalda, A.; Occhi, L.; Rossi, C. Evaluation of postnatal growth of preterm infants. J. Matern. Fetal Neonatal Med. 2011, 24 (Suppl. S2), 9–11. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus Based Definition of Growth Restriction in the Newborn. J. Pediatr. 2018, 196, 71–76.e1. [Google Scholar] [CrossRef]
- Alur, P. Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants. Front. Pediatr. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kierans, W.J.; Kendall, P.R.W.; Foster, L.T.; Liston, R.M.; Tuk, T. New birth weight and gestational age charts for the British Columbia population. Gender-specific data are now available to help evaluate the health of infants born in BC. BC Med. J. 2006, 48, 28–32. [Google Scholar]
- Pawlus, B.; Wiśniewski, A.; Kubik, P.; Milde, K.; Gmyrek, L.; Pęsko, E. Birth body length, birth body weight and birth head circumference in neonates born in a single centre between 2011 and 2016. Ginekol. Pol. 2017, 88, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.E.; Giuliano, M.A. The neonate with macrosomia. Clin. Obstet. Gynecol. 2000, 43, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Schluchter, M.; Cartar, L.; Rahman, M.; Cuttler, L.; Borawski, E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003, 112, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.L.; Overpeck, M.D.; Maurer, K.R.; Kuczmarski, R.J.; McGlynn, A.; Davis, W.W. Growth of infants and young children born small or large for gestational age: Findings from the Third National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 1998, 152, 1225–1231. [Google Scholar] [CrossRef]
- Lubchenco, L.O.; Hansman, C.; Boyd, E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics 1966, 37, 403–408. [Google Scholar] [CrossRef]
- Voldner, N.; Frey Frøslie, K.; Godang, K.; Bollerslev, J.; Henriksen, T. Determinants of birth weight in boys and girls. Hum. Ontogenet. 2009, 3, 7–12. [Google Scholar] [CrossRef]
- Available online: http://www.sck.gov.tr/wp-content/uploads/2020/08/TNSA2018_ana_Rapor.pdf (accessed on 1 February 2025).






| n = 17,693 | ||
| Gender (n)/(%) | Female | 8700 (49.2%) |
| Male | 8993 (50.8%) | |
| Route of delivery (n)/(%) | NSVD | 9589 (54.2%) |
| C/S | 8104 (45.8%) | |
| APGAR score (min.–max./median) | 1st min | 1–9 (8) |
