Abstract
Background/Objectives: Sleep-disordered breathing (SDB) is a primary concern in children’s health. Research suggests that repeated oxygen drops during sleep—common in SDB—may harm the brainstem’s breathing control centres. This damage likely occurs through oxidative stress, inflammation, and cell death, which weaken the brain’s ability to regulate breathing. Over time, these effects could lead to functional changes (e.g., disrupted chemical signalling) and physical damage in critical brain regions, creating a cycle of unstable breathing. However, much of this evidence comes from animal or lab studies, leaving gaps in our understanding of how these mechanisms work in humans. This review synthesises existing research on how breathing disruptions during sleep—particularly episodes of intermittent hypoxia—affect the brain’s ability to control respiration in children and adolescents. Methods: We analysed studies from medical databases PubMed, Scopus, and Web of Science, focusing on how SDB (obstructive or central sleep apnoea) impacts the brain’s respiratory centres in young populations. Animal studies and research involving children on mechanical ventilation were excluded to focus on natural sleep patterns. Results: After removing duplicates, 54 studies remained. Additionally, 43 record were excluded for various reasons. Ultimately, 11 articles were selected for the final analysis, including three that focused on genetic conditions, such as Down syndrome, Prader–Willi syndrome, and Pierre Robin sequence. The findings suggest that repeated oxygen dips during sleep may harm the brainstem’s respiratory control areas, especially during critical developmental stages. This damage could lead to long-term issues, such as unstable breathing, cardiovascular strain, or neurological problems. However, most studies only captured the immediate effects of low oxygen, leaving uncertainty about permanent harm due to a lack of long-term follow-up. Conclusions: Repeated oxygen deprivation during sleep appears to damage the brainstem and disrupt breathing regulation. However, small study sizes and short observation periods limit the strength of these conclusions. Future research should use advanced imaging tools to clarify long-term risks, develop effective treatments, and track children over extended periods. More significantly, longer-term studies are urgently needed to guide clinical care for vulnerable populations.
1. Introduction
Sleep-disordered breathing (SDB), including central sleep apnoea (CSA) and obstructive sleep apnoea (OSA), is a significant health issue in children and teens. It disrupts the balance between the nervous and respiratory systems, potentially hindering healthy development [1]. In OSA, repeated breathing pauses occur during sleep due to blocked airways or faulty brain signals controlling respiration [2].
Low oxygen levels can harm the brainstem’s respiratory centres, especially in developing children or those with genetic risks. This damage includes imbalances in brain chemicals, malfunctioning oxygen sensors, and structural changes like a buildup of star-like brain cells (astrogliosis) [3,4]. Studies also show abnormal brain development in kids with OSA, affecting areas critical for breathing [5].
The brainstem contains specialised cells that act as oxygen sensors, activating breathing when oxygen levels fall [6]. During abrupt oxygen shortages, the body responds in two distinct phases: an initial surge in breathing effort, followed by a rapid decline as the brainstem’s regulatory circuits become overwhelmed. This pattern mirrors a system pushed to its limits—briefly compensating before faltering under strain [7,8,9]. Over time, repeated oxygen deprivation can harm breathing, heart health [10], and brain function [9,11]. In adults with OSA, brain scans reveal damage to white matter—the “wiring” connecting areas like the limbic system, cerebellum, and cortex [12,13].
While SDB research has advanced, scientists still struggle to understand how the brainstem controls breathing in children. Specifically, two areas—the medulla and pons—play a critical role in maintaining breathing rhythms during sleep and adapting to chemical cues like oxygen and carbon dioxide levels in the blood. These regions act like a “breathing control centre”, fine-tuning respiration based on the body’s needs, but much about their function in kids remains unclear [14,15]. However, in kids with SDB, repeated breathing pauses may damage these areas, making them less responsive to oxygen changes or airway shifts [16,17]. For example, chronic low oxygen in OSA may blunt the brainstem’s oxygen sensors, worsening breathing instability [18].
Emerging research links SDB’s body-wide effects—like poor brain oxygenation and irregular heart function—to harmful changes in brainstem regions in paediatric patients [19,20]. These changes might explain why some children still struggle with SDB even after surgery to fix airway blockages [21,22]. This highlights the need to differentiate between physical airway issues and nervous system dysfunction when treating SDB [23,24].
Aim of the Study
This study reviews the existing literature on the link between SDB, intermittent hypoxaemia, and the function of the neurological centres that regulate respiration during sleep in children and adolescents.
2. Materials and Methods
We searched three major databases—MEDLINE (PubMed), Scopus, and Web of Science—for English-language studies published from their earliest records up to 20 January 2025. Custom search terms were tailored for each database, combining the following keywords:
Inclusion terms: sleep-disordered breathing, sleep apnea, obstructive sleep apnea, upper airway obstruction paired with neurorespiratory, central respiratory, medullary respiratory, pontine respiratory, and age-related terms (children, infants, adolescents).
Exclusion terms: case reports, animal studies, mechanical ventilation techniques, and studies focused on adults or elderly populations.
