Neonatal and Two-Year Prognosis of Eutrophic Newborns from Monochorionic Diamniotic Twin Pregnancies Complicated by Selective Intrauterine Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
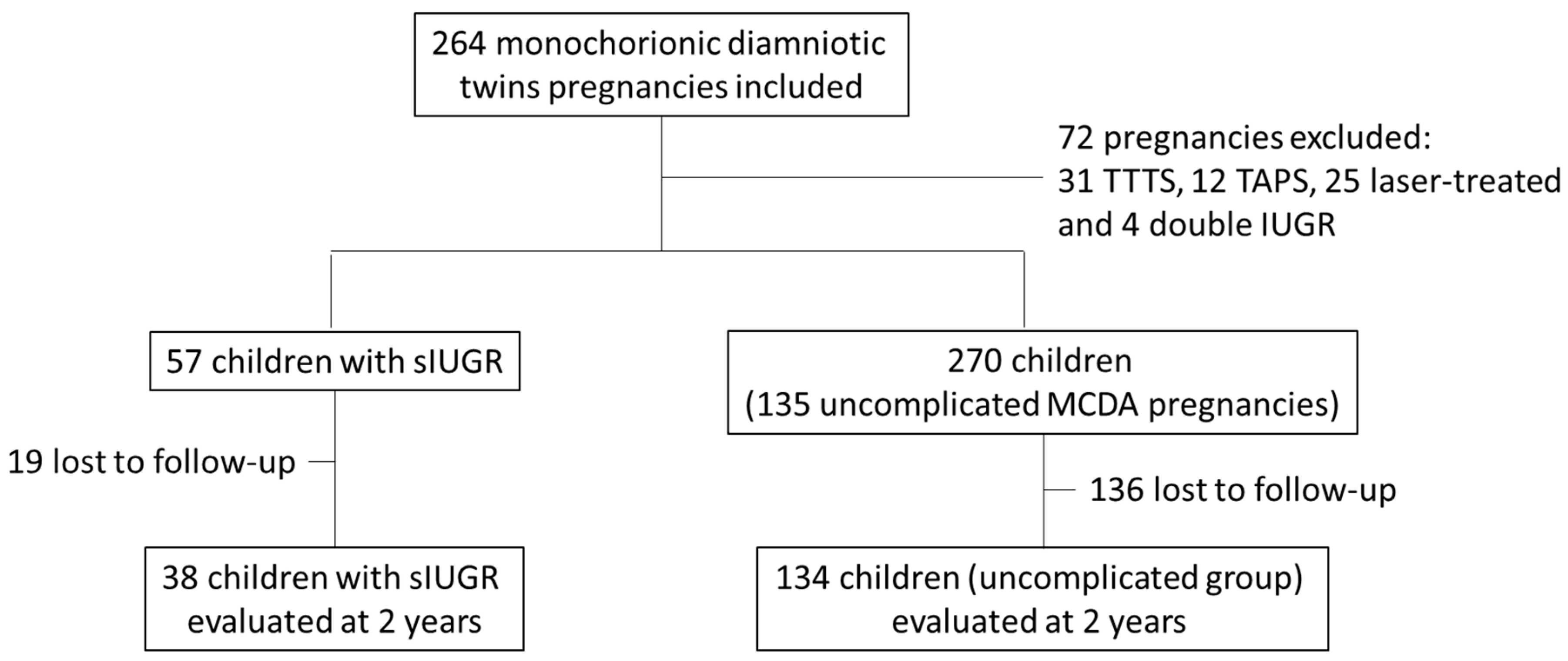
2.1. Study Population
2.2. Study Objectives
2.3. Perinatal and Neonatal Data
2.4. Development at 2 Years
2.5. Statistical Analysis
3. Results
3.1. Perinatal Characteristics of the Population
3.2. Neonatal Assessment
3.3. Neurodevelopmental Assessment at 2 Years
3.4. Comparison Between the Eutrophic Twin and the IUGR Co-Twin
Neonatal Data
3.5. ASQ at 2 Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennasar, M.; Eixarch, E.; Martinez, J.M.; Gratacós, E. Selective intrauterine growth restriction in monochorionic diamniotic twin pregnancies. Semin. Fetal Neonatal Med. 2017, 22, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Leduc, L.; Takser, L.; Rinfret, D. Persistence of adverse obstetric and neonatal outcomes in monochorionic twins after exclusion of disorders unique to monochorionic placentation. Am. J. Obstet. Gynecol. 2005, 193, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Chauhan, S.P. Epidemiology of twinning in developed countries. Semin. Perinatol. 2012, 36, 156–161. [Google Scholar] [CrossRef]
- Blondel, B. Increase in twin maternities and consequences on health. J. Gynecol. Obstet. Biol. Reprod. 2009, 38, S7–S17. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Scardo, J.; Hayes, E.; Abuhamad, A.Z.; Berghella, V. Twins: Prevalence, problems, and preterm births. Am. J. Obstet. Gynecol. 2010, 203, 305–315. [Google Scholar] [CrossRef]
- Ingram Cooke, R. Does neonatal and infant neurodevelopmental morbidity of multiples and singletons differ? Semin. Fetal Neonatal Med. 2010, 15, 362–366. [Google Scholar] [CrossRef]
- Ray, B.; Platt, M. Mortality of twins and singleton livebirths under 30 weeks gestation: A population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F140–F143. [Google Scholar] [CrossRef]
- Corsello, G.; Piro, E. The world of twins: An update. J. Matern. Fetal Neonatal Med. 2010, 23, 59–62. [Google Scholar] [CrossRef]
- Bonellie, S.R.; Currie, D.; Chalmers, J. Comparison of risk factors for cerebral palsy in twins and singletons. Dev. Med. Child. Neurol. 2005, 47, 587–591. [Google Scholar] [CrossRef]
- Guellec, I.; Lapillonne, A.; Marret, S.; Picaud, J.C.; Mitanchez, D.; Charkaluk, M.L.; Fresson, J.; Arnaud, C.; Flamand, C.; Cambonie, G.; et al. Effect of intra- and extrauterine growth on long term neurologic outcomes of very preterm infants. J. Pediatr. 2016, 175, 93–99. [Google Scholar] [CrossRef]
- Bodeau-Livinec, F.; Zeitlin, J.; Blondel, B.; Arnaud, C.; Fresson, J.; Burguet, A.; Subtil, D.; Marret, S.; Rozé, J.C.; Marchand-Martin, L.; et al. Do very preterm twins and singletons differ in their neurodevelopment at 5 years of age? Arch. Dis. Child Fetal Neonatal Ed. 2013, 98, F480–F487. [Google Scholar] [CrossRef] [PubMed]
- Hack, K.; Derks, J.; Elias, S.; Franx, A.; Roos, E.J.; Voerman, S.K.; Bode, C.L.; Koopman-Esseboom, C.; Visser, G.H.A. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: Clinical implications of a large Dutch cohort study. BJOG 2008, 115, 58–67. [Google Scholar] [CrossRef]
