Lipid Profile Alterations in Pediatric Patients with Vitamin D Deficiency
Abstract
1. Introduction
2. Materials and Methods
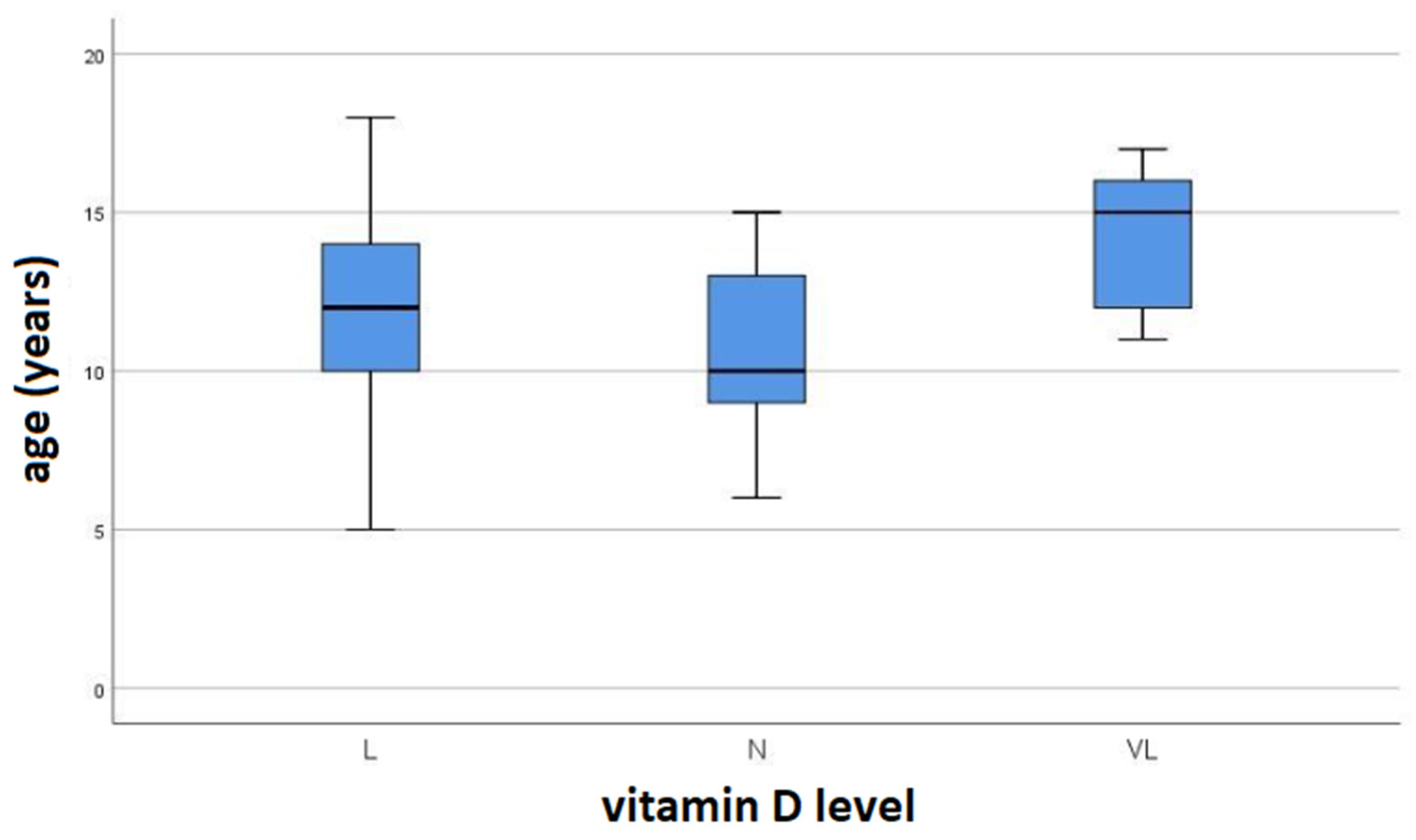
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAG | Triacylglycerols (triglycerides); |
| CHOL | Cholesterol; |
| HDL | High-density lipoprotein; |
| LDL | Low-density lipoprotein; |
| VLDL | Very-low-density lipoprotein; |
| BMI | Body mass index; |
| CVD | Cardiovascular diseases; |
| RNI | Reference nutrient intake; |
| PTH | Parathyroid hormone; |
| DGE | Deutsche Gesellschaft für Ernährung (German Nutrition Society). |
References
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, 213–256. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Figenschau, Y.; Hutchinson, M.; Emaus, N.; Grimnes, G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur. J. Clin. Nutr. 2010, 64, 1457–1464. [Google Scholar] [CrossRef]
- Kim, M.R.; Jeong, S.J. Relationship between vitamin D level and lipid profile in non-obese children. Metabolites 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition. Vitamin D and Health. 2016. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 17 January 2025).
- Radkhah, N.; Zarezadeh, M.; Jamilian, P.; Ostadrahimi, A. The Effect of Vitamin D Supplementation on Lipid Profiles: An Umbrella Review of Meta-Analyses. Adv. Nutr. 2023, 14, 1479–1498. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines. Vitamin D Deficiency. 2020. Available online: https://www.rch.org.au/clinicalguide/guideline_index/Vitamin_D_deficiency/ (accessed on 17 January 2025).
