Obesity and Overweight Conditions in Children and Adolescents (6–18 Years) and Their Impact on Craniofacial Morphology: A Systematic Review
Abstract
1. Introduction
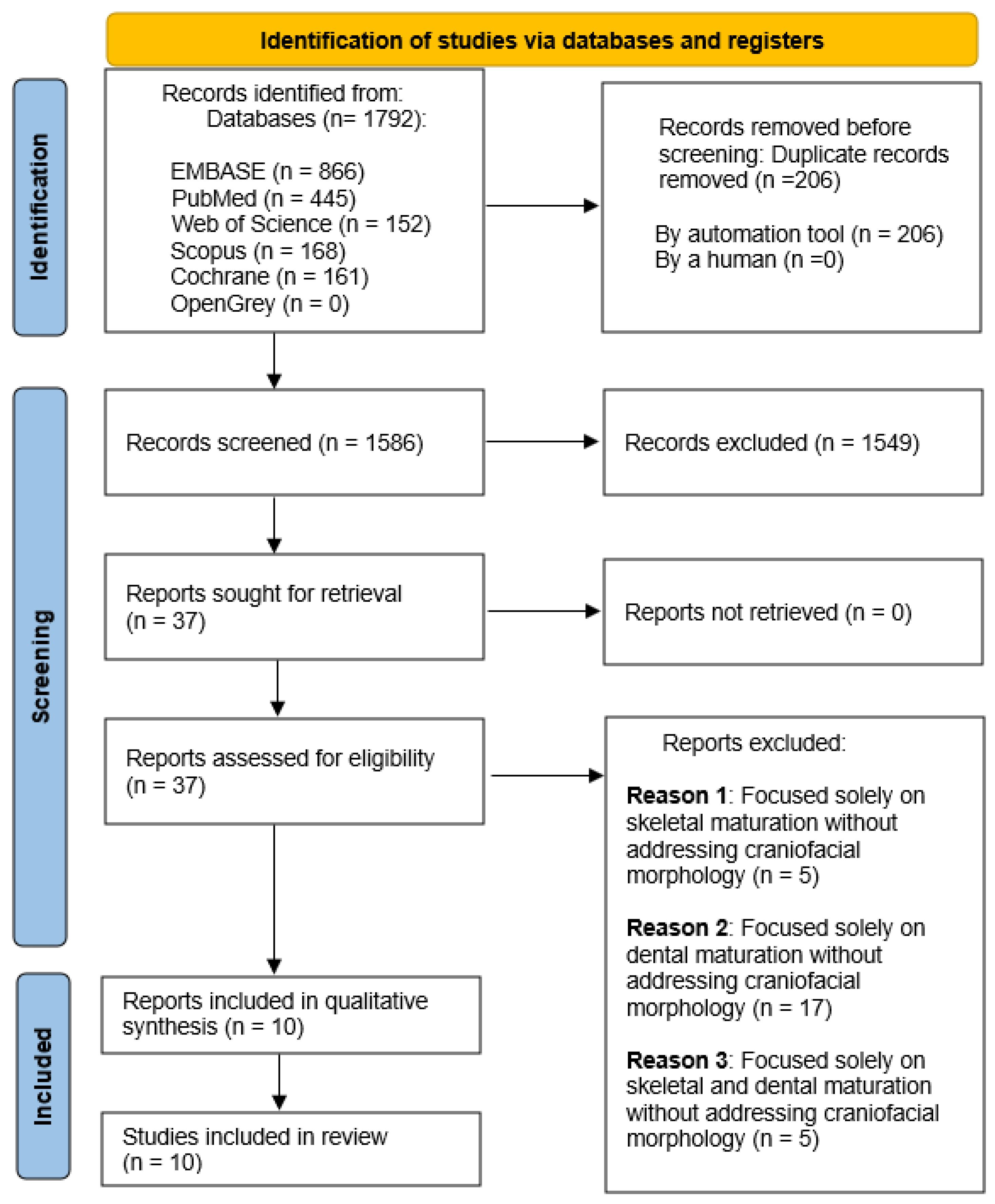
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
- Population (P): Children and adolescents aged 6 to 18 years with obesity or overweight conditions.