- Acostas-Rojas, R.; Becker, J.; Munoz-Abellana, B.; Ruiz, C.; Carreras, E.; Gratacos, E.; Catalunya and Balears Monochorionic Network. Twin chorionicity and the risk of adverse perinatal outcome. Int. J. Gynaecol. Obstet. 2007, 96, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Ishii, K.; Yonetani, N.; Mabuchi, A.; Hayashi, S.; Mitsuda, N. Significance of chorionicity on long-term outcome of low birthweight infants of <1500g in twin pregnancies. J. Obstet. Gynaecol. Res. 2015, 41, 1185–1192. [Google Scholar] [PubMed]
- Hack, K.; Koopman-Esseboom, C.; Derks, J.; Elias, S.G.; de Kleine, M.J.; Baerts, W.; Go, A.T.J.I.; Schaap, A.H.P.; van der Hoeven, M.A.H.B.M.; Eggink, A.J.; et al. Long-term neurodevelopmental outcome of monochorionic and matched dichorionic twins. PLoS ONE 2009, 28, e6815. [Google Scholar] [CrossRef]
- Ichinomiya, K.; Maruyama, K.; Koizumi, A.; Inoue, F.; Fukuda, K.; Kaburagi, K.; Miyakawa, Y. Comparaison of neurodevelopmental outcomes between monochorionic and dichorionic twins with birth weight ≤ 1500 g in Japan: A register-based cohort study. J. Perinatol. 2018, 38, 1407–1413. [Google Scholar] [CrossRef]
- Fick, A.L.; Feldstein, V.A.; Norton, M.E.; Wassel Fyr, C.; Caughey, A.B.; Machin, G.A. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am. J. Obstet. Gynecol. 2006, 195, 178–183. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, S.; Chao, A.; Hsieh, P.C.C.; Wang, C.; Wang, T. Clinical outcome and placental territory ratio of monochorionic twin pregnancies and selective intrauterine growth restriction with different types of umbilical artery Doppler. Prenat. Diagn. 2009, 29, 253–256. [Google Scholar] [CrossRef]
- Russell, Z.; Quintero, R.A.; Kontopoulos, E.V. Intrauterine growth restriction in monochorionic twins. Semin. Fetal Neonatal Med. 2007, 12, 439–449. [Google Scholar] [CrossRef]
- Lopriore, E.; Sluimers, C.; Pasman, S.A.; Middeldorp, J.M.; Oepkes, D.; Walther, F.J. Neonatal Morbidity in Growth-Discordant Monochorionic Twins: Comparison Between the Larger and the Smaller Twin. Twin Res. Hum. Genet. 2012, 15, 541–546. [Google Scholar] [CrossRef]
- Denbow, M.L.; Cox, P.; Taylor, M.; Hammal, D.M.; Fisk, N.M. Placental angioarchitecture in monochorionic twin pregnancies: Relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am. J. Obstet. Gynecol. 2000, 182, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Deprest, J.; Hecher, K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am. J. Obstet. Gynecol. 2013, 208, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Gratacós, E.; Lewi, L.; Muñoz, B.; Acosta-Rojas, R.; Hernandez-Andrade, E.; Martinez, J.M.; Carreras, E.; Deprest, J. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet. Gynecol. 2007, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Lopriore, E.; Slaghekke, F.; Oepkes, D.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.J.C.M.; Tan, R.N.G.B.; Rijken, M.; Van Klink, J.M.M. High risk of long-term neurodevelopmental impairment in donor twins with spontaneous twin anemia–polycythemia sequence. Ultrasound Obstet. Gynecol. 2020, 55, 39–46. [Google Scholar] [CrossRef]
