Timing and Indications for Liver Transplantation for Children with Chronic Liver Disease
Abstract
1. Introduction
2. Chronic Liver Disease
3. Indications of Liver Transplantation for Children with CLD
3.1. Disease-Specific Indications
3.1.1. Biliary Atresia
3.1.2. Inborn Errors of Metabolism
3.2. Malignancy
3.3. Genetic Conditions
3.4. New Indications for Transplantation
3.5. Mitochondrial Diseases
3.6. Acute on Chronic Liver Failure (ACLF)
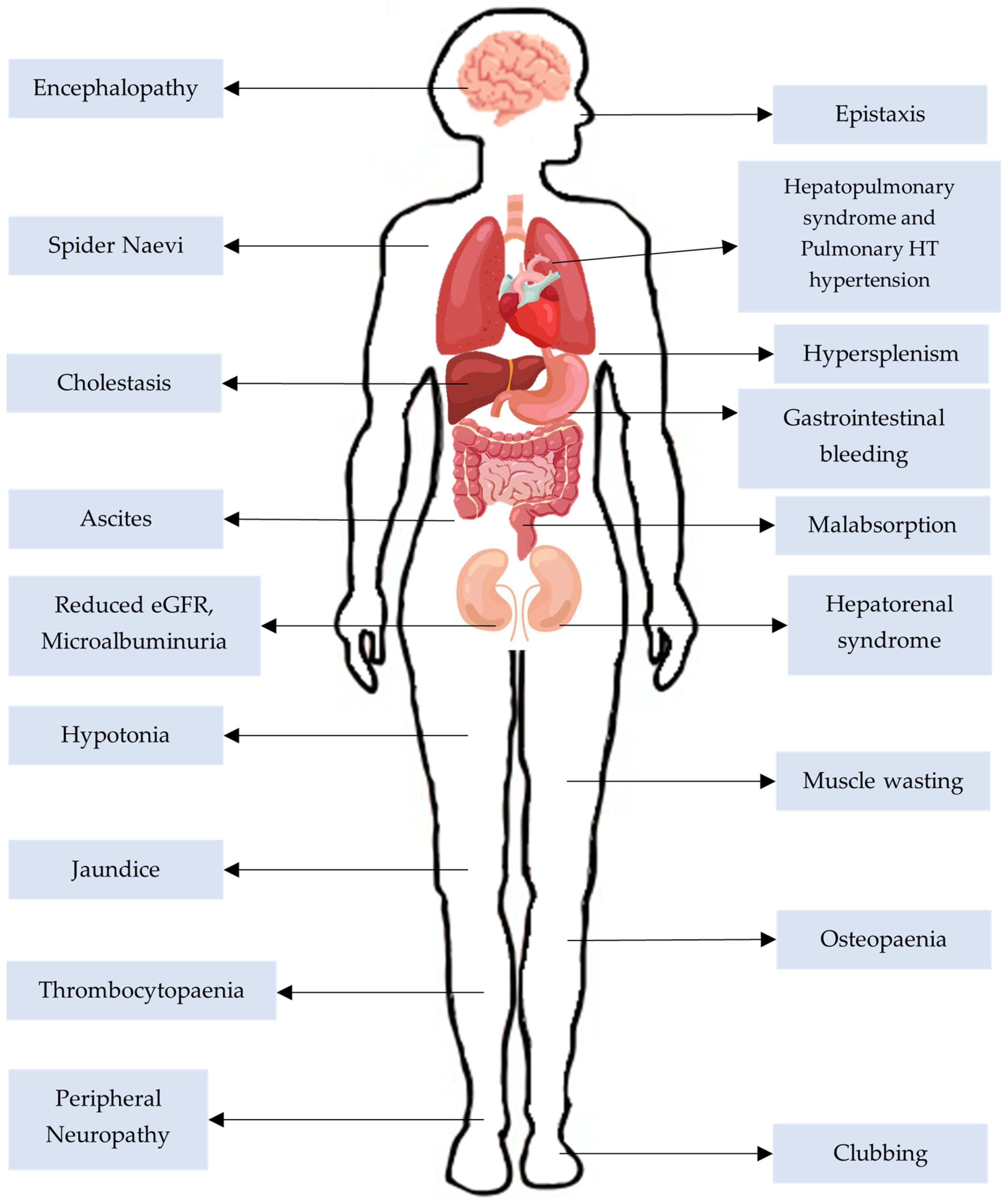
3.7. Systemic Complications of CLD
- Mean pulmonary artery pressure of 20 mmHg or more;
- Pulmonary artery wedge pressure 15 mmHg or less;
- Pulmonary vascular resistance 3WU or more.
