The Occurrence of Supernumerary Umbilical Cord Vessels: A Review for Practicing Clinicians
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vafaei, H.; Rafeei, K.; Dalili, M.; Asadi, N.; Seirfar, N.; Akbarzadeh-Jahromi, M. Prevalence of single umbilical artery, clinical outcomes and its risk factors: A cross-sectional study. Int. J. Reprod. Biomed. 2021, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wolman, I.; Gull, I.; Fait, G.; Amster, R.; Kupferminc, M.J.; Lessing, J.B.; Jaffa, A.J. Persistent right umbilical vein: Incidence and significance. Ultrasound Obs. Gynecol. 2002, 19, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowski, A.; Swatowski, D.; Gęca, T.; Kwiatek, M.; Stupak, A.; Woźniak, S.; Kwaśniewska, A. Prenatal diagnosis of persistent right umbilical vein—Incidence and clinical impact. A prospective study. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, G.; Keles, A.; Yücel Celik, O.; Yucel, A.; Sahin, D. Prenatal diagnosis of the persistent right umbilical vein, incidence and clinical significance. J. Obstet. Gynaecol. 2022, 42, 443–446. [Google Scholar] [CrossRef]
- Puvabanditsin, S.; Garrow, E.; Bhatt, M.; Kathiravan, S.; Gowda, S.; Wong, R.; Nagar, M. Four-vessel umbilical cord associated with multiple congenital anomalies: A case report and literature review. Fetal. Pediatr. Pathol. 2011, 30, 98–105. [Google Scholar] [CrossRef]
- Lide, B.; Lindsley, W.; Foster, M.J.; Hale, R.; Haeri, S. Intrahepatic Persistent Right Umbilical Vein and Associated Outcomes: A Systematic Review of the Literature. J. Ultrasound Med. 2016, 35, 1–5. [Google Scholar] [CrossRef]
- Koolhaas, G.D.; Hollander, M.H.; Molendijk, H. A case of a four-vessel umbilical cord: Don’t stop counting at three! Case Rep. Perinat. Med. 2012, 1, 87–90. [Google Scholar] [CrossRef]
- Toscano, P.; Saccone, G.; Di Meglio, L.; Di Meglio, L.; Mastantuoni, E.; Riccardi, C.; Raffone, A.; Zullo, F.; Locci, M.; Di Meglio, A. Intrahepatic persistent fetal right umbilical vein: A retrospective study. J. Matern. Fetal Neonatal. Med. 2021, 34, 4025–4028. [Google Scholar] [CrossRef]
- Mohapatra, I.; Samantaray, S.R. Persistent Right Umbilical Vein in Association With Single Umbilical Artery: A Case Report and Review of Literature. Cureus 2023, 15, e36544. [Google Scholar] [CrossRef]
- Leonidas, J.C.; Fellows, R.A. Congenital absence of the ductus venosus: With direct connection between the umbilical vein and the distal inferior vena cava. Am. J. Roentgenol. 1976, 126, 892–895. [Google Scholar] [CrossRef]