- Graef, C.; Ellenrieder, B.; Hecher, K.; Hackeloer, B.J.; Huber, A.; Bartmann, P. Long-term neurodevelopmental outcome of 167 children after intrauterine laser treatment for severe twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2006, 194, 303–308. [Google Scholar] [CrossRef]
- Prasad, S.; Beg, S.; Badran, D.; Masciullo, L.; Huddy, C.; Khalil, A. Neurodevelopmental outcome in complicated twin pregnancy: Prospective observational study. Ultrasound Obstet. Gynecol. 2024, 63, 189–197. [Google Scholar] [CrossRef]
- Buca, D.; Pagani, G.; Rizzo, G.; Familiari, A.; Flacco, M.E.; Manzoli, L.; Liberati, M.; Fanfani, F.; Scambia, G.; D’Antonio, F. Outcome of monochorionic twin pregnancy with selective intrauterine growth restriction according to umbilical artery Doppler flow pattern of smaller twin: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 559–568. [Google Scholar] [CrossRef]
- Vedel, C.; Oldenburg, A.; Worda, K.; Larsen, H.; Holmskov, A.; Andreasen, K.R.; Uldbjerg, N.; Ramb, J.; Bødker, B.; Skibsted, L.; et al. Short- and long-term perinatal outcome in twin pregnancies affected by weight discordance. Acta Obstet. Gynecol. Scand. 2017, 96, 233–242. [Google Scholar] [CrossRef]
- Khalil, A.; Beune, I.; Hecher, K.; Wynia, K.; Ganzevoort, W.; Reed, K.; Lewi, L.; Oepkes, D.; Gratacos, E.; Thilaganathan, B.; et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: A Delphi procedure. Ultrasound Obstet. Gynecol. 2019, 53, 47–54. [Google Scholar] [CrossRef]
- Gratacós, E.; Lewi, L.; Carreras, E.; Becker, J.; Higueras, T.; Deprest, J.; Cabero, L. Incidence and characteristics of umbilical artery intermittent absent and/or reversed end-diastolic flow in complicated and uncomplicated monochorionic twin pregnancies. Ultrasound Obstet. Gynecol. 2004, 23, 456–460. [Google Scholar] [CrossRef]
- Inklaar, M.J.; Van Klink, J.M.M.; Stolk, T.T.; Van Zwet, E.W.; Oepkes, D.; Lopriore, E. Cerebral injury in monochorionic twins with selective intrauterine growth restriction: A systematic review. Prenat. Diagn. 2014, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Murakoshi, T.; Takahashi, Y.; Shinno, T.; Matsushita, M.; Naruse, H.; Torii, Y.; Sumie, M.; Nakata, M. Perinatal outcome of monochorionic twins with selective intrauterine growth restriction and different types of umbilical artery Doppler under expectant management. Fetal Diagn. Ther. 2009, 26, 157–161. [Google Scholar] [CrossRef]
- Quintero, R.A.; Bornick, P.W.; Morales, W.J.; Allen, M.H. Selective photocoagulation of communicating vessels in the treatment of monochorionic twins with selective growth retardation. Am. J. Obstet. Gynecol. 2001, 185, 689–696. [Google Scholar] [CrossRef]
- Knijnenburg, P.J.C.; Lopriore, E.; Slaghekke, F.; Van Klink, J.M.M. Long-term follow-up of complicated monochorionic twin pregnancies: Focus on neurodevelopment. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022, 84, 166–178. [Google Scholar] [CrossRef]