3. Results
A total of 92 articles were retrieved from the literature search, among which 38 were identified as duplicates. As a result, 43 studies were excluded (Figure 1). Ultimately, 11 articles were selected for the final analysis, including 3 that focused on syndromic children.
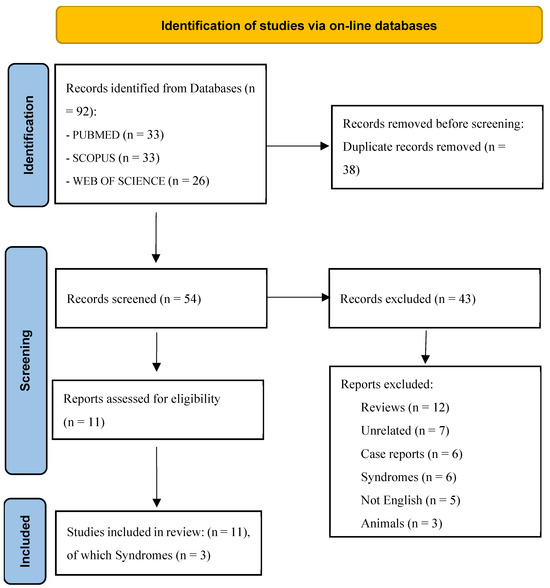
Figure 1.
The PRISMA flow diagram visually represents the study selection process and the number of studies included at each stage. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ (accessed on 22 February 2025).
Table 1 provides details on two studies investigating OSA. One notable study, a crossover trial involving 10 school-aged children [20], demonstrated that exposure to high altitude exacerbates the severity of SDB. Specifically, the study reported an increased AHI, worsening oxygenation (SpO2), increased sleep fragmentation, and heightened cardiovascular stress, as indicated by elevated heart rate [20]. The findings indicate that the low-oxygen conditions of high-altitude environments (hypobaric hypoxia) might interfere with the brain’s ability to regulate breathing—specifically disrupting communication between the medulla and pons, key brainstem regions that act as the body’s “breathing command centre.” Surprisingly, despite the strain on the body, the study found no apparent harm to children’s brain function or heart health [20]. The researchers speculated that this could be because the children were only exposed briefly or because their bodies adapted temporarily to protect them.

Table 1.
Summary of included studies and key findings.
While the study confirmed physical changes like reduced oxygen levels and fragmented sleep, it did not detect clear signs of long-term neurological or cardiovascular damage. This gap between observed stress and visible harm raises questions about whether children’s resilience or the study’s limitations (like short-term observation) masked subtle effects. This discrepancy suggests that children may possess transient adaptive mechanisms that mask potential subclinical neuronal damage [20]. Furthermore, the value of monitoring Tissue Oxygenation Index (TOI) and pulse transit time (PTT) in children [19] supports using portable, multimodal assessment tools to evaluate SDB severity and central nervous system (CNS) involvement in real-world settings.
Another study examined a cohort of 60 children aged 3 to 12, revealing that central respiratory events during non-REM (NREM) sleep led to reductions in cerebral oxygenation like those observed in obstructive events [19]. These episodes coincided with changes in peripheral blood pressure and heart rate, suggesting that central apnoea might lead to brief periods of cerebral hypoxia [19]. This observation supports previous findings showing that intermittent hypoxia, common in SDB, can harm neurological development and cardiovascular health, even without obvious immediate symptoms. PTT, a non-invasive measure of autonomic activity, significantly drops during breathing disruptions, reflecting increased sympathetic nervous system activity [19]. Similarly, the TOI falls during apnoea events, showing reduced oxygen supply to tissues and underscoring how respiratory regulation affects overall blood flow [19]. These physiological markers provide insight into how central respiratory control dysfunction can trigger a cascade of autonomic and cardiovascular responses mediated by brainstem nuclei and higher cortical networks. Finally, the finding that central and obstructive respiratory events similarly reduce cerebral oxygenation [19] underscores the need for a shift in perspective regarding SDB management.
Table 2 provides an overview of studies examining the effects of predominantly central SDB on sleep physiology in children, comprising three studies focusing on infants and three on older children, all within specific clinical contexts.

Table 2.
Summary of studies on central sleep-disordered breathing and sleep physiology in children.
A histopathological investigation of the medulla oblongata in newborns showed notable changes in dendritic spine density within respiratory control centres before and after birth [25]. These results emphasise the importance of neuronal maturation in establishing robust respiratory regulation, highlighting how early susceptibility to SDB may stem from the developmental progression of these critical neural networks [25].
An experimental approach was proposed to counteract central respiratory insufficiency in neonates, using a combination of sensory stimuli, including light exposure and the controlled modulation of O2 and CO2 levels, to stabilise breathing during sleep [26]. While promising, this technique requires further clinical studies to assess its efficacy in preventing hypoxaemia in high-risk newborns [26].
A higher incidence of CA was observed in neonates classified as small for gestational age (SGA), attributing this phenomenon to neurological alterations associated with intrauterine growth restriction (IUGR) [27]. These findings highlight how prenatal factors influence respiratory stability after birth and the likelihood of developing SDB [27]. Progress in diagnostic tools has been driven by Fukumizu (2004) and Foo et al. (2005, 2008), who confirmed that PTT reliably detects central breathing disruptions in children non-invasively [28,29,30]. Their work marks significant progress in bridging research and clinical practice, offering practical methods to monitor how breathing and brain activity interact during sleep without disturbing natural sleep patterns.