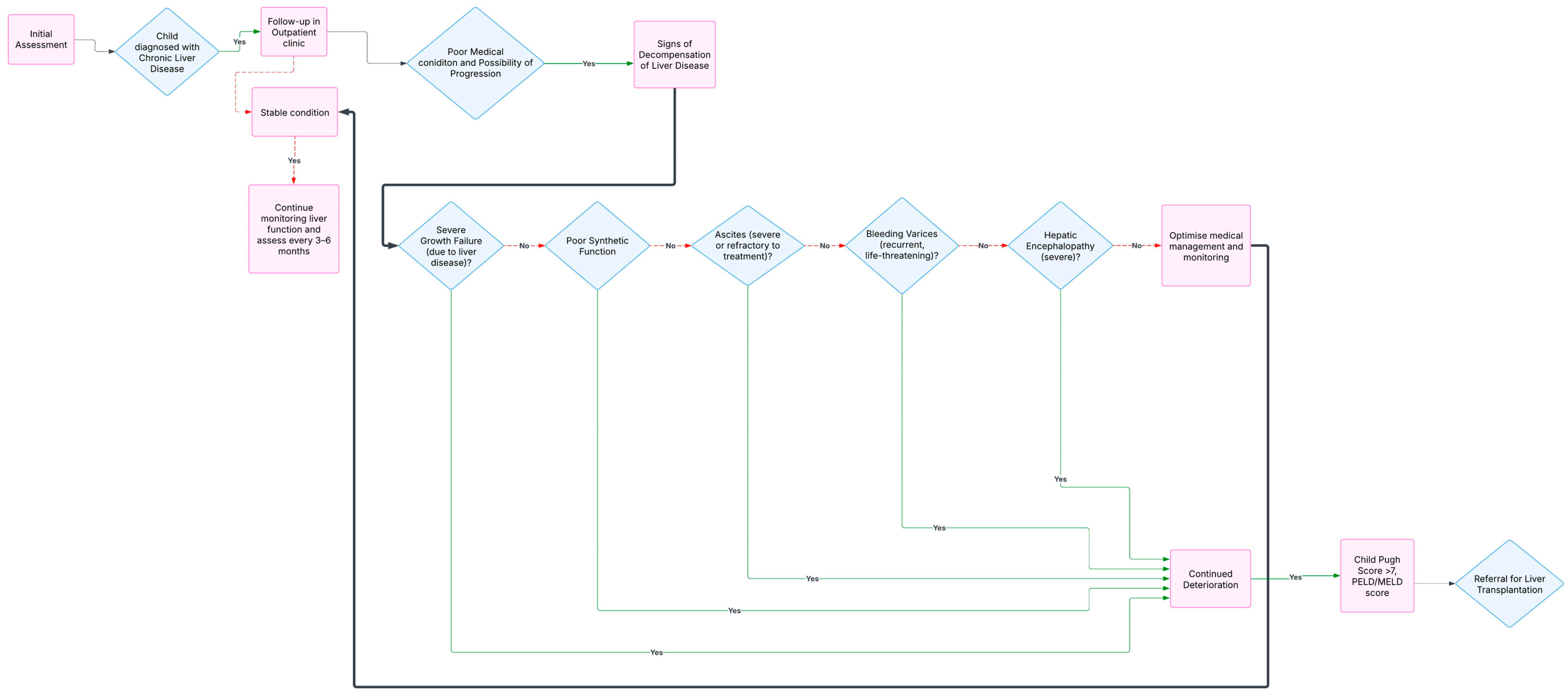
3.8. Timing and Referral for a Liver Transplantation
3.8.1. The PELD Scoring System
Loge (total bilirubin mg/dL) + 1.87 × Loge (INR) + 0.667 (Growth failure
(<−2 Std. Deviations present))
3.8.2. Referral Process
4. Contraindications
5. Liver Transplantation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tewari, S.; Parida, S.; Mishra, S.; Sinha, N.K. Prevention of chronic liver disease by applying therapeutic diet. Pharma Innov. 2019, 8, 437–440. [Google Scholar]
- Asrani, S.K.; Kouznetsova, M.; Ogola, G.; Taylor, T.; Masica, A.; Pope, B.; Trotter, J.; Kamath, P.; Kanwal, F. Increasing health care burden of chronic liver disease compared with other chronic diseases, 2004–2013. Gastroenterology 2018, 155, 719–729. [Google Scholar]
- Esquivel, C.O.; Iwatsuki, S.; Gordon, R.D.; Marsh, W.W., Jr.; Koneru, B.; Makowka, L.; Tzakis, A.G.; Todo, S.; Starzl, T.E. Indications for pediatric liver transplantation. J. Pediatr. 1987, 111 Pt 2, 1039–1045. [Google Scholar] [PubMed]
- Spada, M.; Riva, S.; Maggiore, G.; Cintorino, D.; Gridelli, B. Pediatric liver transplantation. World J. Gastroenterol. 2009, 15, 648–674. [Google Scholar]
- Engelmann, G.; Schmidt, J.; Oh, J.; Lenhartz, H.; Wenning, D.; Teufel, U.; Buchler, M.W.; Hoffmann, G.F.; Meyburg, J. Indications for pediatric liver transplantation. Data from the Heidelberg pediatric liver transplantation program. Nephrol. Dial. Transplant. 2007, 22, viii23–viii28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuenca, A.G.; Yeh, H. Improving Patient Outcomes Following Pediatric Liver Transplant: Current Perspectives. Transpl. Res. Risk Manag. 2019, 11, 69–78. [Google Scholar]
- Hussaini, T.; Erb, S.; Yoshida, E.M. Immunosuppressive pharmacotherapy in liver transplantation. AME Med. J. 2018, 3, 18. [Google Scholar]
