Fiber in the Treatment of Dyslipidemia in Pediatric Patients
Abstract
1. Introduction
1.1. Fibers and Healthy Diet
1.2. Fibers and Disease
1.3. Dyslipidemia and Atherosclerosis in Pediatric Patients
1.4. Manuscript Purpose and Characteristics
2. Materials and Methods
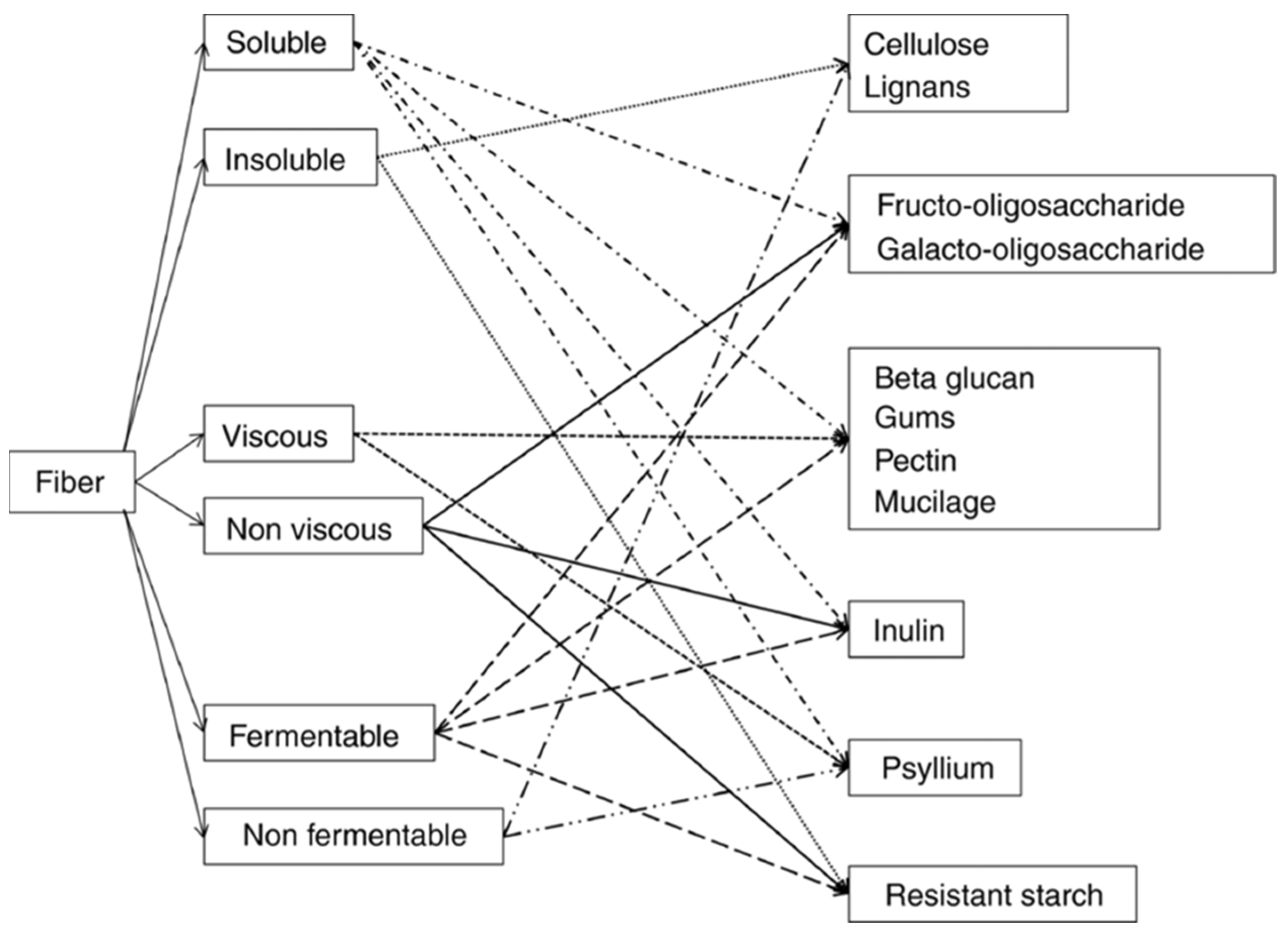
3. Fibers: Definition and Characteristic
3.1. Definition
- o
- Resistant oligosaccharides (RO), indigestible, with MU between 3 and 9.
- o
- Non-starch polysaccharides (NSP) derived from fruits, vegetables, cereals, and tubers, whether intrinsic, extracted, chemically, physically, and/or enzymatically modified, or of synthetic origin (MU ≥ 10).