| 5th min | 5–10 (9) | |
| Gestational week * | 38.21 ± 5.2 | |
| Birth weight (g) * | 3188.49 ± 579.62 | |
| Birth length (cm) * | 49.74 ± 5.7 | |
| Head circumference (cm) * | 34.90 ± 2.6 | |
| Birth Weight Percentages (g) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational Age | Gender | L | M | S | 3rd | 5th | 10th | 15th | 25th | 50th | 75th | 85th | 90th | 95th | 97th |
| 24–26 | Female | −0.319 | 790 | 0.186 | 435 | 545 | 595 | 600 | 650 | 790 | 900 | 930 | 935 | 946 | 1025 |
| Male | 0.079 | 715 | 0.34 | 450 | 475 | 550 | 600 | 655 | 715 | 895 | 960 | 1005 | 1040 | 1240 | |
| 27–29 | Female | 2.832 | 1000 | 0.374 | 515 | 590 | 650 | 680 | 817 | 1000 | 1145 | 1235 | 1510 | 1600 | 1800 |
| Male | 2.943 | 1200 | 0.332 | 650 | 735 | 865 | 900 | 1040 | 1200 | 1345 | 1415 | 1500 | 1690 | 1785 | |
| 30–31 | Female | 1.776 | 1725 | 0.342 | 930 | 1010 | 1130 | 1285 | 1410 | 1725 | 1800 | 1900 | 2000 | 2200 | 2300 |
| Male | 0.815 | 1520 | 0.216 | 850 | 990 | 1115 | 1240 | 1330 | 1520 | 1740 | 1870 | 1985 | 2075 | 2175 | |
| 32 | Female | 0.781 | 1830 | 0.230 | 1170 | 1245 | 1410 | 1470 | 1580 | 1830 | 2150 | 2350 | 2400 | 2630 | 2700 |
| Male | 1.245 | 1830 | 0.285 | 1120 | 1205 | 1385 | 1425 | 1585 | 1830 | 1970 | 2130 | 2240 | 2390 | 2540 | |
| 33 | Female | 1.153 | 2080 | 0.212 | 1490 | 1545 | 1680 | 1730 | 1910 | 2080 | 2350 | 2540 | 2660 | 2855 | 3010 |
| Male | 0.029 | 2210 | 0.176 | 1490 | 1530 | 1620 | 1780 | 1930 | 2210 | 2490 | 2540 | 2640 | 2930 | 3000 | |
| 34 | Female | 0.205 | 2285 | 0.163 | 1585 | 1680 | 1870 | 1960 | 2078 | 2285 | 2490 | 2560 | 2870 | 2990 | 3150 |
| Male | 0.240 | 2390 | 0.157 | 1630 | 1700 | 1885 | 1960 | 2150 | 2390 | 2615 | 2805 | 2870 | 3060 | 3160 | |
| 35 | Female | 0.446 | 2530 | 0.156 | 1870 | 1920 | 2090 | 2170 | 2330 | 2530 | 2825 | 2970 | 3100 | 3250 | 3360 |
| Male | 0.112 | 2565 | 0.169 | 1780 | 1825 | 2050 | 2150 | 2310 | 2565 | 2840 | 2975 | 3070 | 3390 | 3480 | |
| 36 | Female | 0.037 | 2805 | 0.151 | 1970 | 2080 | 2270 | 2355 | 2525 | 2805 | 3040 | 3180 | 3350 | 3485 | 3610 |
| Male | 0.112 | 2565 | 0.169 | 1780 | 1825 | 2050 | 2150 | 2310 | 2565 | 2840 | 2975 | 3070 | 3390 | 3480 | |
| 37 | Female | 0.148 | 3000 | 0.142 | 2190 | 2345 | 2490 | 2620 | 2750 | 3000 | 3235 | 3300 | 3590 | 3730 | 3800 |