- Intervention (I): No intervention applied.
- Comparison (C): Normal weight children and adolescents within the same age range.
- Outcome (O): Differences in the development of craniofacial structures among growing individuals with obesity or overweight conditions.
2.3. Data Extraction and Synthesis
2.4. Quality Assessment
3. Results
3.1. Description of the Included Studies
3.1.1. Country and Study Design
3.1.2. Sample Characteristics
3.1.3. Body Mass Categories
3.1.4. Craniofacial Morphology and Maturation Parameters
3.1.5. Body Mass Assessment
3.2. Results of Cephalometric Analysis
3.2.1. Maxillofacial Length Parameters
3.2.2. Jaw Projection and Prognathism Parameters
3.2.3. Skeletal Divergence Parameters
3.2.4. Dental Positions
3.2.5. Facial Soft Tissue Thickness
3.2.6. Airway Analysis
3.3. Risk of Bias Assessment
4. Discussion
- The majority of included studies are cross-sectional, which limits the ability to establish causal relationships.
- Many studies do not account for racial and ethnic variations in craniofacial morphology, potentially influencing generalizability.
- The wide age range considered in some studies may introduce developmental variability, affecting the interpretation of growth patterns.
- Specific hormonal analyses to determine the precise physiopathological etiology of craniofacial changes were not conducted.
- There is a lack of long-term prospective studies, which are essential for understanding the sustained effects of obesity on craniofacial development and orthodontic outcomes. Given the rising prevalence of childhood obesity, orthodontic diagnostics should integrate metabolic and hormonal considerations to optimize treatment outcomes. Future investigations should focus on longitudinal studies assessing the enduring effects of obesity on craniofacial development, along with further elucidation of the molecular pathways linking adipokine activity to orthodontic and skeletal dynamics.
5. Conclusions
- Obesity is associated with increased craniofacial dimensions, including maxillary and mandibular length, as well as increased jaw projections and jaw prognathism.
- Obese and overweight individuals exhibit greater facial hyperdivergence.
- Soft tissue thickness is significantly greater in obese individuals.
- Despite these findings, further longitudinal and prospective studies are necessary to establish causal relationships and refine clinical guidelines for orthodontic treatment in obese patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet Diabetes Endocrinology. Childhood obesity: A growing pandemic. Lancet Diabetes Endocrinol. 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Morales Camacho, W.J.; Molina Díaz, J.M.; Plata Ortiz, S.; Plata Ortiz, J.E.; Morales Camacho, M.A.; Calderón, B.P. Childhood obesity: Aetiology, comorbidities, and treatment. Diabetes Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef] [PubMed]
- Güngör, N.K. Overweight and obesity in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef]
- de Groot, C.J.; van den Berg, A.; Ballieux, B.E.P.B.; Kroon, H.M.; Rings, E.H.H.M.; Wit, J.M.; van den Akker, E.L.T. Determinants of Advanced Bone Age in Childhood Obesity. Horm. Res. Paediatr. 2017, 87, 254–263. [Google Scholar] [CrossRef]