- o
- Resistant starch (RS) with MU ≥ 10.
3.2. Classification
- Insoluble fibers: not soluble in water, with little fermented in the intestine. They may have a possible mechanical laxative effect.
- Non-viscous soluble fibers (inulin, dextrins, oligosaccharides): non-viscous, rapidly fermented, do not increase viscosity, and are completely fermented by the intestinal microbiota. They can exert a prebiotic effect, but without any laxative effect.
- Fermentable viscous soluble fibers (β-glucan, guar gum, pectin): they create a viscous gel in water and increase the viscosity of the chyme, slowing down the absorption of nutrients. They are rapidly fermented in the intestine and lose their laxative effect.
- Non-fermentable viscous soluble fibers (psyllium, multicellulose): they reduce nutrient absorption and, due to their viscosity, can exert a laxative effect.
3.3. Mechanism of Action
- Colonic function: reduction of transit time, increase in fecal volume, and fermentation in the colon (production of short-chain fatty acids, SCFA). These effects are mainly associated with insoluble fibers such as cellulose, hemicellulose, and psyllium. A diet rich in legumes and whole grains is particularly effective in reducing intestinal transit time.
- Reduction of cholesterol in the blood, with particular mention of β-glucans, which increase viscosity and reduce the reabsorption of bile acids in the small intestine, resulting in a decrease in circulating cholesterol levels.
- Reduction of glucose in the blood and in the small intestine: soluble fibers trap sugars, and the increase in viscosity creates a barrier that slows down glucose absorption, inhibits amylase, and reduces starch digestion, thus improving insulin sensitivity.
- Increased satiety and consequent weight loss: soluble fibers mix with partially digested food in the stomach, slowing its emptying. β-glucans can also stimulate the release of appetite-suppressing substances, such as cholecystokinin, thus increasing the feeling of fullness.
3.4. Nutritional Fibers’ Sources
4. Fibers in the Treatment of Dyslipidemia
4.1. Key Consensus Pediatric Documents
4.2. Main Evidence from the Literature
5. Fibers in Pediatric Patients
5.1. Heterozygous Familial Hypercholesterolemia
5.2. Weight Excess-Related Dyslipidemia
5.3. Familial Chylomicronemia Syndrome
5.4. Sitosterolemia
6. Conclusive Considerations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ApoA5 | Apolipoprotein A5 |
| ApoC2 | Apolipoprotein C2 |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CVD | Cardiovascular Disease |
| EFSA | European Food Safety Authority |
| ESPGHAN | European Society of Paediatric Gastroenterology, Hepatology and Nutrition |
| FAO | Food and Agricultural Organization |
| FCS | Familial Chylomicronemia Syndrome |
| FH | Familial Hypercholesterolemia |
| GPIHBP1 | Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 |
| HDL | High-Density Lipoprotein |
| HoFH | Homozygous Familial Hypercholesterolemia |
| LDL | Low-Density Lipoprotein |
| LDLR | Low-Density Lipoprotein Receptor |
| LDLRAP1 | Low-Density Lipoprotein Receptor Adaptor Protein 1 |
| LMF1 | Lipase maturation factor 1 |
| LPL | Lipoprotein Lipase |
| MCT | Middle Chain Triglycerides |
| MU | Monomeric Units |
| MUO | Metabolically Unhealthy Obesity |
| NHANES | National Health and Nutrition Examination Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NSP | Non-starch Polysaccharides |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin type 9 |
| RS | Resistant Starch |
| SCFA | Short-Chain Fatty Acids |
| TC | Total Cholesterol |
| WHO | World Health Organization |
References