- Al-Oanzi, Z.H.; Alenazy, F.O.; Alhassan, H.H.; Alruwaili, Y.; Alessa, A.I.; Alfarm, N.B.; Alanazi, M.O.; Alghofaili, S.I. The Role of Vitamin D in Reducing the Risk of Metabolic Disturbances That Cause Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 209. [Google Scholar] [CrossRef]
- Nikparvar, M.; Khaladeh, M.; Yousefi, H.; Vahidi, F.M.; Moayedi, B.; Kheirandish, M. Dyslipidemia and its associated factors in southern Iranian women, Bandare-Kong Cohort study, a cross-sectional survey. Sci. Rep. 2021, 11, 9125. [Google Scholar] [CrossRef]
- Dos Santos, S.F.; Dos Reis Costa, P.N.; Gouvêa, T.G.; de Almeida, N.F.A.; Cardoso, F.S. Influence of hypovitaminosis D during pregnancy on glycemic and lipid profile, inflammatory indicators and anthropometry of pregnant and newborn. Clin. Nutr. ESPEN 2023, 54, 81–93. [Google Scholar] [CrossRef]
- Song, S.J.; Si, S.; Liu, J.; Chen, X.; Zhou, L.; Jia, G.; Liu, G.; Niu, Y.; Wu, J.; Zhang, W.; et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013, 16, 687–692. [Google Scholar] [CrossRef]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Gonzalez-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Sodero, G.; Rigante, D.; Pane, L.C.; Sessa, L.; Quarta, L.; Candelli, M.; Cipolla, C. Cardiometabolic Risk Assessment in a Cohort of Children and Adolescents Diagnosed with Hyperinsulinemia. Diseases 2024, 12, 119. [Google Scholar] [CrossRef]
- López-Gil, J.F.; García-Hermoso, A.; Smith, L.; Firth, J.; Trott, M.; Mesas, A.E.; Jiménez-López, E.; Gutiérrez-Espinoza, H.; Tárraga-López, P.J.; Victoria-Montesinos, D. Global Proportion of Disordered Eating in Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 363–372. [Google Scholar] [CrossRef]
- Magge, S.N.; Goodman, E.; Armstrong, S.C.; Committee on Nutrition; Section on Endocrinology; Section on Obesity. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics 2017, 140, 20171603. [Google Scholar] [CrossRef]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef]
- Isa, H.; Almaliki, M.; Alsabea, A.; Mohamed, A. Vitamin D deficiency in healthy children in Bahrain: Do gender and age matter? East. Mediterr. Health J. 2020, 26, 260–267. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Wei, Y.; Fu, J.; Feng, Y.; Chen, D.; Xu, D. Status and influential factors of vitamin D among children aged 0 to 6 years in a Chinese population. BMC Public Health 2020, 20, 429. [Google Scholar] [CrossRef] [PubMed]
- German Nutrition Society. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef] [PubMed]
| Parameter (mmol/L) | N (n = 130) | L (n = 114) | VL (n = 88) | p * |
|---|---|---|---|---|
| TAG | 0.93 ± 0.12 | 1.10 ± 0.11 | 1.13 ± 0.21 | 0.033 a,b,c |
| CHOL | 4.48 ± 0.26 | 4.49 ± 0.22 | 4.53 ± 0.54 | ns |
| HDL | 1.30 ± 0.12 | 1.29 ± 0.09 | 1.26 ± 0.1 | 0.042 b,c |
| LDL | 2.76 ± 0.16 | 2.70 ± 0.17 | 2.76 ± 0.4 | ns |
| VLDL | 0.42 ± 0.07 | 0.50 ± 0.05 | 0.51 ± 0.1 | 0.038 a |
| TAG:HDL-C | 0.72 ± 0.13 | 0.86 ± 0.11 | 0.90 ± 0.18 | <0.001 a,b,c |
| Vitamin D | 60.11 ± 13.4 | 39.71 ± 5.36 | 22.5 ± 4.09 | <0.001 a,b,c |
| Parameter | N | L | VL | p * |
|---|---|---|---|---|
| BMI | 21.74 ± 6.46 | 19.6 (17.25–22.25) | 19.9 (18.72–27.85) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanić, J.; Dobrijević, D. Lipid Profile Alterations in Pediatric Patients with Vitamin D Deficiency. Children 2025, 12, 546. https://doi.org/10.3390/children12050546
Katanić J, Dobrijević D. Lipid Profile Alterations in Pediatric Patients with Vitamin D Deficiency. Children. 2025; 12(5):546. https://doi.org/10.3390/children12050546
Chicago/Turabian StyleKatanić, Jasmina, and Dejan Dobrijević. 2025. "Lipid Profile Alterations in Pediatric Patients with Vitamin D Deficiency" Children 12, no. 5: 546. https://doi.org/10.3390/children12050546
APA StyleKatanić, J., & Dobrijević, D. (2025). Lipid Profile Alterations in Pediatric Patients with Vitamin D Deficiency. Children, 12(5), 546. https://doi.org/10.3390/children12050546