- Park, T.H.; Lin, J.H.; Chung, C.H.; Zheng, Z.; Li, C. The skeletal and dental age advancements of children and adolescents with overweight and obesity: A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 325–339. [Google Scholar] [CrossRef]
- Traver, C.; Miralles, L.; Barcia, J.M. Association between Molecular Mechanisms and Tooth Eruption in Children with Obesity. Children 2022, 9, 1209. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The Bones of Children with Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Silva, M.A.F.S.; Dechichi, P.; Limirio, P.H.J.O. Impact of Childhood Obesity on Bone Metabolism. Pediatr. Endocrinol. Rev. 2020, 17, 308–316. [Google Scholar]
- Dimitri, P. The Impact of Childhood Obesity on Skeletal Health and Development. J. Obes. Metab. Syndr. 2019, 28, 4–17. [Google Scholar] [CrossRef]
- Costacurta, M.; Sicuro, L.; Di Renzo, L.; Condò, R.; De Lorenzo, A.; Docimo, R. Childhood obesity and skeletal-dental maturity. Eur. J. Paediatr. Dent. 2012, 13, 128–132. [Google Scholar]
- Traver-Ferrando, C.; Barcia-González, J. Early permanent dental eruption in obese/overweight schoolchildren. J. Clin. Exp. Dent. 2022, 14, e199–e204. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Phillips, S.M.; Tybor, D.J.; Lividini, K.; Hayes, C. The association between childhood obesity and tooth eruption. Obesity 2012, 20, 2070–2074. [Google Scholar] [CrossRef]
- Manohar, N.; Hayen, A.; Fahey, P.; Arora, A. Obesity and dental caries in early childhood: A systematic review and meta-analyses. Obes. Rev. 2020, 21, e12960. [Google Scholar] [CrossRef] [PubMed]
- Saloom, H.F.; Papageorgiou, S.N.; Carpenter, G.H.; Cobourne, M.T. Impact of Obesity on Orthodontic Tooth Movement in Adolescents: A Prospective Clinical Cohort Study. J. Dent. Res. 2017, 96, 547–554. [Google Scholar] [CrossRef]
- Consolaro, A. Obesity and orthodontic treatment: Is there any direct relationship? Dent. Press J. Orthod. 2017, 22, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Saloom, H.F.; Boustan, R.; Seehra, J.; Papageorgiou, S.N.; Carpenter, G.H.; Cobourne, M.T. The impact of obesity on orthodontic treatment outcome in adolescents: A prospective clinical cohort study. Eur. J. Orthod. 2021, 43, 165–172. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Eapen, N. Childhood Obesity. Prim. Care 2021, 48, 505–515. [Google Scholar] [CrossRef]
- Giglione, E.; Lapolla, R.; Cianfarani, S.; Faienza, M.F.; Fintini, D.; Weber, G.; Delvecchio, M.; Valerio, G. Linear growth and puberty in childhood obesity: What is new? Minerva Pediatr. 2021, 73, 563–571. [Google Scholar] [CrossRef]
- Bilello, G.; Currò, G.; Fregapane, A. Correlazione tra status nutrizionale e malocclusione in un gruppo di bambini tra i 3 e i 12 anni d’età. Correggere lo stile di vita. Recent. Prog. Med. 2012, 103, 205–207. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Ohrn, K.; Al-Kahlili, B.; Huggare, J.; Forsberg, C.M.; Marcus, C.; Dahllöf, G. Craniofacial morphology in obese adolescents. Acta Odontol. Scand. 2002, 60, 193–197. [Google Scholar] [CrossRef]