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, L.-G.; Wu, Q.-J.; Ma, X.; Xiang, Y.-B. Association between dietary fiber and lower risk of all-cause mortality: A meta-analysis of cohort studies. Am. J. Epidemiol. 2015, 181, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, C.; Capra, M.E.; Biasucci, G.; Banderali, G.; Fabrizi, E.; Gazzotti, M.; Casula, M.; Catapano, A.L.; Marcello, A.; Maurizio, A.; et al. Lipoprotein(a) and family history for cardiovascular disease in paediatric patients: A new frontier in cardiovascular risk stratification. Data from the LIPIGEN paediatric group. Atherosclerosis 2022, 349, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.E.; Monopoli, D.; Decarolis, N.M.; Giudice, A.; Stanyevic, B.; Esposito, S.; Biasucci, G. Dietary Models and Cardiovascular Risk Prevention in Pediatric Patients. Nutrients 2023, 15, 3664. [Google Scholar] [CrossRef]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef]
- Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B.; Buckland, G.; et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar]
- Akesson, A.; Andersen, L.F.; Kristjansdottir, A.G.; Roos, E.; Trolle, E.; Voutilainen, E.; Wirfält, E. Health effects associated with foods characteristic of the Nordic diet: A systematic literature review. Food Nutr. Res. 2013, 57, 22790. [Google Scholar] [CrossRef]
- Giacco, R.; Clemente, G.; Cipriano, D.; Luongo, D.; Viscovo, D.; Patti, L.; Di Marino, L.; Giacco, A.; Naviglio, D.; Bianchi, M.; et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 186–194. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fiber intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fiber intake and risk of first stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1360–1368. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Jovanovski, E.; Ho, H.V.T.; Marques, A.C.R.; Zurbau, A.; Mejia, S.B.; Sievenpiper, J.L.; Vuksan, V. The effect of viscous soluble fiber on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid intima-media thickness in adulthood: The cardiovascular risk in young Finns study. JAMA 2003, 290, 2277–2283. [Google Scholar]
- National Heart, Lung, and Blood Institute; Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reductionin Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128, S213–S256. [Google Scholar]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar]
- Banderali, G.; Capra, M.E.; Biasucci, G.; Stracquadaino, R.; Viggiano, C.; Pederiva, C. Detecting Familial hypercholesterolemia in children and adolescents: Potential and challenges. Ital. J. Pediatr. 2022, 48, 115. [Google Scholar]
- Kusters, D.M.; Wiegman, A.; Kastelein, J.J.; Hutten, B.A. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ. Res. 2014, 114, 307–310. [Google Scholar]
- European Food Safety Authority. Scientific opinion on dietary reference values for carbohydrates and dietary fiber. EFSA J. 2010, 8, 1462. [Google Scholar]
- Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre1, EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA)2, 3 European Food Safety Authority (EFSA). EFSA J. 2010, 8, 1462.
- McRorie, J.W., Jr. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [PubMed]
- European Commission. Regulations Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fiber in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar]
- Grooms, K.N.; Ommerborn, M.J.; Pham, D.Q.; Djousse, L.; Clark, C.R. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999–2010. Am. J. Med. 2013, 126, 1059–1067. [Google Scholar]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar]
- USDA. National Nutrient Database for Standard Reference. 2018. Available online: https://fdc.nal.usda.gov/ (accessed on 10 March 2025).
- Capra, M.E.; Biasucci, G.; Crivellaro, E.; Banderali, G.; Pederiva, C. Dietary intervention for children and adolescents with familial hypercholesterolaemia. Ital. J. Pediatr. 2023, 49, 77. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; Report of a Joint WHO/FAO Expert Consultation; Technical Report Series 916; WHO: Geneva, Switzerland, 2003.