| Male | 0.273 | 3085 | 0.133 | 2350 | 2450 | 2630 | 2700 | 2830 | 3085 | 3340 | 3510 | 3645 | 3830 | 3940 | |
| 38 | Female | 0.189 | 3170 | 0.127 | 2450 | 2540 | 2680 | 2790 | 2930 | 3170 | 3430 | 3590 | 3780 | 3840 | 3950 |
| Male | 0.203 | 3300 | 0.122 | 2570 | 2690 | 2825 | 2920 | 3050 | 3300 | 3570 | 3730 | 3845 | 4000 | 4120 | |
| 39 | Female | 0.080 | 3290 | 0.132 | 2500 | 2625 | 2760 | 2850 | 3010 | 3290 | 3580 | 3740 | 3900 | 3990 | 4100 |
| Male | −0.119 | 3450 | 0.119 | 2605 | 2720 | 2910 | 3028 | 3170 | 3450 | 3710 | 3860 | 3950 | 4080 | 4375 | |
| 40 | Female | −0.117 | 3380 | 0.127 | 2670 | 2745 | 2830 | 3015 | 3130 | 3380 | 3685 | 3830 | 3970 | 4140 | 4270 |
| Male | 0.358 | 3530 | 0.126 | 2810,0 | 2880 | 3030 | 3140 | 3260 | 3530 | 3840 | 4030 | 4160 | 4380 | 4500 | |
| 41 | Female | 0.136 | 3480 | 0.124 | 2725 | 2805 | 2995 | 3100 | 3225 | 3480 | 3790 | 4000 | 4100 | 4235 | 4420 |
| Male | −0.018 | 3660 | 0.123 | 2990 | 3055 | 3170 | 3270 | 3360 | 3660 | 3960 | 4130 | 4225 | 4415 | 4630 | |
| 42 | Female | 0.233 | 3550 | 0.136 | 2845 | 2970 | 3025 | 3115 | 3375 | 3550 | 3890 | 4260 | 4270 | 4420 | 4585 |
| Male | 0.792 | 3775 | 0.152 | 3132 | 3200 | 3280 | 3350 | 3490 | 3775 | 4165 | 4260 | 4450 | 4675 | 4890 | |
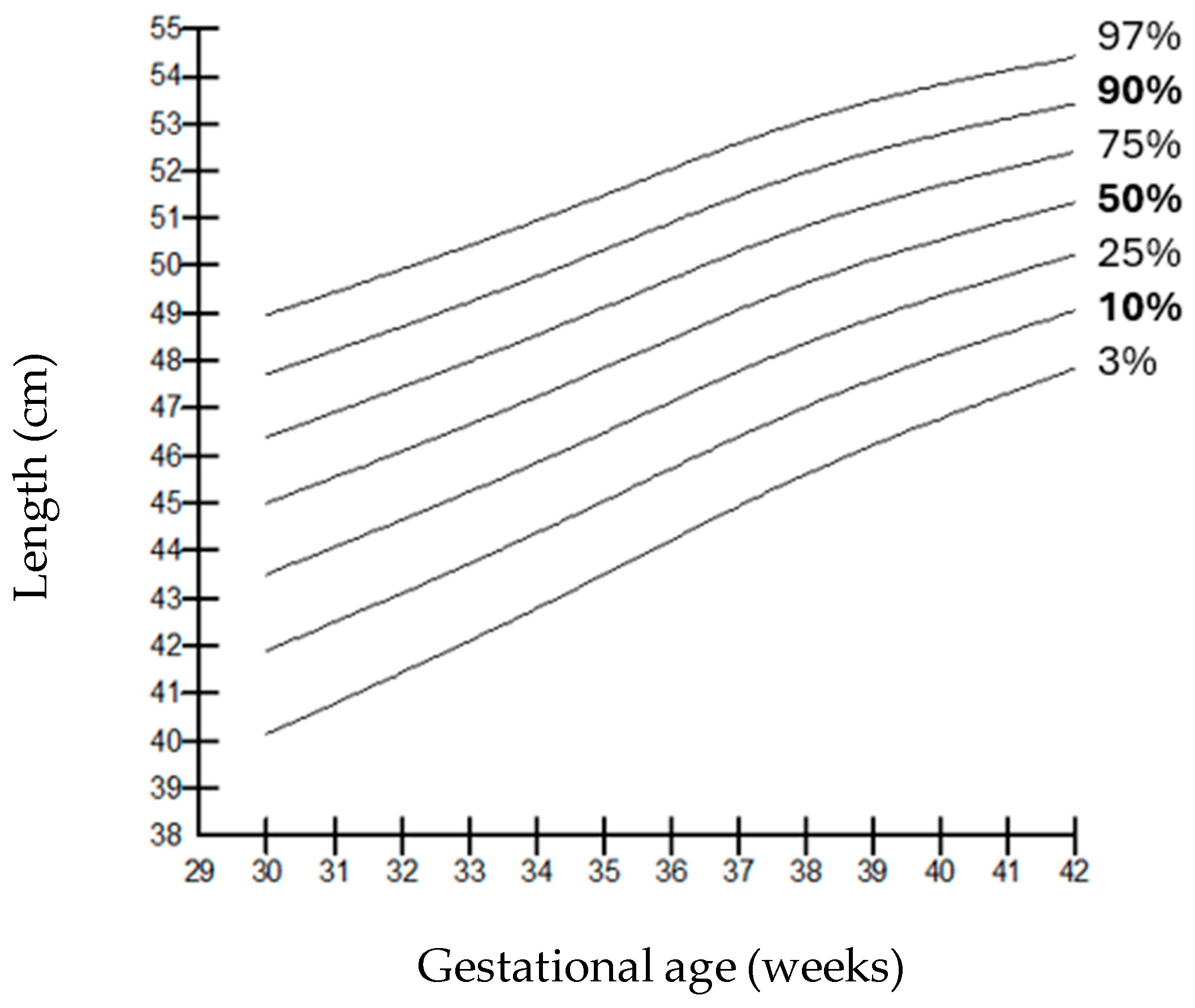
| Birth Length Percentages (g) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational Age | Gender | L | M | S | 3rd | 5th | 10th | 15th | 25th | 50th | 75th | 85th | 90th | 95th | 97th |
| 24–26 | Female | −0.529 | 32.0 | 0.120 | 25.2 | 26.7 | 27.1 | 28.0 | 29.3 | 32.0 | 34.8 | 35.0 | 37.0 | 38.0 | 38.0 |
| Male | −0.428 | 32.5 | 0.081 | 27.0 | 27.5 | 28.0 | 28.4 | 30.0 | 32.5 | 34.0 | 35.0 | 37.0 | 38.2 | 38.4 | |
| 27–29 | Female | 1.543 | 35.0 | 0.106 | 29.5 | 30.0 | 32.0 | 32.0 | 33.0 | 35.0 | 36.0 | 38.3 | 39.7 | 41.0 | 42.7 |
| Male | 1.544 | 36.0 | 0.093 | 32.0 | 32.7 | 33.0 | 34.0 | 35.0 | 36.0 | 38.0 | 40.1 | 41.7 | 43.4 | 46.9 | |
| 30–31 | Female | −0.188 | 42.0 | 0.106 | 32.6 | 33.0 | 36.1 | 38.7 | 39.8 | 41.0 | 43.0 | 44.4 | 45.0 | 45.4 | 46.0 |
| Male | −0.041 | 41.5 | 0.071 | 36.0 | 36.7 | 37.5 | 38.0 | 39.0 | 40.5 | 42.8 | 44.0 | 46.0 | 46.8 | 48.1 | |
| 32 | Female | 1.146 | 43.0 | 0.084 | 35.7 | 38.6 | 39.0 | 39.4 | 41.0 | 43.0 | 44.0 | 46.0 | 47.4 | 48.0 | 48.4 |