- Sadeghianrizi, A.; Forsberg, C.M.; Marcus, C.; Dahllöf, G. Craniofacial development in obese adolescents. Eur. J. Orthod. 2005, 27, 550–555. [Google Scholar] [CrossRef]
- Giuca, M.R.; Giannotti, L.; Saggese, R.; Vanni, A.; Pasini, M. Evaluation of cephalometric, hormonal and enzymatic parameters in young obese subjects. Eur. J. Paediatr. Dent. 2013, 14, 175–180. [Google Scholar] [PubMed]
- Buyuk, S.K.; Genc, E.; Simsek, H.; Karaman, A. Analysis of facial soft tissue values and cranial skeletal widths in different body mass index percentile adolescent subjects. CRANIO® 2019, 37, 223–230. [Google Scholar] [CrossRef]
- Danze, A.; Jacox, L.A.; Bocklage, C.; Whitley, J.; Moss, K.; Hardigan, P.; Garcia-Godoy, C.E.; Jackson, T.H. Influence of BMI percentile on craniofacial morphology and development in children and adolescents. Eur. J. Orthod. 2021, 43, 184–192. [Google Scholar] [CrossRef]
- Gordon, L.A.; Miller, S.F.; Caplin, J.; Galang-Boquiren, M.T.; Alrayyes, S.; Nicholas, C.L. Childhood obesity may accelerate timing of human facial growth. Arch. Oral Biol. 2021, 121, 104964. [Google Scholar] [CrossRef]
- Karaman, A.; Genc, E. Evaluation of facial soft-tissue values and craniofacial morphology in obese adolescent patients with different skeletal classes. APOS Trends Orthod. 2022, 11, 270–278. [Google Scholar] [CrossRef]
- Vora, S.R.; Tam, S.; Katsube, M.; Pliska, B.; Heda, K. Craniofacial form differences between obese and nonobese children. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 744–752.e3. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Carmack, A.; Kocher, M.; Rezende Silva, E.; Sulkowski, T.; Nanney, E.; Graves, C.; Mitchell, K.; Jacox, L.A. Influence of BMI percentile on craniofacial morphology and development in adolescents, Part II: Elevated BMI is associated with larger final facial dimensions. Eur. J. Orthod. 2024, 46, cjad043. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, A.; Torre, I.C.; Diaz, I.M.; Sanz, V.G.; Mesa, Y.G.; Cobo, T.; Gallardo, V.P. Analysis of the Relationship Between Body Mass Index (BMI) and Dento-Skeletal Maturation: A Cross-Sectional Case-Control Study. Dent. J. 2024, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Franchi, L.; McNamara, J.A. The Cervical Vertebral Maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin. Orthod. 2005, 11, 119–129. [Google Scholar] [CrossRef]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A new system of dental age assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Extended Growth Charts. Available online: https://www.cdc.gov/growth-chart-training/hcp/extended-growth-charts/index.html (accessed on 26 December 2024).
- World Health Organization (WHO). BMI-for-Age Growth Reference Data. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 26 December 2024).
- Shalitin, S.; Kiess, W. Putative Effects of Obesity on Linear Growth and Puberty. Horm. Res. Paediatr. 2017, 88, 101–110. [Google Scholar] [CrossRef]