- Gidding, S.S.; Dennison, B.A.; Birch, L.L.; Daniels, S.R.; Gilman, M.W.; Lichtenstein, A.H.; Rattay, K.T.; Steinberger, J.; Stettler, N.; Van Horn, L.; et al. Dietary recommendations for children and adolescents: A guide for practitioners: Consensus statement from the American Heart Association. Circulation 2005, 112, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the Europe. Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- WHO. Carbohydrate Intake for Adults and Children: WHO Guidline; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Burr, M.L.; Fehily, A.M.; Rogers, S.; Welsby, E.; King, S.; Sandham, S. Diet and reinfarction trial (DART): Design, recruitment, and compliance. Eur. Heart J. 1989, 10, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Basora-Gallisá, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Escoda, R.; et al. Effects of dietary fiber intake on risk factors for cardiovascular disease in subjects at high risk. J. Epidemiol. Community Health 2009, 63, 582–588. [Google Scholar] [CrossRef]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Jovanovski, E.; Zurbau, A.; Blanco Mejia, S.; Sievenpiper, J.L.; Au-Yeung, F.; Jenkins, A.L.; Duvnjak, L.; Leiter, L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am. J. Clin. Nutr. 2017, 105, 1239–1247. [Google Scholar] [CrossRef]
- Ghavami, A.; Ziaei, R.; Talebi, S.; Barghchi, H.; Nattagh-Eshtivani, E.; Moradi, S.; Rahbarinejad, P.; Mohammadi, H.; Ghasemi-Tehrani, H.; Marx, W.; et al. Soluble Fiber Supplementation and Serum Lipid Profile: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 465–474. [Google Scholar] [CrossRef]
- Jakobsen, D.D.; Brader, L.; Bruun, J.M. Association between Food, Beverages and Overweight/Obesity in Children and Adolescents-A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 764. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883. [Google Scholar] [CrossRef]
- Hui, S.; Liu, K.; Lang, H.; Liu, Y.; Wang, X.; Zhu, X.; Doucette, S.; Yi, L.; Mi, M. Comparative effects of different whole grains and brans on blood lipid: A network meta-analysis. Eur. J. Nutr. 2019, 58, 2779–2787. [Google Scholar] [PubMed]
- SINU-Società Italiana di Nutrizione Umana. Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana, 5th ed.; Biomedia: Milan, Italy, 2024. [Google Scholar]
- Schiess, S.; Grote, V.; Scaglioni, S.; Luque, V.; Martin, F.; Stolarczyk, A.; Vecchi, F.; Koletzko, B. Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 92–98. [Google Scholar] [PubMed]
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; Van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fiber in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar]
- Zavoral, J.H.; Hannan, P.; Fields, D.J.; Hanson, M.N.; Frantz, I.D.; Kuba, K.; Elmer, P.; Jacobs, D.R. The hypolipidemic effect of locust bean gum food products in familial hypercholesterolemic adults and children. Am. J. Clin. Nutr. 1983, 38, 285–294. [Google Scholar] [PubMed]
- Glassman, M.; Spark, A.; Berezin, S.; Schwarz, S.; Medow, M.; Newman, L.J. Treatment of type IIa hyperlipidemia in childhood by a simplified American Heart Association diet and fiber supplementation. Am. J. Dis. Child. 1990, 144, 973–976. [Google Scholar]
- Dennison, B.A.; Levine, D.M. Randomized, double-blind, placebo-controlled, two-period cross-over clinical trial of psyllium fiber in children with hypercholesterolemia. J. Pediatr. 1993, 123, 24–29. [Google Scholar]
- Davidson, M.H.; Dugan, L.D.; Burns, J.H.; Sugimoto, D.; Story, K.; Drennan, K. A psyllium-enriched cereal for the treatment of hypercholesterolemia in children: A controlled, double-blind, cross-over study. Am. J. Clin. Nutr. 1996, 63, 96–102. [Google Scholar]
- Ribas, S.A.; Cunha, D.B.; Sichieri, R.; Santana da Silva, L.C. Effects of psyllium on LDL-cholesterol concentrations in Brazilian children and adolescents: A randomized, placebo-controlled, parallel clinical trial. Br. J. Nutr. 2015, 113, 134–141. [Google Scholar]
- Fogacci, F.; ALGhasab, N.S.; Di Micoli, V.; Giovannini, M.; Cicero, A.F.G. Cholesterol-Lowering Bioactive Foods and Nutraceuticals in Pediatrics: Clinical Evidence of Efficacy and Safety. Nutrients 2024, 16, 1526. [Google Scholar] [CrossRef]
- Sood, N.; Baker, W.L.; Coleman, C.I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2008, 88, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Puddu, P.E.; Pannarale, G.; Colantoni, C.; Martino, E.; Niglio, T.; Zanoni, C.; Barillà, F. Low dose chromiumpolynicotinate or policosanol is effective in hypercholesterolemic children only in combination with glucomannan. Atherosclerosis 2013, 228, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, O.; Abello, F.; Cagliero, P.; Visioli, F. Could dyslipidemic children benefit from glucomannan intake? Nutrition 2013, 29, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar]