- Rustico, M.A.; Consonni, D.; Lanna, M.; Faiola, S.; Schena, V.; Scelsa, B.; Introvini, P.; Righini, A.; Parazzini, C.; Lista, G.; et al. Selective intrauterine growth restriction in monochorionic twins: Changing patterns in umbilical artery Doppler flow and outcomes. Ultrasound Obstet. Gynecol. 2017, 49, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Gremillet, L.; Netter, A.; Chau, C.; Gire, C.; Tosello, B. Neonatal and Long-Term Prognosis of Monochorionic Diamniotic Pregnancies Complicated by Selective Growth Restriction. Children 2022, 9, 708. [Google Scholar] [CrossRef]
- Vayssière, C.; Sentilhes, L.; Ego, A.; Bernard, C.; Cambourieu, D.; Flamant, C.; Gascoin, G.; Gaudineau, A.; Grangé, G.; Houfflin-Debarge, V.; et al. Fetal growth restriction and intra-uterine growth restriction: Guidelines for clinical practice from the French College of Gynecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 10–18. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 g. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Ternberg, J.; Feigin, R.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis: Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Jobe, A. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2011, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.; Bricker, D.; Potter, L. Revision of a Parent-Completed Developmental Screening Tool: Ages and Stages Questionnaires. J. Pediatr. Psychol. 1997, 22, 313–328. [Google Scholar] [CrossRef]
- Hoarau, D.; Tosello, B.; Blanc, J.; Lorthe, E.; Foix-L’helias, L.; D’ercole, C.; Winer, N.; Subtil, D.; Goffinet, F.; Kayem, G.; et al. Chorionicity and neurodevelopmental outcomes at 5½ years among twins born preterm: The EPIPAGE2 cohort study. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1047–1058. [Google Scholar] [CrossRef]
- Tosello, B.; Garbi, A.; Blanc, J.; Lorthe, E.; Foix-L’helias, L.; D’ercole, C.; Winer, N.; Subtil, D.; Goffinet, F.; Kayem, G.; et al. The impact of chorionicity on pregnancy outcome and neurodevelopment at 2 years old among twins born preterm: The EPIPAGE-2 cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 281–291. [Google Scholar] [CrossRef]
- Wintermark, P. The role of brain MRI scanning in the newborn. Paediatr. Child. Health. 2012, 22, 155–159. [Google Scholar] [CrossRef]
- Rutherford, M.; Pennock, J.; Schwieso, J.; Cowan, F.; Dubowitz, L. Hypoxic-ischemic encephalopathy: Early and late magnetic resonance imaging findings in relation to outcome. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 75, F145–F151. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.; Biarge, M.M.; Allsop, J.; Counsell, S.; Cowan, F. MRI of perinatal brain injury. Pediatr. Radiol. 2010, 40, 819–833. [Google Scholar] [CrossRef]
- Troude, P.; Squires, J.; L’Helias, L.F.; Bouyer, J.; de La Rochebrochard, E. Ages and stages questionnaires: Feasibility of postal surveys for child follow-up. Early Hum. Dev. 2011, 87, 671–676. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Shi, L.; Daniel, L.M.; Yang, P.H.; Khoo, P.C.; Quek, B.H.; Zheng, Q.; Rajadurai, V.S. Prospective evaluation of the Ages and Stages Questionnaire 3rd Edition in very-low-birthweight infants. Dev. Med. Child. Neurol. 2017, 59, 484–489. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Xie, H.; Sathyapalan Rema, A.S.; Rajadurai, V.S.; Lim, S.B.; Meaney, M.; Daniel, L.M. Evaluation of the Ages and Stages Questionnaire (ASQ 3) as a developmental screener at 9, 18, and 24 months. Early Hum. Dev. 2020, 147, 105081. [Google Scholar] [CrossRef]