- Moore, L.; Toi, A.; Chitayat, D. Abnormalities of the intra-abdominal fetal umbilical vein: Reports of four cases and a review of the literature. Ultrasound Obstet. Gynecol. 1996, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Keeling, J.W. Fetal Hydrops. In Fetal and Neonatal Pathology, 1st ed.; Keeling, J.W., Ed.; Springer: London, UK, 1987; pp. 211–228. [Google Scholar]
- Sivén, M.; Ley, D.; Hägerstrand, I.; Svenningsen, N. Agenesis of the ductus venosus and its correlation to hydrops fetalis and the fetal hepatic circulation: Case reports and review of the literature. Pediatr. Pathol. Lab. Med. 1995, 15, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Firdouse, M.; Agarwal, A.; Pindiprolu, B.; Mondal, T. Two ductus venosus: A previously unreported anomaly. J. Ultrasound 2014, 17, 293–296. [Google Scholar] [PubMed]
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O. The fetal venous system, part I: Normal embryology, anatomy, hemodynamics, ultrasound evaluation and Doppler investigation. Ultrasound Obstet. Gynecol. 2010, 35, 741–750. [Google Scholar] [CrossRef]
- Painter, D.; Russell, P. Four-vessel umbilical cord associated with multiple congenital anomalies. Obstet Gynecol. 1977, 50, 505–507. [Google Scholar]
- Hoppen, T.; Hofstaetter, C.; Plath, H.; Kau, N.; Bartmann, P. Agenesis of the ductus venosus and its correlation to hydrops fetalis. J. Perinat. Med. 2000, 28, 69–73. [Google Scholar] [CrossRef]
- Karatza, A.; Tsamandas, A.; Varvarigou, A.; Davlouros, P.; Pavlou, V.; Mantagos, S. Supernumerary umbilical vein in a hydropic neonate with hypertrophic cardiomyopathy. Fetal. Pediatr. Pathol. 2011, 30, 173–176. [Google Scholar]
- Degirmencioglu, H.; Oncel, M.Y.; Yurttutan, S.; Calisici, E.; Erdeve, O.; Zergeroglu, S.; Dilmen, U. A four-vessel umbilical cord with omphalomesenteric duct in trisomy 18. Genet. Couns. 2012, 23, 431–433. [Google Scholar]
- Premkumar, S.; Manikandan, P.; Kamalarathnam, C. Four Vessel Umbilical Cord -Three Arteries and One Vein. Pediatr. Neonatal Biol. Open Access. 2023, 8, 000184. [Google Scholar]
- Murdoch, D.E. Umbilical-cord doubling. Report of a case. Obstet. Gynecol. 1966, 27, 555–557. [Google Scholar]
- Rodriguez, M.A. Four-vessel umbilical cord without congenital abnormalities. South Med. J. 1984, 77, 539. [Google Scholar] [PubMed]