| Male | 0.185 | 43.0 | 0.081 | 37.0 | 37.5 | 38.5 | 40.0 | 41.0 | 43.0 | 45.0 | 46.0 | 47.0 | 48.0 | 50.1 | |
| 33 | Female | −0.331 | 45.0 | 0.070 | 38.0 | 40.0 | 41.0 | 42.0 | 44.0 | 44.5 | 47.0 | 48.0 | 48.4 | 50.8 | 51.0 |
| Male | −0.982 | 45.0 | 0.077 | 37.9 | 39.0 | 40.3 | 41.0 | 43.0 | 45.0 | 47.0 | 48.0 | 48.7 | 49.9 | 50.5 | |
| 34 | Female | −0.493 | 46.0 | 0.053 | 40.0 | 42.0 | 42.4 | 43.0 | 44.0 | 46.0 | 47.3 | 48.0 | 49.0 | 49.2 | 50.0 |
| Male | −0.930 | 47.0 | 0.061 | 40.0 | 41.0 | 42.0 | 44.0 | 45.0 | 47.0 | 48.0 | 49.0 | 49.0 | 50.7 | 51.0 | |
| 35 | Female | −0.399 | 47.5 | 0.048 | 42.0 | 43.0 | 43.8 | 45.0 | 46.0 | 47.5 | 49.0 | 49.4 | 50.0 | 51.0 | 51.5 |
| Male | −0.687 | 48.0 | 0.057 | 42.0 | 42.5 | 44.0 | 44.0 | 46.0 | 48.0 | 49.0 | 50.0 | 50.0 | 51.5 | 52.0 | |
| 36 | Female | −1.159 | 48.0 | 0.050 | 43.0 | 44.0 | 45.0 | 46.0 | 47.0 | 48.5 | 50.0 | 50.0 | 51.0 | 51.0 | 52.0 |
| Male | −0.213 | 49.0 | 0.043 | 45.0 | 45.2 | 46.0 | 47.0 | 48.0 | 49.0 | 50.0 | 51.0 | 51.0 | 52.0 | 53.0 | |
| 37 | Female | −1.362 | 49.0 | 0.045 | 45.0 | 46.0 | 46.0 | 47.0 | 48.2 | 49.5 | 50.0 | 51.0 | 52.0 | 52.0 | 53.0 |
| Male | −0.434 | 50.0 | 0.040 | 46.0 | 46.5 | 47.0 | 48.0 | 48.0 | 50.0 | 51.0 | 52.0 | 52.0 | 52.4 | 53.0 | |
| 38 | Female | −0.435 | 50.0 | 0.040 | 46.0 | 46.3 | 47.0 | 48.0 | 48.6 | 50.3 | 51.0 | 52.0 | 52.8 | 53.0 | 53.5 |
| Male | −0.078 | 50.0 | 0.038 | 47.0 | 47.3 | 48.0 | 48.0 | 49.0 | 50.0 | 52.0 | 52.0 | 53.0 | 53.0 | 54.0 | |
| 39 | Female | −0.658 | 50.0 | 0.040 | 46.3 | 47.0 | 48.0 | 48.4 | 49.0 | 51.0 | 51.4 | 52.4 | 53.0 | 53.5 | 54.0 |
| Male | −0.340 | 51.0 | 0.038 | 47.5 | 48.0 | 49.0 | 49.0 | 50.0 | 51.0 | 52.0 | 53.0 | 53.5 | 54.0 | 54.0 | |
| 40 | Female | −0.306 | 51.0 | 0.039 | 47.0 | 48.0 | 49.0 | 49.2 | 50.0 | 51.4 | 52.0 | 53.0 | 53.5 | 54.0 | 54.5 |
| Male | −0.835 | 51.0 | 0.040 | 48.0 | 49.0 | 49.0 | 49.5 | 50.0 | 51.0 | 52.5 | 53.0 | 54.0 | 54.5 | 55.0 | |
| 41 | Female | −0.096 | 51.0 | 0.036 | 47.2 | 48.4 | 50.0 | 50.4 | 51.0 | 52.4 | 52.6 | 53.0 | 54.0 | 54.5 | 55.2 |
| Male | −0.256 | 52.0 | 0.037 | 48.5 | 49.0 | 50.0 | 50.0 | 51.0 | 52.0 | 53.0 | 53.5 | 54.2 | 55.0 | 55.0 | |
| 42 | Female | 0.382 | 51.0 | 0.044 | 47.6 | 49.8 | 51.0 | 51.2 | 51.8 | 53.2 | 53.6 | 54.0 | 54.5 | 55.2 | 55.8 |