Table 3 shows the effects of SDB, particularly sleep apnoea, on central respiratory control and its interactions with the nervous and cardiovascular systems, with special attention to the paediatric implications of the studies summarised in Table 2.

Table 3.
Literature review on sleep-disordered breathing and its impact on central respiratory control.
The crucial role of dendritic spine development in the respiratory centres of the medulla oblongata has been highlighted, linking neuronal maturation to respiratory control in neonates. This study provides valuable insights into the mechanisms underlying primary apnoea in preterm infants and sleep apnoea associated with sudden infant death syndrome (SIDS), reinforcing the importance of neurodevelopmental monitoring in high-risk neonates [25].
An innovative method to prevent low oxygen levels in newborns was proposed, involving sensory interventions like light exposure and adjustments to breathing gases, paired with the precise tracking of oxygen and carbon dioxide levels. Though promising, more clinical trials are needed to verify its usefulness in newborn care [26].
Infants born SGA showed more frequent breathing pauses, likely due to immature respiratory centres associated with growth restriction during pregnancy. This research stresses the importance of specialised respiratory monitoring for these high-risk infants [27].
The connection between central breathing irregularities (like pauses and sighs) and the brain’s control of respiration has been explored, revealing that breathing regulation shifts with age and sleep phases. These insights reinforce the idea that respiratory control evolves dynamically as the nervous system matures [28].
Finally, studies by Foo et al. validated the use of plethysmography and PTT as non-invasive tools for studying CSA. Their work has helped clarify the interactions between respiratory variability, oxygenation, and arterial stiffness, contributing to improved research methodologies for SDB in paediatric populations [29,30].
The evidence in Table 4 shows how SDB affects the brain’s control of breathing in children with genetic syndromes, exploring how genes, body structures, and bodily functions contribute to these challenges.

Table 4.
Impact of sleep-disordered breathing on central respiratory control in children with genetic syndromes.
In children with Prader–Willi syndrome, sleep studies by Schlüter et al. (1997) found that the main issue lies in how the brain regulates breathing, likely due to faulty brain signals and physical airway blockages [31]. Similarly, research comparing children with Down syndrome to others revealed that CSA is more common in this group, possibly tied to impaired brainstem function [32]. These findings illustrate how genetic risks and physical problems interact to worsen SDB.
A study on children with Pierre Robin sequence [33] observed uncoordinated breathing muscle activity during sleep, even when the brainstem appeared normal. This suggests that respiratory issues in some syndromes may stem from glitches in how specific brain networks operate rather than visible structural damage [33].
These patterns mirror what doctors see in children with brainstem injuries—whether present at birth or occurring later—where breathing pauses and shallow breathing are common. Such insights stress the importance of specialised care and close breathing monitoring in these vulnerable groups.
4. Discussion
Existing evidence suggests that even brief bouts of hypoxia—such as those encountered at high altitudes—can significantly worsen SDB indices (for instance, the AHI), increase sleep fragmentation, and place a heavier strain on the cardiovascular system in paediatric patients [20]. However, in adults, OSA becomes CSA as chemoreceptor sensitivity increases, and central respiratory motor output instability occurs during a sojourn at high altitudes [34]. These findings point to the possibility that hypobaric hypoxia may disrupt respiratory control mechanisms at the level of the brainstem.
Studies in children using advanced measurement techniques (including TOI and PTT) also indicate that both CSA and OSA can lower cerebral oxygenation and activate the sympathetic nervous system, demonstrating that central events—often considered less severe—can trigger notable autonomic and cardiovascular responses [19,29,30].
Furthermore, breathing regulation in infancy and childhood involves substantial maturational and structural components [26,27,28,29,30]. Histopathological data highlight the critical role of dendritic development in the bulbar centres. At the same time, prenatal factors (such as IUGR, leading to SGA status) appear to increase the likelihood of respiratory irregularities in paediatric age, including premature babies [25,27]. Finally, children with specific genetic syndromes—such as Prader–Willi [31], Down [35], or Pierre Robin sequence [33]—have been shown to experience dysfunctional central respiratory control stemming from both structural and functional abnormalities.
However, the heterogeneity of the included studies, such as small sample sizes [20,29,31,32] and varying methodologies (PSG, transcutaneous pO2 monitoring, plethysmography, and PTT analysis), limits the generalizability of the results. Additionally, the scarcity of neuroimaging data in most studies precludes a direct correlation between physiological markers and brainstem alterations.
The exacerbation of SDB at high altitudes [20] underscores the sensitivity of paediatric respiratory centres to hypobaric hypoxia. Observed increases in the AHI, sleep fragmentation, and cardiovascular stress in this context suggest that hypoxia compromises the function of medullary chemoreceptors in experimental data [36,37] and the integration of autonomic feedback at the pons in models [38] and mammals [39], both crucial for maintaining rhythmic breathing [40]. The absence of overt neurocognitive deficits in this cohort could reflect transient adaptive plasticity, such as increased serotonergic signalling in the brainstem in the mouse model [41] and temporarily stabilising respiratory drive in models [18]. However, prolonged or recurrent exposure to hypoxia could overwhelm compensatory capabilities in adults [42] and using animal models [43], in line with evidence suggesting that intermittent hypoxia induces oxidative stress in brainstem neurons [44], impairing synaptic plasticity over time [45].