- Yagasaki, Y.; Yamaguchi, T.; Watahiki, J.; Konishi, M.; Katoh, H.; Maki, K. The role of craniofacial growth in leptin-deficient (ob/ob) mice. Orthod. Craniofac. Res. 2003, 6, 233–241. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Tan, X.; Ye, Y.; Huang, L.; Dai, H.; Mei, L. Osteopenic effects of high-fat diet-induced obesity on mechanically induced alveolar bone remodeling. Oral Dis. 2021, 27, 1243–1256. [Google Scholar] [CrossRef]
- Damanaki, A.; Memmert, S.; Nokhbehsaim, M.; Sanyal, A.; Gnad, T.; Pfeifer, A.; Deschner, J. Impact of obesity and aging on crestal alveolar bone height in mice. Ann. Anat. 2018, 218, 227–235. [Google Scholar] [CrossRef]
- Tuncer, B.B. Obesity and Craniofacial Morphology. Turk. J. Orthod. 2007, 20, 174–180. [Google Scholar]
- Zhao, T.; Tao, Z.; Zhang, G.; Zhu, J.; Du, M.; Hua, F.; He, H. Fat mass and obesity-associated protein (FTO) affects midpalatal suture bone remodeling during rapid maxillary expansion. Eur. J. Orthod. 2024, 46, cjae009. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.L.; Thalji, G.N.; Richter, A. Childhood Obesity and Accelerated Timing of Dental Development: A Critical Review. Forensic Anthropol. 2018, 1, 170–179. [Google Scholar] [CrossRef]
- Mohamedhussein, N.; Busuttil-Naudi, A.; Mohammed, H.; UlHaq, A. Association of obesity with the eruption of first and second permanent molars in children: A systematic review. Eur. Arch. Paediatr. Dent. 2020, 21, 13–23. [Google Scholar] [CrossRef]
- Thongudomporn, U.; Chongsuvivatwong, V.; Geater, A.F. The effect of maximum bite force on alveolar bone morphology. Orthod. Craniofac. Res. 2009, 12, 1–8. [Google Scholar] [CrossRef]
- Araujo, D.S.; Marquezin, M.C.S.; Barbosa, T.d.S.; Gavião, M.B.; Castelo, P.M. Evaluation of masticatory parameters in overweight and obese children. Eur. J. Orthod. 2016, 38, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Conith, A.J.; Lam, D.T.; Albertson, R.C. Muscle-induced loading as an important source of variation in craniofacial skeletal shape. Genesis 2019, 57, e23263. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X. The interaction of obesity and craniofacial deformity in obstructive sleep apnea. Dentomaxillofac. Radiol. 2021, 50, 20200425. [Google Scholar] [CrossRef]
- Ruiz-Heiland, G.; Yong, J.W.; von Bremen, J.; Ruf, S. Leptin reduces in vitro cementoblast mineralization and survival as well as induces PGE2 release by ERK1/2 commitment. Clin. Oral Investig. 2021, 25, 1933–1944. [Google Scholar] [CrossRef]
- Fudalej, P.; Bollen, A.M. Effectiveness of the cervical vertebral maturation method to predict postpeak circumpubertal growth of craniofacial structures. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 59–65. [Google Scholar] [CrossRef]
- Li, L.W.; Wong, H.M.; McGrath, C.P. Longitudinal association between obesity and periodontal diseases among secondary school students in Hong Kong: A prospective cohort study. BMC Oral Health 2018, 18, 189. [Google Scholar] [CrossRef]
- Neeley, W.W., 2nd; Gonzales, D.A. Obesity in adolescence: Implications in orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 581–588. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Strategy |
|---|---|