| Male | −0.234 | 52.0 | 0.041 | 49.0 | 49.4 | 50.0 | 50.0 | 51.0 | 52.5 | 53.8 | 54.3 | 54.8 | 55.7 | 56.0 | |
| Head Circumference Percentiles (cm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational Age | Gender | L | M | S | 3rd | 5th | 10th | 15th | 25th | 50th | 75th | 85th | 90th | 95th | 97th |
| 24–26 | Female | 0.218 | 23.3 | 0.095 | 19.1 | 19.9 | 20.0 | 20.6 | 21.3 | 23.3 | 24.5 | 25.5 | 26.0 | 26.5 | 28.7 |
| Male | 0.406 | 23.0 | 0.092 | 20.0 | 20.0 | 20.5 | 21.0 | 21.3 | 23.0 | 24.8 | 25.3 | 25.8 | 26.0 | 26.5 | |
| 27–29 | Female | 1.285 | 25.8 | 0.100 | 23.6 | 23.8 | 24.0 | 24.4 | 24.8 | 25.8 | 26.9 | 28.0 | 29.3 | 32.0 | 33.1 |
| Male | 1.179 | 27.0 | 0.085 | 24.0 | 23.7 | 24.2 | 25.0 | 25.3 | 27.0 | 28.0 | 29.0 | 29.0 | 32.0 | 33.0 | |
| 30–31 | Female | −0.430 | 30.0 | 0.085 | 26.6 | 26.8 | 26.9 | 27.0 | 27.2 | 27.6 | 31.0 | 31.4 | 32.0 | 33.0 | 33.8 |
| Male | −0.147 | 30.0 | 0.060 | 26.3 | 27.0 | 27.2 | 27.8 | 28.5 | 30.0 | 31.0 | 31.2 | 32.2 | 33.0 | 34.0 | |
| 32 | Female | 0.231 | 30.0 | 0.059 | 27.8 | 28.0 | 28.2 | 28.4 | 29.0 | 29.8 | 31.4 | 32.0 | 32.3 | 33.4 | 34.6 |
| Male | 0.278 | 31.0 | 0.073 | 27.0 | 27.3 | 28.0 | 28.3 | 29.0 | 31.0 | 32.9 | 33.0 | 33.8 | 34.5 | 35.0 | |
| 33 | Female | −1.915 | 32.0 | 0.067 | 28.4 | 29.0 | 29.2 | 30.0 | 31.0 | 31.4 | 32.8 | 33.0 | 33.4 | 34.8 | 35.0 |
| Male | −0.064 | 32.0 | 0.050 | 27.5 | 28.0 | 29.0 | 30.0 | 31.0 | 32.0 | 33.0 | 34.0 | 34.0 | 35.3 | 36.0 | |
| 34 | Female | 2.655 | 32.0 | 0.074 | 29.0 | 29.2 | 30.0 | 30.8 | 31.6 | 32.1 | 33.0 | 34.0 | 34.4 | 35.1 | 36.0 |
| Male | −0.029 | 33.0 | 0.049 | 28.0 | 30.0 | 31.0 | 31.0 | 32.0 | 33.0 | 34.0 | 34.0 | 34.4 | 36.0 | 36.5 | |
| 35 | Female | −0.069 | 33.0 | 0.043 | 29.6 | 30.0 | 30.8 | 32.0 | 32.2 | 33.0 | 34.0 | 34.4 | 35.2 | 36.0 | 36.4 |
| Male | −0.367 | 34.0 | 0.046 | 30.0 | 31.0 | 32.0 | 32.0 | 33.0 | 34.0 | 35.0 | 35.0 | 35.0 | 36.5 | 36.9 | |
| 36 | Female | 1.580 | 34.0 | 0.044 | 30.2 | 30.8 | 31.5 | 32.4 | 33.0 | 33.8 | 35.0 | 35.4 | 35.9 | 36.2 | 36.8 |
| Male | −0.172 | 34.0 | 0.042 | 31.0 | 32.0 | 33.0 | 33.0 | 33.5 | 34.0 | 35.0 | 36.0 | 36.2 | 37.0 | 37.5 | |
| 37 | Female | 1.429 | 34.0 | 0.046 | 30.8 | 31.0 | 32.2 | 33.0 | 33.8 | 34.5 | 35.4 | 36.0 | 36.4 | 36.5 | 37.0 |