While OSA is often seen as a problem of blocked airways, the issue goes beyond anatomy. The nervous system’s ability to maintain steady airflow is equally essential [46]. For instance, the brainstem’s emergency wake-up reaction—triggered by low oxygen—interrupts healthy sleep patterns [47], creating a harmful cycle of oxygen drops and abrupt awakenings [2,39]. Over time, these repeated disruptions may gradually alter brain structure, shrinking grey matter in critical regions like the prefrontal cortex, hippocampus, and cerebellum, affecting thinking, behaviour, and learning [13,48].
During non-REM sleep, CSA reduces brain oxygen levels like OSA [49], questioning the traditional focus on fixing airway mechanics alone in children [19]. This hints that flaws in brain networks governing breathing rhythms and arousal—specifically in the pons and medulla—might explain OSA and CSA [50,51]. Post-mortem studies support this idea, showing that brainstem damage directly disrupts breathing control [52], while brain scans reveal weaker connections between the pons and prefrontal cortex in people with CSA [53].
The dynamic development of medullary respiratory nuclei indicates a critical phase in childhood where the formation of dendritic spines is essential for establishing stable respiratory control [25]. Disruptions in this process, such as those caused by IUGR [27], can predispose infants to CSA, highlighting the long-term impact of prenatal insults on respiratory stability [54]. Similarly, the efficacy of sensory stimulation in reducing hypoxemia [26] suggests that immature brainstem circuits in infants can be externally modulated [55,56]. However, the clinical translation of these techniques requires further validation.
In children with Prader–Willi syndrome, two mechanisms underlie SDB [31]: mechanical obstruction of the airways and reduced CO2 sensitivity in medullary chemoreceptors. This duality mirrors observations in the Pierre Robin sequence [33], where the functional disorganisation of pontomedullary networks, rather than overt structural anomalies, has been identified as the cause of respiratory dysfunction. These studies demonstrate that SDB arises from a complex interaction between brainstem dysfunctions and anatomical vulnerabilities in genetic syndromes [57]. In adult patients (aged 47.8 ± 12.3 years) with genetic abnormalities, the instabilities in the ventilatory control system can generate the temporary arrest of the respiratory drive (CSA), which is also a consequence of the obstruction of the airway [17]
In paediatric age, it has been observed that PTT and TOI [28,29,30] reflect the integration between autonomic responses and CNS involvement in SDB. A reduction in PTT during apnoea indicates sympathetic activation [58], while changes in TOI highlight alterations in oxygen distribution, both processes mediated by brainstem nuclei. The combined use of these tools and home PSG in children [20] allows for assessing SDB severity and central involvement in real-world contexts, bridging the gap between laboratory research and clinical practice.
Current evidence supports the hypothesis that intermittent hypoxia in children with OSA may contribute to damage to the brainstem respiratory centre through oxidative stress [59], inflammation [60,61], and apoptosis, leading to compromised ventilatory control. Chronic intermittent hypoxia promotes a pro-oxidative state that destabilises rhythmogenesis in the preBötzinger complex, leading to sporadic failures in transmission to the XII nerve. These effects could perpetuate apnoea and respiratory instability [62]. However, causality is often inferred from animal models [63,64,65], where the roles of oxidative stress and apoptosis-related neural injury are confirmed [66]. Therefore, OSA worsens neurological outcomes in mice and increases neuronal death by enhancing neural apoptosis and neuroinflammation [67].
This comprehensive body of evidence—spanning pathophysiology, neurophysiological parameters, and clinical observations—clearly shows how intermittent hypoxia and SDB can disrupt the normal functioning of the brainstem’s respiratory centres (medulla oblongata and pons). Children can be especially vulnerable, with these alterations often manifesting during sleep.
Repeated episodes of low oxygen (or intermittent hypoxia) can directly damage the brainstem regions responsible for controlling breathing and lead to broader health issues that weaken the body’s ability to regulate respiration. Over time, these disturbances may disrupt sleep and waking hours, making it harder to maintain steady breathing patterns.
This study faces some important limitations, starting with the limited number of available studies and the differences in research protocols, such as variations in diagnostic criteria, sample sizes, and population characteristics. These factors make it difficult to draw definitive conclusions. Another challenge is that most existing research is based on short-term observations and lacks advanced neuroimaging techniques. This would help provide a clearer understanding of how SDB affects central respiratory control. Many studies also rely on indirect physiological markers like TOI or PTT. While these tools are valuable and non-invasive, they may not be enough to capture the complexity of brainstem function in children fully.
A standardised approach is essential to ensure that studies produce reliable and comparable results, ultimately driving progress in this field. Another key step is the use of advanced neuroimaging techniques, such as MRI and functional MRI, which can provide deeper insights into the structural and functional changes affecting the brainstem and related areas. Expanding research through large-scale, long-term, multicentre studies would help paint a more comprehensive picture of both the immediate and long-term neurological effects of sleep-disordered breathing (SDB).