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C.; Bodeau-Livinec, F.; Morgan, A.S.; Goffinet, F.; Marret, S.; et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 2017, 358, j3448. [Google Scholar] [CrossRef] [PubMed]
- von Stumm, S.; Plomin, R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence 2015, 48, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.G.; McCandliss, B.D.; Farah, M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007, 10, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.O.; Bergh, E.P.; Johnson, A.; Ruano, R.; Hernandez-Andrade, E.; Papanna, R. The Delphi definition for selective fetal growth restriction may not improve detection of pathologic growth discordance in monochorionic twins. Am. J. Obstet. Gynecol. MFM 2022, 4, 100561. [Google Scholar] [CrossRef]
| sIUGR Group | Uncomplicated Group | p Value | |
|---|---|---|---|
| Number of pregnancies, n | 57 | 135 | |
| Age, years, median (min–max) | 30.9 (20.2–44.3) | 29.9 (18.7–44.3) | 0.75 |
| Gravidity, n, median (min–max) | 2 (1–10) | 2 (1–10) | 0.92 |
| Parity, n, median (min–max) | 1 (0–4) | 1 (0–8) | 0.26 |
| BMI, kg/m2, median (min–max) | 22.4 (16.4–43.0) | 24.1 (16.3–45.0) | 0.08 |
| Fetal sex, n (%) | |||
| Male | 28 (49%) | 75 (56%) | 0.27 |
| Female | 29 (51%) | 60 (44%) | |
| Antenatal corticosteroid therapy, n (%) | |||
| Not administered | 11 (19%) | 70 (52%) | <0.001 |
| Incomplete | 0 (0%) | 4 (3%) | |
| Complete | 46 (81%) | 61 (45%) | |
| Mode of delivery, n (%) | |||
| Vaginal delivery | 9 (16%) | 68 (50%) | <0.001 |
| Cesarean section | 48 (84%) | 67 (50%) | |
| Indication for delivery, n (%) | |||
| Spontaneous | 13 | 62 | |
| Abnormal fetal heart rate | 24 | 3 | |
| Doppler abnormalities | 15 | 3 | |
| Growth restriction | 12 | 7 | |
| Other | 5 | 41 | |
| Gestational age at birth, weeks of amenorrhea, median (min–max) | 33 (25–38) | 36 (24–39) | <0.001 |
| Number of newborns, n | 114 | 270 | |
| Birth weight, grams, mean ± SD | 1914 ± 473 | 2306 ± 576 | <0.001 |
| Head circumference at birth, centimeters, mean ± SD | 29 ± 2 | 31 ± 3 | 0.001 |
| Birth length, centimeters, mean ± SD | 42 ± 3 | 44 ± 4 | 0.001 |
| Growth discordance at birth, %, mean ± SD | 26 ± 10.4 | 7.8 ± 5.3 | <0.001 |
| Birth weight percentile, %, mean ± SD | 44.3 ± 32 | 38.3 ± 25 | 0.69 |
| Apgar score, median, (min–max) | |||
| 1 min | 9 (0–10) | 10 (0–10) | 0.24 |
| 5 min | 9 (5–10) | 10 (3–10) | 0.06 |
| 10 min | 10 (8–10) | 10 (5–10) | 0.003 |
| Apgar score < 7, n/N (%) | |||
| 1 min | 8/57 (14%) | 41/270 (15%) | 0.7 |
| 5 min | 4/57 (7%) | 14/270 (5%) | 0.7 |
| 10 min | 0/57 (0%) | 1/270 (0.3%) | 0.25 |
| pH at birth, median, (min–max) | 7.34 (7.2–7.4) | 7.31 (7.0–7.5) | 0.003 |
| pH < 7.15, n/N (%) | 0/49 | 16/258 (6%) | 0.06 |
| Lactate levels at birth, mmol/L, median, (min–max) | 2.3 (1.3–6.5) | 2.9 (1.0–10.0) | 0.06 |
| sIUGR Group | Uncomplicated Group | p Value Adjusted * | |
|---|---|---|---|
| Hospitalization in intensive care, n/N (%) | 36/57 (63%) | 66/270 (24%) | <0.001 |