- Horvat, N.; Marcelino, A.S.Z.; Horvat, J.V.; Yamanari, T.R.; Batista Araújo-Filho, J.A.; Panizza, P.; Seda-Neto, J.; Antunes da Fonseca, E.; Carnevale, F.C.; Mendes de Oliveira Cerri, L.; et al. Pediatric Liver Transplant: Techniques and Complications. Radiographics 2017, 37, 1612–1631. [Google Scholar]
- Squires, R.H.; Ng, V.; Romero, R.; Ekong, U.; Hardikar, W.; Emre, S.; Mazariegos, G.V. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology 2014, 60, 362–398. [Google Scholar]
- National Institutes of Health. Consensus Development Conference Statement Liver transplantation: Leure 20–23, 1983. Hepatology 1984, 4 (Suppl. S1), 1075.
- Starzl, T.E.; Iwatsuki, S.; Shaw, B.W., Jr.; Gordon, R.D.; Esquivel, C. Immunosuppression and other non-surgical factors in the improved results of liver transplantation. Semin. Liver Dis. 1985, 5, 334–343. [Google Scholar]
- Siddiqui, A.I.; Ahmad, T. Biliary Atresia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537262/ (accessed on 14 March 2025).
- Emre, S.; Umman, V.; Cimsit, B.; Rosencrantz, R. Current concepts in pediatric liver transplantation. Mt. Sinai J. Med. 2012, 79, 199–213. [Google Scholar]
- Venkat, V.; Ng, V.L.; Magee, J.C.; Ye, W.; Hawthorne, K.; Harpavat, S.; Molleston, J.P.; Murray, K.F.; Wang, K.S.; Soufi, N.; et al. Modeling Outcomes in Children with Biliary Atresia With Native Liver After 2 Years of Age. Hepatol. Commun. 2020, 4, 1824–1834. [Google Scholar] [PubMed]
- Bennett, J.; Bromley, P. Paediatric liver transplantation: Assessment and intraoperative care. In Oxford Textbook of Transplant Anaesthesia and Critical Care; Pretto, E.A., Jr., Biancofiore, G., Slinger, P.D., Eds.; Oxford Academic: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [PubMed]
- Teckman, J.H.; Jain, A. Advances in alpha-1-antitrypsin deficiency liver disease. Curr. Gastroenterol. Rep. 2014, 16, 367. [Google Scholar]
- Meseeha, M.; Attia, M. Alpha-1 Antitrypsin Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442030/ (accessed on 14 March 2025).