| Male | −0.445 | 35.0 | 0.041 | 32.0 | 33.0 | 33.0 | 33.5 | 34.0 | 35.0 | 36.0 | 36.0 | 36.5 | 37.4 | 37.8 | |
| 38 | Female | 0.424 | 35.0 | 0.037 | 31.4 | 31.8 | 32.7 | 33.5 | 34.0 | 35.0 | 36.0 | 36.2 | 36.7 | 36.9 | 37.5 |
| Male | −0.175 | 35.0 | 0.037 | 33.0 | 33.0 | 34.0 | 34.0 | 34.0 | 35.0 | 36.0 | 36.9 | 37.0 | 37.8 | 38.0 | |
| 39 | Female | −0.014 | 35.0 | 0.041 | 32.0 | 32.4 | 33.2 | 33.8 | 34.2 | 35.5 | 36.2 | 36.7 | 36.9 | 37.0 | 37.8 |
| Male | −0.015 | 35.5 | 0.038 | 33.0 | 33.0 | 34.0 | 34.5 | 35.0 | 35.5 | 36.0 | 37.0 | 37.5 | 38.0 | 38.5 | |
| 40 | Female | 3.440 | 35.0 | 0.046 | 32.6 | 32.8 | 33.7 | 34.0 | 34.6 | 35.8 | 36.6 | 36.9 | 37.2 | 37.4 | 38.0 |
| Male | 1.469 | 36.0 | 0.040 | 33.0 | 34.0 | 34.0 | 34.0 | 35.0 | 36.0 | 36.0 | 37.0 | 37.8 | 38.5 | 39.0 | |
| 41 | Female | 0.048 | 35.0 | 0.037 | 33.0 | 33.2 | 34.2 | 34.4 | 35.0 | 36.2 | 36.8 | 37.0 | 37.5 | 37.8 | 38.2 |
| Male | −1.240 | 36.0 | 0.039 | 33.0 | 34.0 | 34.0 | 35.0 | 35.0 | 36.0 | 37.0 | 37.0 | 38.0 | 38.0 | 39.5 | |
| 42 | Female | −1.104 | 35.0 | 0.042 | 33.4 | 33.7 | 34.7 | 34.8 | 35.4 | 36.4 | 37.0 | 37.5 | 37.8 | 38.0 | 38.8 |
| Male | 0.111 | 36.0 | 0.035 | 34.0 | 34.0 | 34.4 | 35.0 | 35.0 | 36.0 | 37.0 | 38.0 | 38.5 | 39.0 | 40.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, N.; Arman, D.; Gül, A.; Erol, K.E.; Cömert, S. Evaluation of Anthropometric Measurements of 17,693 Newborns: Have Percentile Cut-Off Values Changed? Children 2025, 12, 644. https://doi.org/10.3390/children12050644
Kara N, Arman D, Gül A, Erol KE, Cömert S. Evaluation of Anthropometric Measurements of 17,693 Newborns: Have Percentile Cut-Off Values Changed? Children. 2025; 12(5):644. https://doi.org/10.3390/children12050644
Chicago/Turabian StyleKara, Nursu, Didem Arman, Adem Gül, Kudret Ebru Erol, and Serdar Cömert. 2025. "Evaluation of Anthropometric Measurements of 17,693 Newborns: Have Percentile Cut-Off Values Changed?" Children 12, no. 5: 644. https://doi.org/10.3390/children12050644
APA StyleKara, N., Arman, D., Gül, A., Erol, K. E., & Cömert, S. (2025). Evaluation of Anthropometric Measurements of 17,693 Newborns: Have Percentile Cut-Off Values Changed? Children, 12(5), 644. https://doi.org/10.3390/children12050644