Collecting detailed data on factors that influence respiratory control—such as coexisting conditions or genetic syndromes—would also be crucial in helping researchers gain a more accurate understanding of the true impact of these disorders.
When SDB is caused by anatomical obstructions, such as adenotonsillar hypertrophy or craniofacial anomalies, targeted interventions can significantly improve nighttime breathing. However, these treatments may not fully address underlying dysfunctions in central respiratory control. In cases where neurological impairment plays a dominant role, as seen in genetic syndromes or brainstem abnormalities, focusing solely on airway management may not be sufficient. Moreover, it is important to recognise that repeated episodes of intermittent hypoxia caused by SDB could further worsen pre-existing dysfunctions in central respiratory control, making it even harder for the body to regulate breathing effectively.
While numerous studies focus on various interventions in treating OSA (anti-inflammatory drugs, surgery for adenotonsillar hypertrophy, or CPAP), little evidence supports therapies that directly modulate central respiratory drive in children. Some isolated neonate reports have evaluated sensory stimulation approaches—combining specific light exposure with controlled O2/CO2 adjustments [68,69]. However, these methods remain experimental [26]. Likewise, some paediatric studies have investigated the use of respiratory stimulants, such as caffeine, for apnoea of prematurity [70]. However, these approaches have not been considered for older children with OSA or central apnoea.
In children with genetic conditions involving abnormal brainstem function (e.g., Prader–Willi syndrome or Down syndrome), addressing structural airway issues may partially alleviate SDB [71,72] but often does not fully correct the underlying central component [31,32]. Consequently, while non-invasive ventilation (e.g., BiPAP) may compensate for the inadequate respiratory central drive [73], evidence supporting solid neurologic therapy (e.g., pharmaceuticals or neuromodulation devices) in the paediatric population remains insufficient. Large-scale, long-term studies utilising advanced neuroimaging are needed to determine whether future therapeutic approaches for central respiratory control (e.g., brainstem-targeted drugs, sensory stimulation, or neural pacing devices) could be effective and safe for children [20,26].
Given the combined anatomical and neurological factors involved in SDB, a multidisciplinary approach is essential, involving specialists in paediatrics, otolaryngology, neurology, and sleep medicine. Tailoring treatment to the patient’s underlying cause will likely yield better clinical outcomes than a standardised approach.
Identifying and treating the root causes of intermittent hypoxia is essential to safeguard the development and function of these critical breathing control areas in children.
5. Conclusions
Research shows that even short periods of low oxygen—like those occurring at high altitudes—can worsen markers of SDB, disrupt sleep quality, and put extra stress on the heart and blood vessels. These effects may weaken the brainstem’s ability to regulate breathing. Both types of breathing pauses (central and obstructive) reduce oxygen flow to the brain and spark overactive “fight-or-flight” nervous system responses, with children being particularly vulnerable. Given the limitations of existing studies in children, future research—using tools like advanced brain imaging—is crucial to fully grasp these issues’ lasting impacts and create better prevention and treatment plans.
Author Contributions
Conceptualisation, M.Z. and A.P.; methodology, L.N.; software, M.Z.; validation, G.F., E.R. and S.G.; formal analysis, M.Z.; investigation, M.Z.; resources G.P.; data curation, A.P. and G.P.; writing—original draft preparation, M.Z.; writing—review and editing, G.F., E.R. and S.G.; visualisation, G.P. and L.N.; supervision, G.P. and A.P.; project administration, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are derived from public domain resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nosetti, L.; Zaffanello, M.; Simoncini, D.; Dellea, G.; Vitali, M.; Amoudi, H.; Agosti, M. Prioritising Polysomnography in Children with Suspected Obstructive Sleep Apnoea: Key Roles of Symptom Onset and Sleep Questionnaire Scores. Children 2024, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, A.C.; Lafontaine, A.L.; Kimoff, R.J.; Kaminska, M. Obstructive Sleep Apnea in Neurodegenerative Disorders: Current Evidence in Support of Benefit from Sleep Apnea Treatment. J. Clin. Med. 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hashimoto, T.; Iwata, H.; Takahashi, K.; Fukumizu, M.; Sasaki, M.; Hanaoka, S.; Sugai, K. Apneustic breathing in children with brainstem damage due to hypoxic-ischemic encephalopathy. Dev. Med. Child. Neurol. 1999, 41, 560–567. [Google Scholar] [CrossRef]