- Aoki, S.; Hata, T.; Ariyuki, Y.; Makihara, K.; Hata, K.; Kitao, M. Antenatal diagnosis of aberrant umbilical vessels. Gynecol. Obstet. Investig. 1997, 43, 232–235. [Google Scholar]
- Schimmel, M.S.; Eidelman, A.I. Supernumerary umbilical vein resulting in a four-vessel umbilical cord. Am. J. Perinatol. 1998, 15, 299–301. [Google Scholar] [PubMed]
- Paize, F.; Yoxall, C.W. Supernumerary umbilical vein demonstrated by radiography of a 27-week-gestation neonate. Pediatr. Radiol. 2006, 36, 570. [Google Scholar]
- Pérez-Cosio, C.; Sheiner, E.; Abramowicz, J.S. Four-vessel umbilical cord: Not always a dire prognosis. J. Ultrasound Med. 2008, 27, 1389–1391. [Google Scholar]
- Avnet, H.; Shen, O.; Mazaki, E.; Yagel, S.; Daniel-Spiegel, E. Four-vessel umbilical cord. Ultrasound Obstet. Gynecol. 2011, 38, 604–606. [Google Scholar]
- Panda, S.; Jha, V.; Khonglah, Y.; Dey, B. A multivessel umbilical cord with a single umbilical artery. J. Clin. Diagn. Res. 2013, 7, 1453–1454. [Google Scholar]
- Hoh, J.; Boo, H.; Lee, E.; Seo, M.; Lee, J.; Choe, Y.; Keum, J. Four-vessel umbilical cord containing three arteries and one vein. In Proceedings of the Supplement: Abstracts of the 25th World Congress on Ultrasound in Obstetrics and Gynecology, Montréal, QC, Canada, 11–14 October 2015. [Google Scholar]
- Lei, T.; Xie, H.N.; Feng, J.L. Prenatal diagnosis of four-vessel umbilical cord with supernumerary vein varix: A case report and literature review. J. Obstet. Gynaecol. Res. 2017, 43, 1200–1204. [Google Scholar]
- Kurakazu, M.; Kurakazu, M.; Murata, M.; Miyamoto, T.; Takahashi, Y.; Hamasaki, M.; Ohta, E.; Yotsumoto, F.; Miyamoto, S. A partial supernumerary umbilical vein: A case report. J. Med. Case Rep. 2019, 13, 149. [Google Scholar]
- Kaur, P.; Singh, J. Four vessel umbilical cord. Pediatric Oncall J. 2020, 17, 137. [Google Scholar] [CrossRef]
- Damiani, G.R.; Del Boca, G.; Biffi, A. Five-vessel umbilical cord and fetal outcome: An obstetric overview. J. Matern. Fetal Neonatal Med. 2022, 35, 6250–6253. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Arora, P.; Tan-Dy, C. Four-vessel umbilical cord in a neonate with no associated congenital anomalies. BMJ Case Rep. 2022, 15, e251453. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.K.; Minto, O. Four-Vessel Umbilical Cord: Supernumerary Right Umbilical Vein with No Associated Congenital Anomalies. Cureus 2024, 16, e67283. [Google Scholar] [CrossRef] [PubMed]