| Scopus | (TITLE-ABS-KEY (“obesity”) OR TITLE-ABS-KEY (“childhood obesity”) OR TITLE-ABS-KEY (“BMI”) OR TITLE-ABS-KEY (“overweight”) AND TITLE-ABS-KEY (“dental eruption”) OR TITLE-ABS-KEY (“facial growth”) OR TITLE-ABS-KEY (“cervical vertebral maturation”) OR TITLE-ABS-KEY (“skull growth”) OR TITLE-ABS-KEY (“dental maturation”) OR TITLE-ABS-KEY (“orthodontics”)) |
| Web of Science | ALL = ((“obesity” OR “childhood obesity” OR “BMI” OR “overweight”) AND (“dental eruption” OR “facial growth” OR “cervical vertebral maturation” OR “skull growth” OR “dental maturation” OR “orthodontics”)) |
| Embase | (obesity:ti,ab,kw OR ‘childhood obesity’:ti,ab,kw OR ‘body mass’:ti,ab,kw OR ‘overweight’:ti,ab,kw) AND ‘tooth eruption’:ti,ab,kw OR ‘face growth’:ti,ab,kw OR ‘skull development’:ti,ab,kw OR ‘dental maturation’:ti,ab,kw OR ‘cervical vertebral maturation’:ti,ab,kw) |
| Cochrane | (“obesity”):ti,ab,kw OR (“BMI”):ti,ab,kw AND (“facial growth”):ti,ab,kw OR (“cervical vertebral maturation”):ti,ab,kw OR (“dental eruption”):ti,ab,kw |
| Pubmed | (“obesity” [All Fields] OR “childhood obesity” [All Fields] OR “BMI” [All Fields] OR “overweight” [All Fields]) AND (“dental eruption” [All Fields] OR “facial growth” [All Fields] OR “cervical vertebral maturation” [All Fields] OR “skull growth” [All Fields] OR “dental maturation” [All Fields] OR “orthodontics” [All Fields]) |
| Author/Year (Country) | Age/Sex/Ethnicity | Sample Size | OB (N) | OW (N) | NW (N) | UW (N) | Dental and Skeletal Maturation Assessment |
|---|---|---|---|---|---|---|---|
| Ohrn K et al., 2002 (Sweden) [24] | F: 15.7 ± 0.82 y M: 14.6 ± 0.53 y 25 F 14 M control group: 25 F 14 M NR | 78 | 39 | NR | 39 | NR | NR |
| Sadeghianrizi A et al., 2005 (Sweden) [25] | F: 15.6 ± 0.83 y M: 13.9 ± 0.98 y 27 F 23 M NR | 100 | 50 | NR | 50 | NR | NR |
| Giuca MR et al., 2013 (Italy) [26] | 9.8 ± 2.1 y Control group: 9.9 ± 2.5 y 22 F 28 M Caucasian | 50 | 25 | NR | 25 | NR | NR |
| Buyuk SK et al., 2019 (Turkey) [27] | 12.92 to 17.53 y 50 F 30 M NR | 80 | 15 | 18 | 47 | NR | NR |
| Danze A et al., 2021 (United States) [28] | 5.0 to 10.0 y 223 F 177 M African American: 43 Caucasian: 243 Asian: 16 Other: 9 | 400 | OB + OW 200 | 200 | NR | - Demerjian - CVM | |
| Gordon LA et al., 2021 (United States) [29] | 9.0 to 19.0 y 95 F 86 M African American: 20 Asian: 9 Caucasian: 134 Multiracial: 3 Unknown: 15 | 181 | 47 | 35 | 93 | 6 | NR |
| Karaman A et al., 2021 (Turkey) [30] | 14.0 to 18.0 y 15.65 ± 1.16 y 135 F 157 M NR | 292 | 95 | 93 | 104 | NR | NR |
| Vora SR et al., 2022 (United States) [31] | 7.6 to 16.4 y 16 F 32 M NR | 48 | 24 | NR | 24 | NR | - Demerjian - CVM |
| Hancock S et al., 2024 (United States) [32] | 8 to 14 y 183 F 143 M Control group: 86 F, 72 M African American 34 Caucasian 192 Hispanic 82 Others 19 | 326 | 168 OB + OW without distinction | 168 OB + OW without distinction | 158 | NR | - NR - CVM |
| Verdecchia A. et al., 2024 (Spain) [33] | 11.96 ± 2.44 y 94 F 84 M Caucasian | 178 | 26 | 37 | 115 | NR | - Demerjian - CVM |
| Craniofacial Parameters | Significant Cephalometric Differences (OB, OW vs. NW)—p < 0.05 | Authors/Year | |
|---|---|---|---|