- Moss, I.R. Canadian Association of Neuroscience Review: Respiratory control and behavior in humans: Lessons from imaging and experiments of nature. Can. J. Neurol. Sci. 2005, 32, 287–297. [Google Scholar] [CrossRef]
- Philby, M.F.; Macey, P.M.; Ma, R.A.; Kumar, R.; Gozal, D.; Kheirandish-Gozal, L. Reduced Regional Grey Matter Volumes in Pediatric Obstructive Sleep Apnea. Sci. Rep. 2017, 7, 44566. [Google Scholar] [CrossRef]
- Gourine, A.V.; Funk, G.D. On the existence of a central respiratory oxygen sensor. J. Appl. Physiol. (1985) 2017, 123, 1344–1349. [Google Scholar] [CrossRef]
- Rehan, V.; Haider, A.Z.; Alvaro, R.E.; Nowaczyk, B.; Cates, D.B.; Kwiatkowski, K.; Rigatto, H. The biphasic ventilatory response to hypoxia in preterm infants is not due to a decrease in metabolism. Pediatr. Pulmonol. 1996, 22, 287–294. [Google Scholar] [CrossRef]
- Freislich, Z.; Stoecklin, B.; Hemy, N.; Pillow, J.J.; Hall, G.L.; Wilson, A.C.; Simpson, S.J. The ventilatory response to hypoxia is blunted in some preterm infants during the second year of life. Front. Pediatr. 2022, 10, 974643. [Google Scholar] [CrossRef]
- Joubert, F.; Loiseau, C.; Perrin-Terrin, A.S.; Cayetanot, F.; Frugière, A.; Voituron, N.; Bodineau, L. Key Brainstem Structures Activated during Hypoxic Exposure in One-day-old Mice Highlight Characteristics for Modeling Breathing Network in Premature Infants. Front. Physiol. 2016, 7, 609. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ersu, R.H.; Nosetti, L.; Beretta, G.; Agosti, M.; Piacentini, G. Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review. Children 2024, 11, 208. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J. Clin. Med. 2023, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, S.K.; Kim, S.; Kim, R.E.Y.; Nam, H.R.; Siddiquee, A.T.; Thomas, R.J.; Hwang, I.; Yoon, J.E.; Yun, C.H.; et al. Association of Obstructive Sleep Apnea with White Matter Integrity and Cognitive Performance Over a 4-Year Period in Middle to Late Adulthood. JAMA Netw. Open 2022, 5, e2222999. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Kumar, R.; Woo, M.A.; Valladares, E.M.; Yan-Go, F.L.; Harper, R.M. Brain structural changes in obstructive sleep apnea. Sleep 2008, 31, 967–977. [Google Scholar] [PubMed]
- Wang, J.; Christensen, D.; Coombes, S.A.; Wang, Z. Cognitive and brain morphological deviations in middle-to-old aged autistic adults: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 163, 105782. [Google Scholar] [CrossRef]
- Fukushi, I.; Yokota, S.; Okada, Y. The role of the hypothalamus in modulation of respiration. Respir. Physiol. Neurobiol. 2019, 265, 172–179. [Google Scholar] [CrossRef]
- Choudhary, S.S.; Choudhary, S.R. Sleep effects on breathing and respiratory diseases. Lung India 2009, 26, 117–122. [Google Scholar] [CrossRef]
- Si, L.; Zhang, J.; Wang, Y.; Cao, J.; Chen, B.Y.; Guo, H.J. Obstructive sleep apnea and respiratory center regulation abnormality. Sleep. Breath. 2021, 25, 563–570. [Google Scholar] [CrossRef]
- Duffin, J.; Mahamed, S. Adaptation in the respiratory control system. Can. J. Physiol. Pharmacol. 2003, 81, 765–773. [Google Scholar] [CrossRef]
- Tamanyan, K.; Weichard, A.; Biggs, S.N.; Davey, M.J.; Nixon, G.M.; Walter, L.M.; Horne, R.S.C. The impact of central and obstructive respiratory events on cerebral oxygenation in children with sleep disordered breathing. Sleep 2019, 42, zsz044. [Google Scholar] [CrossRef]
- Hughes, B.H.; Brinton, J.T.; Ingram, D.G.; Halbower, A.C. The Impact of Altitude on Sleep-Disordered Breathing in Children Dwelling at High Altitude: A Crossover Study. Sleep 2017, 40, zsx120. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Vicini, C.; Cammaroto, G. Treatment of sleep disordered breathing relapse after surgery. Acta Otorhinolaryngol. Ital. 2023, 43, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Kumar, R.; Ogren, J.A.; Macey, P.M. Sleep-disordered breathing: Effects on brain structure and function. Respir. Physiol. Neurobiol. 2013, 188, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Ramar, K. Neurologic aspects of sleep apnea: Is obstructive sleep apnea a neurologic disorder? Semin. Neurol. 2009, 29, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Becker, L.E. Prenatal and postnatal maturation of medullary ‘respiratory centers’. Brain Res. 1986, 391, 173–177. [Google Scholar] [CrossRef]
- Schlaefke, M.E.; Schaefer, T.; Kronberg, H.; Ullrich, G.J.; Hopmeier, J. Transcutaneous monitoring as trigger for therapy of hypoxemia during sleep. Adv. Exp. Med. Biol. 1987, 220, 95–100. [Google Scholar] [CrossRef]