- Mari, S.; Polichetti, M.; Castaldi, M.A.; Gargano, V.; Gragnano, E.; Saccone, G. Four-vessel umbilical cord with three arteries and one vein: A case report and literature review. Ital. J. Gynaecol. Obstet. 2024, 36, 292–296. [Google Scholar]
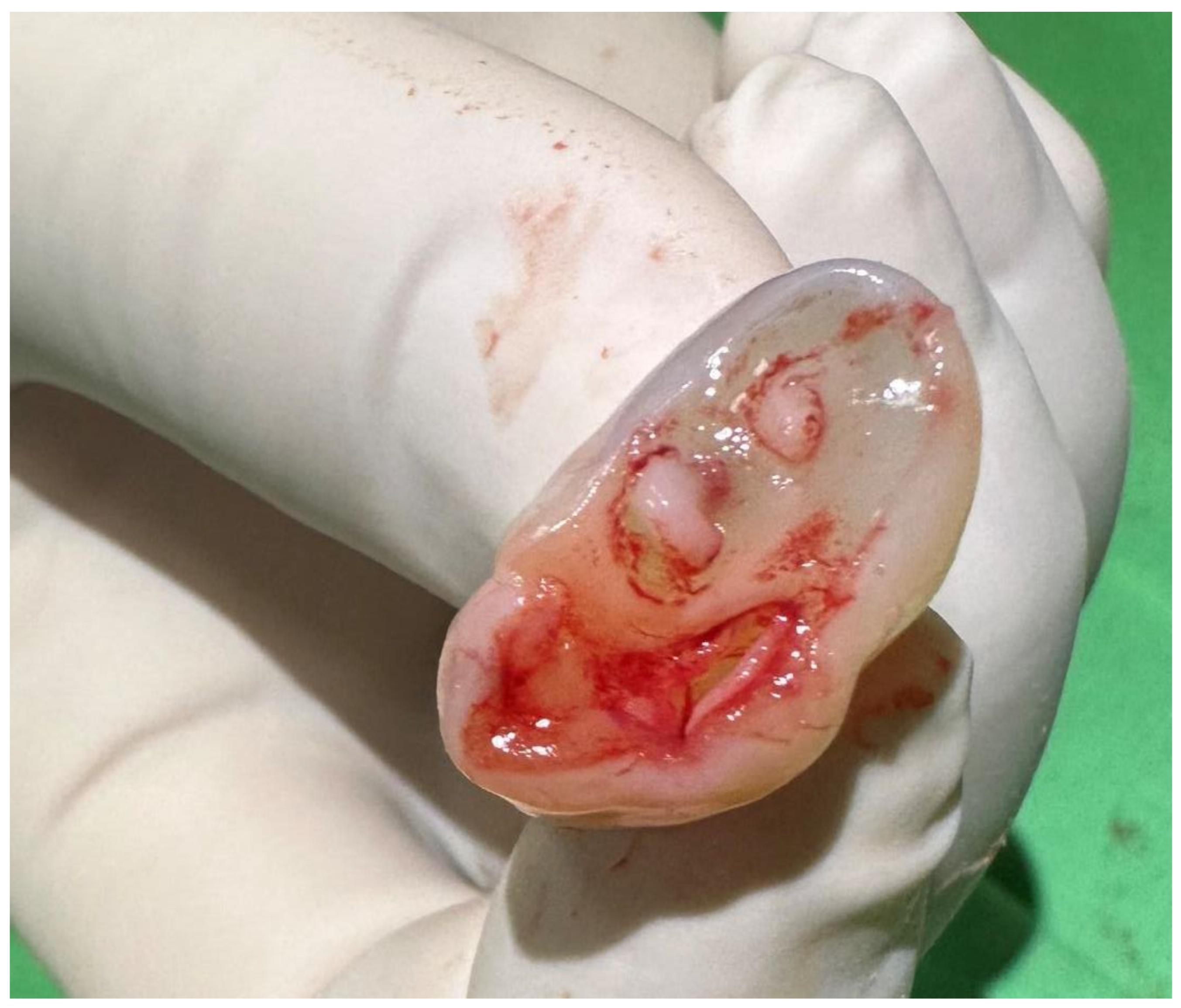
| Article | Age of Mother | Risk Factors | GW at Birth (Week) | Mode of Delivery | Gender | Weight (g) | Apgar Score (1 and 5 min) | Diagnosis of FVUC | Type of FVUC | Associated Mal-Formations | Intrauterine Growth Restriction | Fetal Karyotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Painter (1977) [16] | 32 | NR | 37 | vag | M | 1550 | 1–7 | pp | 2a, 2v | complete thoracic ectopia cordis | NR | IUD |
| asymmetrical bifid liver | ||||||||||||
| severe bilateral cleft lip and palate with absent soft palate and uvula | ||||||||||||
| cecum mobile | ||||||||||||
| Hoppen (2000) [17] | 32 | polyhydramnion | 34 | cs | M | 1940 | 1–7–8 | 34 GW | 2a, 2v | hydrops | no | normal |
| proteinuria | ||||||||||||
| agenesis of ductus venosus | ||||||||||||
| 2 v.umb. drained into the right atria | ||||||||||||
| Karatza (2011) [18] | 16 | NR | 40 | cs | F | NR | 1–2 | pp | 2a, 2v | hydrops | no | normal |
| hypertrophic cardiomyopathy | ||||||||||||
| Puvabanditsin (2011) [5] | 20 | NR | 39 | cs | F | 2730 | 8–9 | pp | 2a, 2v | heterotaxy syndrome | yes | normal |
| Degirmencioglu (2012) [19] | 22 | NR | 32 | cs | F | 1240 | 4–6 | pp | 2a, 2v | esophagus atresia | yes | trisomy 18 |
| hypoplastic corpus callosum | ||||||||||||
| dysmorphic facial features | ||||||||||||
| clenched hands | ||||||||||||
| rocker bottom feet | ||||||||||||
| intracranial hemorrhage | ||||||||||||
| Premkumar (2023) [20] | 26 | none | 37 | vag | M | 2540 | 7–8 | pp | 3a, 1v | d-TGA | NR | |
| single coronary artery | ||||||||||||
| left-side urinary tract dilatation |
| Article | Age of Mother | Risk Factors | GW at Birth (Week) | Mode of Delivery | Gender | Weight (g) | Apgar Score (1 and 5 min) | Time of Diagnosis of FVUC | Type of FVUC | Intrauterine Growth Restriction | Fetal Karyotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Murdoch (1966) [21] | 16 | NR | 39 | forceps | M | 2806 | 10-10 | pp | 2a, 2v | NR | NR |
| Rodrigez (1984) [22] | 30 | di-zygote twin | NR | cs | F | 3090 | 1–7 | pp | 2a, 2v | no | NR |
| Aoki (1997) [23] | NR | NR | 37 | vag | F | 3016 | 9, NR | 29 GW | 2a, 2v | no | NR |