- David, A.R.; Dawn, R.E. Tyrosinemia, Encyclopedia of Gastroenterology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 538–541. [Google Scholar]
- Sahai, I.; Harvey, L.L. 27—Newborn Screening, Avery’s Diseases of the Newborn, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 332–346.e3. [Google Scholar]
- Meyburg, J.; Hoffmann, G.F. Liver transplantation for inborn errors of metabolism. Transplantation 2005, 80 (Suppl. S1), S135–S137. [Google Scholar] [PubMed]
- Camp, K.M.; Parisi, M.A.; Acosta, P.B.; Berry, G.T.; Bilder, D.A.; Blau, N.; Bodamer, O.A.; Brosco, J.P.; Brown, C.S.; Burlina, A.B.; et al. Phenylketonuria Scientific Review Conference: State of the science and future research needs. Mol. Genet. Metab. 2014, 112, 87–122. [Google Scholar]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar]
- Kakos, C.D.; Ziogas, I.A.; Demiri, C.D.; Esagian, S.M.; Economopoulos, K.P.; Moris, D.; Tsoulfas, G.; Alexopoulos, S.P. Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review. Cancers 2022, 14, 1294. [Google Scholar] [CrossRef]
- Murawski, M.; Weeda, V.B.; Maibach, R.; Morland, B.; Roebuck, D.J.; Zimmerman, A.; Casanova, M.; Perilongo, G.; Laithier, V.; Kebudi, R.; et al. Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned from the SIOPEL 2 and 3 Studies. J. Clin. Oncol. 2016, 34, 1050–1056. [Google Scholar] [CrossRef]
- Santos, J.L.; Choquette, M.; Bezerra, J.A. Cholestatic liver disease in children. Curr. Gastroenterol. Rep. 2010, 12, 30–39. [Google Scholar]
- Balistreri, W.F. Intrahepatic Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2002, 35, S17–S23. [Google Scholar] [PubMed]
- Siddiqi, I.A.; Tadi, P. Progressive Familial Intrahepatic Cholestasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559317/ (accessed on 14 March 2025).
- Davit-Spraul, A.; Gonzales, E.; Baussan, C.; Jacquemin, E. Progressive familial intrahepatic cholestasis. Orphanet J. Rare Dis. 2009, 4, 1–2. [Google Scholar] [PubMed]
- Ganschow, R.; Nolkemper, D.; Helmke, K.; Harps, E.; Commentz, J.C.; Broering, D.C.; Pothmann, W.; Rogiers, X.; Hellwege, H.H.; Burdelski, M. Intensive care management after pediatric liver transplantation: A single-center experience. Pediatr. Transplant. 2000, 4, 273–279. [Google Scholar] [PubMed]
- Lane, M.; Boczonadi, V.; Bachtari, S.; Gomez-Duran, A.; Langer, T.; Griffiths, A.; Kleinle, S.; Dineiger, C.; Abicht, A.; Holinski-Feder, E.; et al. Mitochondrial dysfunction in liver failure requiring transplantation. J. Inherit. Metab. Dis. 2016, 39, 427–436. [Google Scholar]
- Naviaux, R.K.; Nguyen, K.V. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann. Neurol. 2004, 55, 706–712. [Google Scholar]
- Uusimaa, J.; Evans, J.; Smith, C.; Butterworth, A.; Craig, K.; Ashley, N.; Liao, C.; Carver, J.; Diot, A.; Macleod, L.; et al. Clinical, biochemical, cellular and molecular characterization of mitochondrial DNA depletion syndrome due to novel mutations in the MPV17 gene. Eur. J. Hum. Genet. 2014, 22, 184–191. [Google Scholar]
- Lee, W.S.; Sokol, R.J. Liver disease in mitochondrial disorders. Semin. Liver Dis. 2007, 27, 259–273. [Google Scholar]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar]