| Length of hospital stay, days, median (min–max) | 4.5 (1–70) | 4 (1–39) | 0.51 |
| Death, n/N (%) | 2/57 (4%) | 7/270 (3%) | 0.81 |
| Age at death, days, median (min–max) | 14 (3–25) | 7 (4–13) | |
| Respiratory complications: | |||
| Need for ventilatory support, n/N (%) | 39/57 (68%) | 69/270 (26%) | <0.001 |
| Duration of ventilatory support, days, median (min–max) | 6 (1–71) | 5.5 (1–55) | 0.99 |
| Hyaline membrane disease, n/N (%) | 33/57 (58%) | 64/270 (24%) | 0.02 |
| Bronchopulmonary dysplasia, n/N (%) | 3/57 (5%) | 7/270 (3%) | 0.34 |
| Sepsis, n/N (%) | 5/55 (9%) | 9/187 (5%) | 0.29 |
| Early-onset sepsis, n/N (%) | 0/55 (0%) | 5/187 (3%) | |
| Late-onset sepsis, n/N (%) | 5/55 (9 %) | 4/185 (2%) | |
| Necrotizing enterocolitis, n/N (%) | 4/57 (7%) | 6/270 (2%) | 0.22 |
| Abnormal Auditory Evoked Potentials, n/N (%) | 1/31 (3%) | 6/95 (6%) | 0.34 |
| Abnormal indirect ophthalmoscopy, n/N (%) | 3/19 (16%) | 6/35 (17%) | 0.92 |
| Abnormal electroencephalogram, n/N (%) | 6/43 (14%) | 3/67 (4%) | 0.23 |
| Abnormal brain MRI, n/N (%) | 9/16 (56%) | 9/23 (39%) | 0.32 |
| Grade III or IV IVH, n/N (%) | 1/16 (6%) | 1/23 (4%) | 0.52 |
| PVL, n/N (%) | 2/16 (13%) | 0/23 (0%) | 0.05 |
| Ventricular dilation, n/N (%) | 2/16 (13%) | 1/23 (4%) | 0.52 |
| Breastfeeding at discharge, n/N (%) | 34/53 (64%) | 98/179 (55%) | 0.22 |
| Composite morbidity and mortality criterion, n/N (%) (including neonatal death, grade III or IV IVH, PVL, BPD, and stage II or III NEC) | 6/57 (11%) | 14/270 (5%) | 0.18 |
| sIUGR Group N = 38 | Uncomplicated Group N = 67 | p Value | |
|---|---|---|---|
| Age, years, median (min–max) | 31 (22–40) | 30 (19–44) | 0.60 |
| Gravidity, n, median (min–max) | 2 (1–5) | 3 (1–7) | 0.04 |
| Parity, n, median (min–max) | 1 (0–4) | 1 (0–3) | 0.19 |
| BMI, kg/m2, median (min–max) | 21.3 (17.4–43) | 24 (16.3–45) | 0.02 |
| Fetal sex, n (%) | 0.12 | ||
| Male | 18 | 40 | |
| Female | 20 | 27 | |
| Antenatal corticosteroid therapy, n (%) | |||
| Not administered | 8 | 36 | 0.002 |
| Incomplete | 0 | 0 | |
| Complete | 30 | 31 | |
| Mode delivery, n (%) | <0.001 | ||
| Vaginal delivery | 6/38 (16%) | 37/67 (55%) | |
| Cesarean section | 32/38 (84%) | 30/67 (45%) | |
| Indication for birth, n (%) | |||
| Spontaneous | 9 | 30 | |
| Abnormal fetal heart rate | 16 | 1 | |
| Doppler abnormalities | 12 | 3 | |
| Growth restriction | 8 | 3 | |
| Other | 3 | 24 | |
| Gestational age at birth, weeks of amenorrhea, median (min–max) | 34 (26–35) | 36 (27–37) | <0.001 |
| Birth weight, grams, mean SD | 1986 ± 456 | 2384 ± 519 | <0.001 |
| Number of newborns, n | 38 | 134 | |
| Head circumference at birth, centimeters, mean SD | 30 ± 1.8 | 31.4 ± 2.3 | 0.002 |
| Length at birth, centimeters, mean SD | 42.5 ± 3.2 | 44.7 ± 3.5 | 0.006 |
| Growth discordance at birth, %, mean SD | 25 ± 27 | 6 ± 7 | <0.001 |
| Birth weight percentile, %, mean SD | 26.5 ± 12 | 36.7 ± 24.5 | 0.55 |
| Apgar score, median, (min–max) | |||
| 1 min | 9 (0–10) | 9.5 (0–10) | 0.12 |
| 5 min | 10 (5–10) | 10 (6–10) | 0.11 |
| 10 min | 10 (8–10) | 10 (7–10) | 0.004 |
| Apgar score < 7, n/N (%) | |||
| 1 min | 3 | 18 | 0.3 |
| 5 min | 3 | 5 | 0.23 |
| 10 min | 0 | 0 | 1 |