| Aioki (1997) [23] | NR | NR | 39 | vag | F | 3588 | 9, NR | 23 GW | 2a, 2v | no | NR |
| Schimmel (1998) [24] | NR | IVF | 30 | NR | NR | 1320 | 1–7 | pp | 2a, 2v | yes | NR |
| Paize (2006) [25] | NR | NR | 27 | vag | M | NR | NR | pp | 2a, 2v | no | NR |
| Perez-Cosio (2008) [26] | 37 | SLE | 38 | cs | M | 3240 | 8–9 | 32 GW | 2a, 2v | no | NR |
| Avent (2011) [27] | 33 | NR | 39 | cs | F | 3780 | 9–10 | 22 GW | 2a, 2v | no | normal |
| Koolhaas (2012) [7] | 26 | meconium-stained amniotic fluid | 41 | vag | M | 4420 | 8–9 | pp | 2a, 2v | yes | NR |
| Panda (2013) [28] | 37 | oligohydramnion | 33 | vag | F | 1400 | NR | pp | 1a, 2v | yes | IUD |
| Hoh (2015) [29] | NR | no | 38 | NR | M | NR | NR | 22 GW | 3a, 1v | NR | NR |
| Lei (2017) [30] | 31 | NR | 38 | forceps | M | 2660 | 10–10 | 36 GW | 2a, 2v | yes | normal |
| Kurakazu (2019) [31] | 37 | NR | 38 | NR | F | 2726 | NR | 36 GW | 2a, 2v | no | NR |
| Kaur (2020) [32] | 24 | none | 34 | vag | NR | 2000 | NR | pp | 2a, 2v | NR | NR |
| Damiani (2021) [33] | NR | IUGR, bicornuate uterus | 37 | NR | NR | 2500 | NR | pp | 4a, 1v | yes | NR |
| Arora (2022) [34] | NR | none | 38 | vag | NR | NR | 5–6–8 | pp | 2a, 2v | NR | NR |
| Allen (2024) [35] | 22 | none | 37 | vag | NR | 2360 | 9–10 | pp | 2a, 2v | NR | NR |
| Our case | 35 | none | 39 | cs | F | 4250 | 9–10–10 | pp | 2a, 2v | no | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth-Varga, É.; Hódi, E.; Pásztor, G.; Katona, M.; Orvos, H.; Gyurkovits, Z. The Occurrence of Supernumerary Umbilical Cord Vessels: A Review for Practicing Clinicians. Children 2025, 12, 418. https://doi.org/10.3390/children12040418
Horváth-Varga É, Hódi E, Pásztor G, Katona M, Orvos H, Gyurkovits Z. The Occurrence of Supernumerary Umbilical Cord Vessels: A Review for Practicing Clinicians. Children. 2025; 12(4):418. https://doi.org/10.3390/children12040418
Chicago/Turabian StyleHorváth-Varga, Éva, Eszter Hódi, Gyula Pásztor, Márta Katona, Hajnalka Orvos, and Zita Gyurkovits. 2025. "The Occurrence of Supernumerary Umbilical Cord Vessels: A Review for Practicing Clinicians" Children 12, no. 4: 418. https://doi.org/10.3390/children12040418
APA StyleHorváth-Varga, É., Hódi, E., Pásztor, G., Katona, M., Orvos, H., & Gyurkovits, Z. (2025). The Occurrence of Supernumerary Umbilical Cord Vessels: A Review for Practicing Clinicians. Children, 12(4), 418. https://doi.org/10.3390/children12040418