- Maki, K.C.; Davidson, M.H.; Ingram, K.A.; Veith, P.E.; Bell, M.; Gugger, E. Lipid responses to consumption of a beta-glucan containing ready-to-eat cereal in children and adolescents with mild-to-moderate primary hypercholesterolemia. Nutr. Res. 2003, 23, 1527–1535. [Google Scholar] [CrossRef]
- Malhotra, A.; Shafiq, N.; Arora, A.; Singh, M.; Kumar, R.; Malhotra, S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia [Review]. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Jane, M.; McKay, J.; Pal, S. Effects of daily consumption of psyllium, oat bran and polyGlycopleX on obesity-related disease risk factors: A critical review. Nutrition 2019, 57, 84–91. [Google Scholar]
- Moreno, L.A.; Tresaco, B.; Bueno, G.; Fleta, J.; Rodríguez, G.; Garagorri, J.M.; Bueno, M.J. Psyllium fiber and the metabolic control of obese children and adolescents. J. Physiol. Biochem. 2003, 59, 235–242. [Google Scholar] [CrossRef]
- Howarth, N.C.; Huang, T.T.; Roberts, S.B.; McCrory, M.A. Dietary fiber and fat are associated with excess weight in young and middle-aged US adults. J. Am. Diet. Assoc. 2005, 105, 1365–1372. [Google Scholar]
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Kelly, L.A.; Lane, C.J.; Byrd-Williams, C.E.; Toledo-Corral, C.M.; Roberts, C.K.; Spruijt-Metz, D.; Weigensberg, M.J.; et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am. J. Clin. Nutr. 2007, 86, 1331–1338. [Google Scholar]
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Toledo-Corral, C.M.; Goran, M.I. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am. J. Clin. Nutr. 2009, 90, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kynde, I.; Johnsen, N.F.; Wedderkopp, N.; Bygbjerg, I.C.; Helge, J.W.; Heitmann, B.L. Intake of total dietary sugar and fiber is associated with insulin resistance among Danish 8-10- and 14-16-year-old girls but not boys. European Youth Heart Studies I and II. Public Health Nutr. 2010, 13, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, L.; Koppes, L.L.J.; Driessen, M.T.; Samoocha, D.; Twisk, J.W.R. Effects of dietary fiber intake during adolescence on the components of the metabolic syndrome at the age of 36 years: The Amsterdam Growth and Health Longitudinal Study. J. Hum. Nutr. Diet. 2010, 23, 601–608. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Zanovec, M.; Cho, S.S.; Kleinman, R. Consumption of whole grains is associated with improved diet quality and nutrient intake in children and adolescents: The National Health and Nutrition Examination Survey 1999–2004. Public Health Nutr. 2011, 14, 347–355. [Google Scholar] [CrossRef]
- Van Gijssel, R.M.A.; Braun, K.V.E.; Kiefte-de Jong, J.C.; Jaddoe, V.W.V.; Franco, O.H.; Voortman, T. Associations between dietary fiber intake in infancy and cardiometabolic health at school age: The generation R study. Nutrients 2016, 8, 531. [Google Scholar] [CrossRef]
- Zalewski, B.M.; Chmielewska, A.; Hania Szajewska, H. The effect of glucomannan on body weight in overweight or obese children and adults: A systematic review of randomized controlled trials. Nutrition 2015, 31, 437–442. [Google Scholar] [CrossRef]
- Zalewski, B.M.; Szajewska, H. Effect of glucomannan supplementation on body weight in overweight and obese children: Protocol of a randomised controlled trial. BMJ Open 2015, 5, e007244. [Google Scholar] [CrossRef]
- Stagi, S.; Lapi, E.; Seminara, S.; Pelosi, P.; Del Greco, P.; Capirchio, L.; Strano, M.; Giglio Chiarelli, F.; de Martino, M. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital. J. Pediatr. 2015, 41, 10. [Google Scholar] [CrossRef]
- Fornari, E.; Morandi, A.; Piona, C.; Tommasi, M.; Corradi, M.; Maffeis, C. Policaptil Gel Retard Intake Reduces Postprandial Triglycerides, Ghrelin and Appetite in Obese Children: A Clinical Trial. Nutrients 2020, 12, 214. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Brauchla, M.; Fleige, L.; Chu, Y.F. Association of whole-grain and dietary fiber intake with cardiometabolic risk in children and adolescents. Nutr. Health 2020, 26, 243–251. [Google Scholar] [CrossRef]