| pH at birth, median, (min–max) | 7.34 (7.19–7.4) | 7.3 (7.03–7.5) | 0.002 |
| PH < 7.15, n/N (%) | 0 | 11 | 0.06 |
| Lactate at birth, mmol/L, median, (min–max) | 2.3 (1.3–5.5) | 2.9 (1–6.9) | 0.15 |
| sIUGR Group | Uncomplicated Group | Adjusted p Value ** | |
|---|---|---|---|
| ASQ score | |||
| Median (IQR) | 252 (226–264) | 240 (220–260) | 0.26 |
| ASQ < 220, n (%) | 8/38 (21%) | 33/134 (25%) | 0.65 |
| ASQ < threshold, n (%) * | 11/38 (29%) | 47/134 (35%) | 0.51 |
| Number of impaired domains, n (%) | 0.32 | ||
| 0 | 32 (80%) | 92 (69%) | |
| 1 | 6 (15%) | 30 (22%) | |
| 2 | 1 (3%) | 12 (9%) | |
| 3 | 1 (3%) | 0 (0%) | |
| 4 or 5 | 0 | 0 | |
| At least one impaired domain, n (%) | 8/38 (21%) | 42/134 (31%) | 0.21 |
| By domain, n (%) | |||
| Communication | 4/38 (11%) | 19/134 (14%) | 0.59 |
| Gross motor skills | 1/38 (3%) | 5/134 (4%) | 0.68 |
| Fine motor skills | 1/38 (3%) | 6/134 (4%) | 0.44 |
| Problem solving | 1/38 (3%) | 0/134 (0%) | 0.21 |
| Social skills | 4/38 (11%) | 24/134 (18%) | 0.30 |
| 26–31 Weeks GA | 32–34 Weeks GA | >35 Weeks GA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| sIUGR Group | Uncomplicated Group | p Value | sIUGR Group | Uncomplicated Group | p Value | sIUGR Group | Uncomplicated Group | p Value | |
| ASQ score, median (IQR) | 250 (220–264) | 240 (230–250) | 0.37 | 245 (219–262) | 232 (209–245) | 0.5 | 260 (242–265) | 240 (220–261) | 0.09 |
| ASQ < 220, n (%) | 3/10 (30%) | 2/10 (20%) | 0.84 | 4/16 (25%) | 7/24 (29%) | 0.92 | 1/12 (8 %) | 24/100 (24%) | 0.17 |
| ASQ < threshold, n (%) | 3/10 (30%) | 2/10 (20%) | 0.84 | 5/16 (31%) | 13/24 (54%) | 0.15 | 1/11 (8%) | 24/100 (24%) | 0.17 |
| Number of impaired domains, n (%) | |||||||||
| 0 | 9 (90%) | 10 (100%) | 1 | 12 (75%) | 13 (54%) | 0.78 | 9 (75%) | 69 (69%) | 0.38 |
| 1 | 1 (10%) | 0 | 2 (13%) | 9 (38%) | 3 (25%) | 21 (21%) | |||
| 2 | 0 | 0 | 1 (6%) | 2 (8%) | 0 | 10 (10%) | |||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 4 or 5 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| At least one impaired domain, n (%) | 1/10 (10%) | 0/10 (0%) | 0.49 | 4/16 (25%) | 11/24 (46%) | 0.15 | 3/12 (25%) | 31/100 (31%) | 0.57 |
| Communication | 1 | 0 | 0.49 | 2/16 (13%) | 4/24 | 0.68 | 1/12 | 15/100 | 0.66 |
| Gross motor skills | 0 | 0 | 1 | 1/16 (6%) | 3/24 | 0.64 | 0/12 | 2/100 | 0.88 |
| Fine motor skills | 0 | 0 | 1 | 0/16 | 1/24 | 1 | 1/12 | 6/100 | 0.94 |
| Problem-solving | 0 | 0 | 1 | 1/16 (6%) | 0/24 | 0.36 | 1/12 | 1/100 | 1 |
| Social and individual aptitudes | 0 | 0 | 1 | 3/16 (19%) | 6/24 | 0.71 | 1/12 | 18/100 | 0.37 |
| Eutrophic Twin | IUGR Twin | p Value | |
|---|---|---|---|
| Hospitalization in intensive care, n/N (%) | 36/57 (63%) | 30/57 (53%) | 0.35 |
| Duration in intensive care, days, median (min–max) | 4.5 (1–70) | 5 (1–63) | 0.75 |
| Duration of hospitalization, days, median (min–max) | 25 (0–113) | 23 (3–113) | 0.96 |
| Death, n/N (%) | 2/57 (4%) | 4/57 (7%) | 0.24 |
| Age at death, days, median (min–max) | 14 (3–25) | 14.5 (6–100) | |
| Respiratory complications: | |||
| Need for ventilatory support, n/N (%) | 39/57 (68%) | 34/57 (60%) | 0.44 |
| Duration of ventilatory support, days, median (min–max) | 6 (1–71) | 6 (1–86) | 0.46 |