- Islek, A.; Tumgor, G. Acute-on-chronic liver failure in children. World J. Hepatol. 2021, 13, 1289–1298. [Google Scholar]
- Kahrilas, P.J.; Shaheen, N.J.; Vaezi, M.F. American Gastroenterological Association Medical Position Statement on the Management of Gastroesophageal Reflux Disease. Gastroenterology 2008, 135, 1383–1391. [Google Scholar] [PubMed]
- Gandhi, K.D.; Taweesedt, P.T.; Sharma, M.; Surani, S. Hepatopulmonary syndrome: An update. World J Hepatol. 2021, 13, 1699–1706. [Google Scholar] [PubMed]
- Yi, H.M.; Wang, G.S.; Yi, S.H.; Yang, Y.; Cai, C.J.; Chen, G.H. Prospective evaluation of postoperative outcome after liver transplantation in hepatopulmonary syndrome patients. Chin. Med. J. 2009, 122, 2598–2602. [Google Scholar]
- Bansal, K.; Gore, M.; Mittal, S. Hepatopulmonary Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562169/ (accessed on 14 March 2025).
- Jasso-Baltazar, E.A.; Peña-Arellano, G.A.; Aguirre-Valadez, J.; Ruiz, I.; Papacristofilou-Riebeling, B.; Jimenez, J.V.; García-Carrera, C.J.; Rivera-López, F.E.; Rodriguez-Andoney, J.; Lima-Lopez, F.C.; et al. Portopulmonary Hypertension: An Updated Review. Transplant. Direct. 2023, 9, e1517. [Google Scholar]
- Bosch, J. Portal hypertension and cirrhosis: From evolving concepts to better therapies. Clin. Liver Dis. 2020, 15 (Suppl. S1), S8–S12. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Saleemi, S. Portopulmonary hypertension. Ann. Thorac. Med. 2010, 5, 5–9. [Google Scholar]
- Gurghean, A.V.; Tudor, I.A. Pulmonary hypertension in patients with hepatic cirrhosis and portal hypertension. An echographic study. Clujul Med. 2017, 90, 161–165. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef]
- Chhabria, M.S.; Boppana, L.K.T.; Manek, G.; Tonelli, A.R. Portopulmonary hypertension: A focused review for the internist. Clevel. Clin. J. Med. 2023, 90, 632–639. [Google Scholar] [CrossRef]
- Ng, C.K.; Chan, M.H.; Tai, M.H.; Lam, C.W. Hepatorenal syndrome. Clin. Biochem. Rev. 2007, 28, 11–17. [Google Scholar]
- Ranasinghe, I.R.; Sharma, B.; Bashir, K. Hepatorenal Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430856/ (accessed on 14 March 2025).
- McDiarmid, S.V.; Merion, R.M.; Dykstra, D.M.; Harper, A.M. Selection of pediatric candidates under the PELD system. Liver Transpl. 2004, 10 (Suppl. S2), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, S.V.; Anand, R.; Lindblad, A.S. Development of a Pediatric End-Stage Liver Disease Score to Predict Poor Outcome in Children Awaiting Liver transplantation. Transplantation 2002, 74, 173–181. [Google Scholar] [CrossRef]
- Shneider, B.L.; Neimark, E.; Frankenberg, T.; Arnott, L.; Suchy, F.J.; Emre, S. Critical analysis of the pediatric end-stage liver disease scoring system: A single center experience. Liver Transpl. 2005, 11, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.; Narang, A.; Minard, C.G.; Hiremath, G.; Goss, J.A.; Shepherd, R. A 10-year united network for organ sharing review of mortality and risk factors in young children awaiting liver transplantation. Liver Transpl. 2016, 22, 1584–1592. [Google Scholar]
- Questions & Answers for Transplant Candidates About MELD and PELD. 2017. Available online: https://www.unos.org/wp-content/uploads/unos/MELD_PELD.pdf (accessed on 1 April 2024).