- Poursalehi, D.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Lotfi, K.; Saneei, P. Total dietary fiber intake is inversely associated with metabolically unhealthy status in adolescents with excess weight. Nutr. Res. 2024, 125, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cacau, L.T.; Hanley-Cook, G.T.; Vandevijvere, S.; Leclercq, C.; De Henauw, S.; Santaliestra-Pasias, A.; Manios, Y.; Mourouti, N.; Díaz, L.E.; Gonzalez-Gross, M.; et al. Association between adherence to the EAT-Lancet sustainable reference diet and cardiovascular health among European adolescents: The HELENA study. Eur. J. Clin. Nutr. 2024, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Bollella, M.C.; Strobino, B.A.; Boccia, L.; Campanaro, L. Plant stanol ester and bran fiber in childhood: Effects on lipids, stool weight and stool frequency in preschool children. J. Am. Coll. Nutr. 1999, 18, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Bollella, M.; Spark, A.; Puder, D. Soluble fiber enhances the hypocholesterolemic effect of the step I diet in childhood. J. Am. Coll. Nutr. 1995, 14, 251–257. [Google Scholar] [CrossRef]
- Sanchez-Bayle, M.; Gonzalez-Requejo, A.; Asensio-Anton, J.; Ruiz-Jarabo, C.; Fernandez-Ruiz, M.L.; Baeza, J. The effect of fiber supplementation on lipid profile in children with hypercholesterolemia. Clin. Pediatr. (Phila) 2001, 40, 291–294. [Google Scholar] [CrossRef]
- Shah, A.S.; Wilson, D.P. Primary hypertriglyceridemia in children and adolescents. J. Clin. Lipidol. 2015, 9, S20–S28. [Google Scholar] [CrossRef]
- Hegele, R.A.; Berberich, A.J.; Ban, M.R.; Wang, J.; Digenio, A.; Alexander, V.J.; D’Erasmo, L.; Arca, M.; Jones, A.; Bruckert, E.; et al. Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. J. Clin. Lipidol. 2018, 12, 920–927. [Google Scholar] [CrossRef]
- Dron, J.S.; Hegele, R.A. Genetics of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 455. [Google Scholar] [CrossRef]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R. Clinical review on triglycerides. Eur. Heart J. 2019, 41, 99–109. [Google Scholar] [CrossRef]
- Williams, L.; Rhodes, K.S.; Karmally, W.; Welstead, L.A.; Alexander, L.; Sutton, L. Familial chylomicronemia syndrome: Bringing to life dietary recommendations throughout the life span. J. Clin. Lipidol. 2018, 12, 908–919. [Google Scholar] [CrossRef]
- Capra, M.E.; Biasucci, G.; Banderali, G.; Pederiva, C. Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet. Nutrients 2023, 15, 1088. [Google Scholar] [CrossRef] [PubMed]
- Rouis, M.; Dugi, K.A.; Previato, L.; Patterson, A.P.; Brunzell, J.D.; Brewer, H.B.; Santamarina-Fojo, S. Therapeutic response tomedium-chain triglycerides and omega-3 fatty acids in a patient with the familial chylomicronemia syndrome. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1400–1406. [Google Scholar] [PubMed]
- Ahmad, Z.; Wilson, D.P. Familial chylomicronemia syndrome and response to medium-chain triglyceride therapy in an infant with novel mutations in GPIHBP1. J. Clin. Lipidol. 2014, 8, 635–639. [Google Scholar]
- Helk, O.; Schreiber, R.; Widhalm, K. Effects of two therapeutic dietary regimens on primary chylomicronemia in paediatric age: A retrospective data analysis. Eur. J. Clin. Nutr. 2016, 70, 1127–1131. [Google Scholar]
- Aljouda, L.; Nagy, L.; Schulze, A. Long-Term Treatment of Lipoprotein Lipase Deficiency with Medium-Chain Triglyceride-Enriched Diet: A Case Series. Nutrients 2023, 15, 3535. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, W.; Du, T.; Gong, Z.; Liang, L.; Wang, R.; Yang, Y.; Zhang, K.; Lu, D.; Chen, X.; et al. Clinical profile, genetic spectrum and therapy evaluation of 19 Chinese pediatric patients with lipoprotein lipase deficiency. J. Clin. Lipidol. 2023, 17, 808–817. [Google Scholar]
- Yoldas Celik, M.; Canda, E.; Yazici, H.; Erdem, F.; Yuksel Yanbolu, A.; Atik Altinok, Y.; Pariltay, E.; Akin, H.; Kalkan Ucar, S.; Coker, M. Long-term clinical outcomes and management of hypertriglyceridemia in children with Apo-CII deficiency. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1798–1806. [Google Scholar]