| Maxillofacial Length Parameters | Anterior Cranial Base Length (S-N) | Increased | Ohrn K et al., 2002 [24]; Sadeghianrizi A et al., 2005 [25]; Giuca MR et al., 2013 [26]; Karaman A et al., 2021 [30] |
| Maxillary Length (Pm-A) | Increased | Ohrn K et al., 2002 [24]; Sadeghianrizi A et al., 2005 [25]; Giuca MR et al., 2013 [26] | |
| Maxillary Length (Co-A) | Increased | Karaman A et al., 2021 [30] | |
| Maxillary Length (PNS-A) | Increased | Vora SR et al., 2022 [31] | |
| Mandibular Length (Cd-Pgn) | Increased | Ohrn K et al., 2002 [24]; Sadeghianrizi A et al., 2005 [25]; Gordon LA et al., 2021 [29] | |
| Mandibular Length (Ar-Gn) | Increased | Danze A et al., 2021 [28] | |
| Mandibular Length (Co-Gn) | Increased | Karaman A et al., 2021 [30]; Vora SR et al., 2022 [31] | |
| Corpus Length (Go-Pg) | Increased | Ohrn K et al., 2002 [24]; Sadeghianrizi A et al., 2005 [25] | |
| Posterior Facial Height (S-Go) | Increased | Ohrn K et al., 2002 [24]; Sadeghianrizi A et al., 2005 [25]; Danze A et al., 2021 [28]; Karaman A et al., 2021 [30] | |
| Anterior Facial Height (N-Me) | Increased/Decreased | Danze A et al., 2021 [28]; Karaman A et al., 2021 [30]; Hancock S et al., 2024 [32]; Vora SR et al., 2022 [31] | |
| Upper Anterior Facial Height (Na-Sp) | Decreased | Ohrn K et al., 2002 [24] | |
| Lower Anterior Facial Height (ANS-Gn) | Increased | Sadeghianrizi A et al., 2005 [25] | |
| Maxillary Dentolabial Height (ANS-Pr) | Increased | Sadeghianrizi A et al., 2005 [25] | |
| Ramus Length | Increased | Gordon LA et al., 2021 [29] | |
| Mandibular Corpus Height | Increased | Gordon LA et al., 2021 [29] | |
| Facial Centroid Size | Increased | Gordon LA et al., 2021 [29] | |
| Mandibular Centroid Size | Increased | Gordon LA et al., 2021 [29] | |
| Vertical Skeletal Dimension (PP/GoMe) | Increased | Karaman A et al., 2021 [30] | |
| Jaws Projection and Prognathism Parameters | Maxillary Projection (SNA, NPerp-A) | Increased | Sadeghianrizi A et al., 2005 [25]; Danze A et al., 2021 [28]; Karaman A et al., 2021 [30]; Hancock S et al., 2024 [32]; Verdecchia A et al., 2024 [33] |
| Mandibular Projection (SNB, NPerp-Pg) | Increased | Sadeghianrizi A et al., 2005 [25]; Danze A et al., 2021 [28]; Gordon LA et al., 2021 [29]; Karaman A et al., 2021 [30]; Hancock S et al., 2024 [32]; Verdecchia A et al., 2024 [33] | |
| Pogonion Projection (SNPg) | Increased | Sadeghianrizi A et al., 2005 [25]; Danze A et al., 2021 [28]; Hancock S et al., 2024 [32] | |
| Maxillary Prognathism (S-Na-Ss) | Increased | Ohrn K et al., 2002 [24]; Gordon LA et al., 2021 [29] | |
| Mandibular Prognathism (S-Na-Sm) | Increased | Ohrn K et al., 2002 [24] | |
| Mandibular Alveolar Prognathism (ML/CL) | Increased | Sadeghianrizi A et al., 2005 [25] | |
| Facial Soft Tissue Thickness | Nasion (N-N’) | Increased | Buyuk SK et al., 2019 [27]; Karaman A et al., 2021 [30] |
| Glabella (G-G’) | Increased | Buyuk SK et al., 2019 [27]; Karaman A et al., 2021 [30] | |
| Pogonion (Pg-Pg’) | Increased | Buyuk SK et al., 2019 [27]; Karaman A et al., 2021 [30] | |
| Gnathion (Gn-Gn’) | Increased | Buyuk SK et al., 2019 [27]; Karaman A et al., 2021 [30] | |
| Rhinion (Rhi-Rhi’) | Increased | Karaman A et al., 2021 [30] | |