| Hyaline membrane disease, n/N (%) | 33/57 (58%) | 26/57 (46%) | 0.18 |
| Bronchopulmonary dysplasia, n/N (%) | 3/57 (5%) | 4/57 (7%) | 0.85 |
| Infectious complications | |||
| Sepsis, n/N (%) | 5/55 (9%) | 7/54 (13%) | 0.5 |
| Early-onset, n/N (%) | 0 | 0 | |
| Late-onset, n/N (%) | 5 | 7 | |
| Necrotizing enterocolitis, n/N (%) | 0.37 | ||
| Stage I, n/N (%) | 4/57 (7%) | 8/57 (14%) | |
| Stage II, n/N (%) | 1 | 3 | |
| Stage III, n/N (%) | 3 | 3 | |
| Stage IV, n/N (%) | 0 | 2 | |
| Neurosensory complications: | |||
| Auditory evoked potential abnormalities, n/N (%) | 1/31 (3%) | 1/34 (3%) | 0.71 |
| Fundus abnormalities, n/N (%) | 3/19 (16%) | 2/17 (12%) | 0.72 |
| Electroencephalogram abnormalities, n/N (%) | 6/43 (13%) | 2/46 (4%) | 0.12 |
| Abnormalities on brain MRI, n/N (%) | 9/16 (56%) | 3/18 (17%) | 0.001 |
| IVH grade III or IV, n/N (%) | 1/16 (6%) | 0/18 (0%) | 0.17 |
| PVL, n/N (%) | 2/16 (13%) | 1/18 (6%) | 0.47 |
| Ventricular dilation, n/N (%) | 2/16 (13%) | 0/18 (0%) | 0.09 |
| Composite morbidity–mortality criterion, n/N (%) (including neonatal death, grade III or IV IVH, PVL, BPD and stage II or III NEC) | 6/57 (11%) | 9/57 (16%) | 0.58 |
| Eutrophic N = 38 | IUGR N = 19 | p Value | |
|---|---|---|---|
| ASQ score | |||
| Median (IQR) | 255 (229–270) | 245 (212–260) | 0.53 |
| ASQ < 220, n (%) | 8/38 (21%) | 6/19 (32%) | 0.70 |
| ASQ < threshold, n (%) | 11/38 (29%) | 8/19 (42%) | 0.60 |
| Number of impaired domains, n (%) | 0.55 | ||
| 0 | 30 | 12 (6%) | |
| 1 | 6 | 5 (26%) | |
| 2 | 1 | 2 (11%) | |
| 3 | 1 | 0 | |
| 4 or 5 | 0 | 0 | |
| At least one altered domain, n (%) | 8/38 (21%) | 7/19 (37%) | 0.38 |
| By domain, n (%) | |||
| Communication | 4 | 5 | |
| Gross motor skills | 1 | 1 | |
| Fine motor skills | 1 | 0 | |
| Problem-solving | 1 | 0 | |
| Social and individual aptitudes | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarry, M.-A.; Topalian, N.; Cosnard, L.; D’Ercole, C.; Chau, C.; Tosello, B. Neonatal and Two-Year Prognosis of Eutrophic Newborns from Monochorionic Diamniotic Twin Pregnancies Complicated by Selective Intrauterine Growth Restriction. Children 2025, 12, 615. https://doi.org/10.3390/children12050615
Jarry M-A, Topalian N, Cosnard L, D’Ercole C, Chau C, Tosello B. Neonatal and Two-Year Prognosis of Eutrophic Newborns from Monochorionic Diamniotic Twin Pregnancies Complicated by Selective Intrauterine Growth Restriction. Children. 2025; 12(5):615. https://doi.org/10.3390/children12050615
Chicago/Turabian StyleJarry, Marie-Anne, Nayri Topalian, Lauréline Cosnard, Claude D’Ercole, Cécile Chau, and Barthélémy Tosello. 2025. "Neonatal and Two-Year Prognosis of Eutrophic Newborns from Monochorionic Diamniotic Twin Pregnancies Complicated by Selective Intrauterine Growth Restriction" Children 12, no. 5: 615. https://doi.org/10.3390/children12050615
APA StyleJarry, M.-A., Topalian, N., Cosnard, L., D’Ercole, C., Chau, C., & Tosello, B. (2025). Neonatal and Two-Year Prognosis of Eutrophic Newborns from Monochorionic Diamniotic Twin Pregnancies Complicated by Selective Intrauterine Growth Restriction. Children, 12(5), 615. https://doi.org/10.3390/children12050615