- Niu, D.M.; Chong, K.W.; Hsu, J.H.; Wu, T.J.; Yu, H.C.; Huang, C.H.; Lo, M.; Kwok, C.F.; Kratz, L.E.; Ho, L. Clinical observations, molecular genetic analysis, and treatment of sitosterolemia in infants and children. J. Inherit. Metab. Dis. 2010, 33, 437–443. [Google Scholar]
- Williams, K.; Segard, A.; Graf, G.A. Sitosterolemia: Twenty Years of Discovery of the Function of ABCG5 ABCG8. Int. J. Mol. Sci. 2021, 22, 2641. [Google Scholar] [CrossRef]
- Tada, H.; Nomura, A.; Ogura, M.; Ikewaki, K.; Ishigaki, Y.; Inagaki, K.; Tsukamoto, K.; Dobashi, K.; Nakamura, K.; Hori, M.; et al. Diagnosis and Management of Sitosterolemia 2021. J. Atheroscler. Thromb. 2021, 28, 791–801. [Google Scholar]
- Parsons, H.G.; Jamal, R.; Baylis, B.; Dias, V.C.; Roncari, D. A marked and sustained reduction in LDL sterols by diet and cholestyramine in betasitosterolemia. Clin. Investig. Med. 1995, 18, 389–400. [Google Scholar]
- Park, J.H.; Chung, I.H.; Kim, D.H.; Choi, M.H.; Garg, A.; Yoo, E.G. Sitosterolemia presenting with severe hypercholesterolemia and intertriginous xanthomas in a breastfed infant: Case report and brief review. J. Clin. Endocrinol. Metab. 2014, 99, 1512–1518. [Google Scholar] [PubMed]
- Mannucci, L.; Guardamagna, O.; Bertucci, P.; Pisciotta, L.; Liberatoscioli, L.; Bertolini, S.; Irace, C.; Gnasso, A.; Federici, G.; Cortese, C. Betasitosterolaemia: A new nonsense mutation in the ABCG5 gene. Eur. J. Clin. Investig. 2007, 37, 997–1000. [Google Scholar]
- Buonuomo, P.S.; Iughetti, L.; Pisciotta, L.; Rabacchi, C.; Papadia, F.; Bruzzi, P.; Tummolo, A.; Bartuli, A.; Cortese, C.; Bertolini, S.; et al. Timely diagnosis of sitosterolemia by next generation sequencing in two children with severe hypercholesterolemia. Atherosclerosis 2017, 262, 71–77. [Google Scholar]
| Types of Dyslipidemia | Etiology |
|---|---|
| Primitive | |
| Monogenic |
|
| Polygenic |
|
| Secondary | |
| Unhealthy dietary habits |
|
| Endocrinological pathologies |
|
| Drugs |
|
| Kidneys |
|
| Hepatic |
|
| Rheumatologic |
|
| Lysosome storage disorder |
|
| Infectious |
|
| Others; |
|
| Cereals and Carbohydrates | Total Fiber/100 g | Nuts and Seeds | Total Fiber/100 g |
|---|---|---|---|
| whole wheat | 13–24 g | peanuts | 7.6 g |
| oats | 8 g | almonds | 7.4 g |
| bread | 7 g | sesame | 7.9 g |
| pasta | 4.2 g | sunflower seeds | 6.0 g |
| Fruit | Total Fiber/100 g | Vegetables | Total Fiber/100 g |
| figs | 6.9 g | lentils | 7.9 g |
| strawberries | 3.8 g | peas | 5.6 g |
| pears | 3.1 g | beans | 4.9 g |
| banana | 2.6 g | broccoli | 2.8 g |
| oranges | 2.4 g | carrots | 2.5 g |
| Source | Age Group | Recommendation |
|---|---|---|
| WHO, 2003 [30] | All children | Increase fiber intake as part of a balanced diet |
| AHA, 2005 [31] | Recommendation of 14 g of fiber per 1000 kcal consumed | |
| ||
| ||
| ||
| EFSA, 2010 [19,20] | >12 months | 2 g of fiber per MJ of energy consumed |
| NHLBL, 2011 [15] | All children | “Age + 5 g” of fiber per day |
| EAS, 2016 [33] | Children with dyslipidemia | 3–5 g of soluble fiber per day (e.g., β-glucans from oats or barley) |
| Adolescents | 25–30 g of fiber per day, adjusted to energy needs | |
| ESPGHAN, 2017 [34] | All children | 2 g of fiber per MJ of energy consumed |
| WHO, 2023 [35] | All children |
|
| Type of Fibres | Type of Study | Sample | Dose and Duration of the Intervention | Effects | Reference |
|---|---|---|---|---|---|
| General Fibres | RCT (DART Study, 1989) | 2033 post-infarction men | 5-year follow-up | No significant effect on secondary prevention. | DART Study, Lancet, 1989 [36] |
| Pooled Analysis | 10 prospective studies | Increase of 10 g/day | ↓ risk of coronary events by 14%; ↓ CHD mortality by 27%. | Pereira et al., Arch. Intern. Med., 2004 [37] | |
| Review | 15 RCTs with 453 participants | Various doses, duration not specified | No effectiveness for primary outcomes due to lack of data. | Malhotra et al., Cochrane Database Syst. Rev. 2014 [60] | |