- Curzi-Dascalova, L.; Peirano, P.; Christova, E. Respiratory characteristics during sleep in healthy small-for- gestational age newborns. Pediatrics 1996, 97, 554–559. [Google Scholar] [CrossRef]
- Fukumizu, M.; Kohyama, J. Central respiratory pauses, sighs, and gross body movements during sleep in children. Physiol. Behav. 2004, 82, 721–726. [Google Scholar] [CrossRef]
- Foo, J.Y.; Parsley, C.L.; Wilson, S.J.; Williams, G.R.; Harris, M.; Cooper, D.M. Detection of central respiratory events using pulse transit time in infants. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 2005, 2579–2582. [Google Scholar] [CrossRef]
- Foo, J.Y.; Wilson, S.J.; Lim, C.S. Use of the pulse transit time trend to relate tidal breathing and central respiratory events. Proc. Inst. Mech. Eng. H 2008, 222, 1005–1011. [Google Scholar] [CrossRef]
- Schlüter, B.; Buschatz, D.; Trowitzsch, E.; Aksu, F.; Andler, W. Respiratory control in children with Prader-Willi syndrome. Eur. J. Pediatr. 1997, 156, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Curzi-Dascalova, L.; Del Gracco, S.; Elia, M.; Musumeci, S.A.; Stefanini, M.C. Respiratory patterns during sleep in Down’s syndrome:importance of central apnoeas. J. Sleep Res. 1997, 6, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Renault, F.; Flores-Guevara, R.; Soupre, V.; Vazquez, M.P.; Baudon, J.J. Neurophysiological brainstem investigations in isolated Pierre Robin sequence. Early Hum. Dev. 2000, 58, 141–152. [Google Scholar] [CrossRef]
- Latshang, T.D.; Nussbaumer-Ochsner, Y.; Henn, R.M.; Ulrich, S.; Lo Cascio, C.M.; Ledergerber, B.; Kohler, M.; Bloch, K.E. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: A randomised controlled trial. JAMA 2012, 308, 2390–2398. [Google Scholar] [CrossRef]
- Ferri, R.; Curzi-Dascalova, L.; Del Gracco, S.; Elia, M.; Musumeci, S.A.; Pettinato, S. Heart rate variability and apnea during sleep in Down’s syndrome. J. Sleep Res. 1998, 7, 282–287. [Google Scholar] [CrossRef]
- Gourine, A.V. On the peripheral and central chemoreception and control of breathing: An emerging role of ATP. J. Physiol. 2005, 568, 715–724. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, D.; Wu, Y.; Xu, Z. Impact of Chronic Intermittent Hypoxia on Cognitive Function and Hippocampal Neurons in Mice: A Study of Inflammatory and Oxidative Stress Pathways. Nat. Sci. Sleep 2024, 16, 2029–2043. [Google Scholar] [CrossRef]
- Molkov, Y.I.; Bacak, B.J.; Dick, T.E.; Rybak, I.A. Control of breathing by interacting pontine and pulmonary feedback loops. Front. Neural Circuits 2013, 7, 16. [Google Scholar] [CrossRef]
- Zoccal, D.B.; Vieira, B.N.; Mendes, L.R.; Evangelista, A.B.; Leirão, I.P. Hypoxia sensing in the body: An update on the peripheral and central mechanisms. Exp. Physiol. 2024, 109, 461–469. [Google Scholar] [CrossRef]
- Smith, D.F.; Vikani, A.R.; Benke, J.R.; Boss, E.F.; Ishman, S.L. Weight gain after adenotonsillectomy is more common in young children. Otolaryngol. Head Neck Surg. 2013, 148, 488–493. [Google Scholar] [CrossRef]
- Papesh, M.A.; Hurley, L.M. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hear. Res. 2016, 332, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.; Twomey, R.; Amann, M. Acute and chronic hypoxia: Implications for cerebral function and exercise tolerance. Fatigue 2014, 2, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, W.; Li, S.; Ding, Y.; Wang, Y.; Ji, X. Intermittent Hypoxia Conditioning: A Potential Multi-Organ Protective Therapeutic Strategy. Int. J. Med. Sci. 2023, 20, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, M.P.; Iturriaga, R. Contribution of Oxidative Stress and Inflammation to the Neurogenic Hypertension Induced by Intermittent Hypoxia. Front. Physiol. 2018, 9, 893. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Fabries, P.; Gomez-Merino, D.; Sauvet, F.; Malgoyre, A.; Koulmann, N.; Chennaoui, M. Sleep loss effects on physiological and cognitive responses to systemic environmental hypoxia. Front. Physiol. 2022, 13, 1046166. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Ji, L.; Su, T.; Cheng, C.; Han, F.; Cox, D.J.; Wang, E.; Chen, R. The complex interplay of hypoxia and sleep disturbance in gray matter structure alterations in obstructive sleep apnea patients. Front. Aging Neurosci. 2023, 15, 1090547. [Google Scholar] [CrossRef]
- Dempsey, J.A. Central sleep apnea: Misunderstood and mistreated! F1000Res 2019, 8, F1000-Faculty. [Google Scholar] [CrossRef]
- Nuding, S.C.; Segers, L.S.; Iceman, K.E.; O’Connor, R.; Dean, J.B.; Bolser, D.C.; Baekey, D.M.; Dick, T.E.; Shannon, R.; Morris, K.F.; et al. Functional connectivity in raphé-pontomedullary circuits supports active suppression of breathing during hypocapnic apnea. J. Neurophysiol. 2015, 114, 2162–2186. [Google Scholar] [CrossRef]