- Starzl, T.E.; Demetris, A.J.; Van Thiel, D. Liver transplantation. N. Engl. J. Med. 1989, 325, 1014–1022. [Google Scholar] [CrossRef]
- NACHRI Reports; National Association of Children’s Hospitals and Related Institutions, Inc.: Alexandria, VA, USA, 1990.
- Emond, J.C.; Whitington, P.F.; Thistlethwaite, J.R.; Alonso, E.M.; Broelsch, C.E. Reduced-size liver transplantation: Use in the Management of Children with Chronic Liver Disease. Hepatology 1989, 10, 867–872. [Google Scholar]
- Whitington, P.F.; Balistreri, W.F. Liver transplantation in pediatrics: Indications, contraindications, and pretransplant management. J. Pediatr. 1991, 118, 169–177. [Google Scholar]
- Otte, J.B.M.; Goyet, J.D.M.V.D.; Sokal, E.M.; Alberti, D.M.; Moulin, D.M.; DE Hemptinne, B.; Veyckemans, F.M.; Van Obbergh, L.; Carlier, M.M.; Clapuyt, P.; et al. Size reduction of the donor liver is a safe way to alleviate the shortage of size- matched organs in pediatric liver transplantation. Ann. Surg. 1990, 211, 146–157. [Google Scholar] [CrossRef]
- Kehar, M.; Raghunathan, V.; Mohan, N. Indications for Liver Transplant in Children: What a Pediatrician Should Know? J. Pediatr. Crit. Care 2018, 5, 36–41. [Google Scholar]
- Jensen, M.K.; Kavan, M.A.; Rodriguez-Davalos, M.; Bezerra, J.A.; Mack, C.L.; Shneider, B.L. Liver Transplantation in Children: Indications and Surgical Aspects. In Liver Disease in Children; Suchy, F.J., Sokol, R.J., Balistreri, W.F., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 801–815. [Google Scholar]
| Cirrhosis from CLD | Metabolic-Associated Steatotic Liver Disease |
|---|---|
| Metabolic Liver Disease | Alpha-1 antitrypsin deficiency |
| Cystic fibrosis-associated liver disease | |
| Cholestatic Liver Diseases | Biliary atresia |
| Alagille syndrome | |
| Progressive familial intrahepatic cholestasis |
| Cirrhosis from CLD | Autoimmune Hepatitis |
|---|---|
| Primary sclerosing cholangitis | |
| Budd Chiari syndrome | |
| Chronic hepatitis B or C viral infection | |
| Cryptogenic liver disease | |
| Malignant Diseases of the Liver | Hepatocellular carcinoma |
| Hepatoblastoma | |
| Metabolic Liver Disease | Wilsons disease |
| Hereditary tyrosinemia | |
| Urea cycle defects | |
| Glycogen storage disease type IV | |
| Familial hypercholesterolemia | |
| Cholestatic Liver Diseases | Sclerosing cholangitis |
| Idiopathic neonatal hepatitis | |
| Portal biliopathy with biliary cirrhosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suthantirakumar, R.L.; Gupte, G.L. Timing and Indications for Liver Transplantation for Children with Chronic Liver Disease. Children 2025, 12, 449. https://doi.org/10.3390/children12040449
Suthantirakumar RL, Gupte GL. Timing and Indications for Liver Transplantation for Children with Chronic Liver Disease. Children. 2025; 12(4):449. https://doi.org/10.3390/children12040449
Chicago/Turabian StyleSuthantirakumar, Risheka Lakshmi, and Girish L. Gupte. 2025. "Timing and Indications for Liver Transplantation for Children with Chronic Liver Disease" Children 12, no. 4: 449. https://doi.org/10.3390/children12040449
APA StyleSuthantirakumar, R. L., & Gupte, G. L. (2025). Timing and Indications for Liver Transplantation for Children with Chronic Liver Disease. Children, 12(4), 449. https://doi.org/10.3390/children12040449