| Guar Gum | Cross-over Design | 28 subjects (11 children and 17 adults): 18 with FH, 10 normal | 15 g/day for 8 weeks | Reduction in total cholesterol and LDL in both affected and healthy adults and children. | Zavoral JH et al., Am. J. Clin. Nutr., 1983 [49] |
| Soluble Fibres | open cross-over study | 2–5 year old preschool children | 4–10 g/day for 13-weeks | ↓ TC: −4%; | Williams, C.L. et al., Am. J. Dis. Child., 1999 [77] |
| Psyllium | RCT | 36 children (3–17 years) with type IIa hypercholesterolemia | ≤7 years: 5 g/day; ≥7 years: 10 g/day; duration: 8 ± 1.1 months | ↓ TC: −18%; ↓ LDL-C: −23% | Glassman M et al., AJDC, 1990 [50] |
| RCT | Children and teenagers with high LDL levels after at least 3 months on diet | 5–17 years, dose not specified | No statistically or clinically significant differences. | Dennison et al., J. Pediatr., 1993 [51] | |
| SB-RCT | 50 children (2–11 years) with LDL-C ≥110 mg/dL | 12-week intervention with cereals containing 3.2 g of psyllium | ↓ TC: −9.6%; ↓ LDL-C: −15.7%; ↑ HDL-C: +9.96% | Williams CL et al., J. Am. Coll. Nutr., 1995 [78] | |
| DB-CO-RCT | 32 children (6–18 years) with LDL-C ≥90th percentile | 8-week diet: 58 g of cereals with 6.4 g psyllium or placebo | ↓ TC: −5%; ↓ LDL-C: −6.8% | Davidson MH et al., Am. J. Clin. Nutr., 1996 [52] | |
| DB-RCT | 51 children (6–19 years) with TC ≥175 mg/dL | 6-week intervention with 7 g/day psyllium vs. 7 g/day cellulose (control) | ↓ TC: −7.7%; ↓ LDL-C: −10.7% | Ribas SA et al., Br. J. Nutr., 2015 [53] | |
| Beta-Glucan | RCT | 29 children (6–14 years) | 3 g/day for 4 weeks | ↓ LDL-C: -5,3% | Maki, K.C. et al., Nutr. Res., 2003 [59] |
| Meta-analysis of RCTs | Adults across multiple trials | 3–10 g/day for various durations | ↓ LDL-C; ↓ TC; (p < 0.00001) | Zhu X. et al., Nutr. Metab. Cardiovasc. Dis., 2015 [58] | |
| Pectin | Non randomized | 51 children (4-18 years) with hyperlipidemia, 33 controls | 50 mg/Kg/day for 3 months | ↓ LDL-C: −17%; ↓ TC: −15% | Sanchez-Bayle, M. et al., Clin. Pediatr. (Phila), 2001 [79] |
| Systematic Review and Meta-Analysis | Adults with hypercholesterolemia | every 5 g/day of soluble fibre | ↓ LDL-C: −8.28 mg/dL; ↓ TC: −10.82 mg/dL; | Ghavami, A. et al., Adv. Nutr., 2023 [41] | |
| Glucomannan | Review | 14 studies | Various doses, duration not specified | Positive effects on TC, LDL, triglycerides, weight, and fasting hyperglycemia, but not on HDL or BP. | Sood N. et al., Am. J. Clin. Nutr., 2008 [55] |
| DB-CO-RCT | 36 FH children (6–15 years) with TC > 90th percentile | 4-week CHILD I diet, 8-week glucomannan or placebo, 4-week washout | ↓ TC: −5.1%; ↓ LDL-C: −7.3% | Guardamagna O et al., Nutrition, 2013 [57] | |
| DR-RCT | 132 children (3–16 years) with TC ≥170 mg/dL or family CVD history | 8-week treatment with 5 neutraceuticals or placebo | GM + CP: ↓ LDL-C: −16%; GM + PC: ↓ LDL-C: −10% | Martino F et al., Atherosclerosis, 2013 [56] | |
| konjac glucomannan (KJM) | Meta-analysis of RCTs | 12 studies (8 in adult, 4 in children) | A dose of around 3 g/day of KJM significantly reduces LDL cholesterol levels (10%). | ↓ LDL-C; ↓ non-HDL-C | Ho et al., Am. J. Clin. Nutr., 2017 [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, M.E.; Biasucci, G.; Travaglia, E.; Sodero, R.; Banderali, G.; Pederiva, C. Fiber in the Treatment of Dyslipidemia in Pediatric Patients. Children 2025, 12, 427. https://doi.org/10.3390/children12040427
Capra ME, Biasucci G, Travaglia E, Sodero R, Banderali G, Pederiva C. Fiber in the Treatment of Dyslipidemia in Pediatric Patients. Children. 2025; 12(4):427. https://doi.org/10.3390/children12040427
Chicago/Turabian StyleCapra, Maria Elena, Giacomo Biasucci, Elisa Travaglia, Roberta Sodero, Giuseppe Banderali, and Cristina Pederiva. 2025. "Fiber in the Treatment of Dyslipidemia in Pediatric Patients" Children 12, no. 4: 427. https://doi.org/10.3390/children12040427
APA StyleCapra, M. E., Biasucci, G., Travaglia, E., Sodero, R., Banderali, G., & Pederiva, C. (2025). Fiber in the Treatment of Dyslipidemia in Pediatric Patients. Children, 12(4), 427. https://doi.org/10.3390/children12040427