- Rybak, I.A.; Shevtsova, N.A.; Paton, J.F.; Dick, T.E.; St-John, W.M.; Mörschel, M.; Dutschmann, M. Modeling the ponto-medullary respiratory network. Respir. Physiol. Neurobiol. 2004, 143, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B. Watershed infarcts in the fetal and neonatal brainstem. An aetiology of central hypoventilation, dysphagia, Möibius syndrome and micrognathia. Eur. J. Paediatr. Neurol. 2004, 8, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Henderson, L.A.; Macey, K.E.; Alger, J.R.; Frysinger, R.C.; Woo, M.A.; Harper, R.K.; Yan-Go, F.L.; Harper, R.M. Brain morphology associated with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 166, 1382–1387. [Google Scholar] [CrossRef]
- Oliveira, B.; Flôr, D.E.L.F.; Rocha, G.; Rodrigues, M.; Ladeiras, R.; Guimarães, H. The impact of intrauterine growth restriction on respiratory outcomes. Minerva Pediatr. 2021, 73, 426–434. [Google Scholar] [CrossRef]
- Geva, R.; Feldman, R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: Implications for prenatal and perinatal risk. J. Child. Psychol. Psychiatry 2008, 49, 1031–1041. [Google Scholar] [CrossRef]
- Geva, R.; Sopher, K.; Kurtzman, L.; Galili, G.; Feldman, R.; Kuint, J. Neonatal brainstem dysfunction risks infant social engagement. Soc. Cogn. Affect. Neurosci. 2013, 8, 158–164. [Google Scholar] [CrossRef][Green Version]
- Oros, M.; Baranga, L.; Plaiasu, V.; Cozma, S.R.; Neagos, A.; Paduraru, L.; Necula, V.; Martu, C.; Dima-Cozma, L.C.; Gheorghe, D.C. Obstructing Sleep Apnea in Children with Genetic Disorders-A Special Need for Early Multidisciplinary Diagnosis and Treatment. J. Clin. Med. 2021, 10, 2156. [Google Scholar] [CrossRef]
- Bisogni, V.; Pengo, M.F.; Maiolino, G.; Rossi, G.P. The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea. J. Thorac. Dis. 2016, 8, 243–254. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Feng, J.; Cao, J.; Chen, B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: The potential roles played by microglia. Neuropsychiatr. Dis. Treat. 2013, 9, 1077–1086. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Zhan, G.; Fenik, P.; Pratico, D.; Veasey, S.C. Inducible nitric oxide synthase in long-term intermittent hypoxia: Hypersomnolence and brain injury. Am. J. Respir. Crit. Care Med. 2005, 171, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J., III; Zanella, S.; Dashevskiy, T.; Khan, S.A.; Khuu, M.A.; Prabhakar, N.R.; Ramirez, J.M. Chronic Intermittent Hypoxia Alters Local Respiratory Circuit Function at the Level of the preBötzinger Complex. Front. Neurosci. 2016, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- O’Nions, E.; Gould, J.; Christie, P.; Gillberg, C.; Viding, E.; Happé, F. Identifying features of ‘pathological demand avoidance’ using the Diagnostic Interview for Social and Communication Disorders (DISCO). Eur. Child. Adolesc. Psychiatry 2016, 25, 407–419. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Pae, E.K.; Chien, P.; Harper, R.M. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci. Lett. 2005, 375, 123–128. [Google Scholar] [CrossRef]
- Xie, H.; Yung, W.H. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: A role for brain-derived neurotrophic factor. Acta Pharmacol. Sin. 2012, 33, 5–10. [Google Scholar] [CrossRef]
- Fei, W.; Jiao, W.; Feng, X.; Chen, X.; Wang, Y. Intermittent hypoxia mimicking obstructive sleep apnea aggravates early brain injury following ICH via neuroinflammation and apoptosis. Mol. Med. Rep. 2021, 24, 824. [Google Scholar] [CrossRef]
- Shogan, M.G.; Schumann, L.L. The effect of environmental lighting on the oxygen saturation of preterm infants in the NICU. Neonatal Netw. 1993, 12, 7–13. [Google Scholar]
- Alvaro, R.E.; Weintraub, Z.; Kwiatkowski, K.; Cates, D.B.; Rigatto, H. A respiratory sensory reflex in response to CO2 inhibits breathing in preterm infants. J. Appl. Physiol. (1985) 1992, 73, 1558–1563. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef]
- Pavone, M.; Caldarelli, V.; Khirani, S.; Colella, M.; Ramirez, A.; Aubertin, G.; Crinò, A.; Brioude, F.; Gastaud, F.; Beydon, N.; et al. Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr. Pulmonol. 2015, 50, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Betavani, V.M.P.; Davey, M.J.; Nixon, G.M.; Walter, L.M.; Horne, R.S.C. Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome. Children 2022, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Amaddeo, A.; Frapin, A.; Fauroux, B. Long-term non-invasive ventilation in children. Lancet Respir. Med. 2016, 4, 999–1008. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).