| Subnasale (ANS-Sn) | Increased | Karaman A et al., 2021 [30] | |
| Labiale Superius (Ls) | Increased | Karaman A et al., 2021 [30] | |
| Stomion (Sto) | Increased | Karaman A et al., 2021 [30] | |
| Labiale Inferius (Li) | Increased | Karaman A et al., 2021 [30] | |
| Labiomentale (B-B’) | Increased | Karaman A et al., 2021 [30] | |
| Chin Prominence | Increased | Gordon LA et al., 2021 [29]; Vora SR et al., 2022 [31] | |
| Facial Soft Tissue Profile (Convexity) | More straight profiles | Sadeghianrizi A et al., 2005 [25] | |
| Skeletal Divergence | Mandibular Growth Direction (Ar-Go-Gn) | Increased | Vora SR et al., 2022 [31] |
| Cranial Base Rotation (N-S-Ba) | More clockwise rotation | Vora SR et al., 2022 [31] | |
| Intermaxillary Plane Angle (NL/ML) | Decreased | Giuca MR et al., 2013 [26] | |
| Jaw Angle (RL/ML) | Increased | Ohrn K et al., 2002 [24] | |
| Maxillary Plane Angle (NL/SN) | Decreased | Sadeghianrizi A et al., 2005 [25] | |
| Dental Positions | Upper Incisor Inclination (U1/NL) | Increased | Sadeghianrizi A et al., 2005 [25] |
| Lower Incisor Inclination (L1/ML) | Increased | Ohrn K et al., 2002 [24] | |
| Airway Analysis | Nasopharyngeal Airway (Pm-Ad2) | Increased | Ohrn K et al., 2002 [24] |
| Author/Year | Selection | Comparability | Outcome | Quality Score (Max 9) |
|---|---|---|---|---|
| Öhrn K et al., 2002 [24] | 3 | 1 | 2 | 6 |
| Sadeghianrizi A et al., 2005 [25] | 3 | 2 | 2 | 7 |
| Giuca M.R. et al., 2013 [26] | 3 | 1 | 2 | 6 |
| Buyuk S.K. et al., 2018 [27] | 3 | 1 | 2 | 6 |
| Danze A. et al., 2020 [28] | 4 | 2 | 2 | 8 |
| Gordon L.A. et al., 2021 [29] | 4 | 2 | 2 | 8 |
| Karaman A. et al., 2021 [30] | 3 | 1 | 3 | 7 |
| Vora S.R. et al., 2021 [31] | 4 | 2 | 2 | 8 |
| Hancock S. et al., 2024 [32] | 4 | 2 | 2 | 8 |
| Verdecchia A. et al., 2024 [33] | 4 | 1 | 2 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdecchia, A.; Suárez-Fernández, C.; Menéndez Diaz, I.; Sanz, V.G.; Spinas, E.; Cobo, T. Obesity and Overweight Conditions in Children and Adolescents (6–18 Years) and Their Impact on Craniofacial Morphology: A Systematic Review. Children 2025, 12, 377. https://doi.org/10.3390/children12030377
Verdecchia A, Suárez-Fernández C, Menéndez Diaz I, Sanz VG, Spinas E, Cobo T. Obesity and Overweight Conditions in Children and Adolescents (6–18 Years) and Their Impact on Craniofacial Morphology: A Systematic Review. Children. 2025; 12(3):377. https://doi.org/10.3390/children12030377
Chicago/Turabian StyleVerdecchia, Alessio, Carlota Suárez-Fernández, Ivan Menéndez Diaz, Veronica García Sanz, Enrico Spinas, and Teresa Cobo. 2025. "Obesity and Overweight Conditions in Children and Adolescents (6–18 Years) and Their Impact on Craniofacial Morphology: A Systematic Review" Children 12, no. 3: 377. https://doi.org/10.3390/children12030377
APA StyleVerdecchia, A., Suárez-Fernández, C., Menéndez Diaz, I., Sanz, V. G., Spinas, E., & Cobo, T. (2025). Obesity and Overweight Conditions in Children and Adolescents (6–18 Years) and Their Impact on Craniofacial Morphology: A Systematic Review. Children, 12(3), 377. https://doi.org/10.3390/children12030377











