Implementation of Parenteral Nutrition Formulations with Increased Calcium and Phosphate Concentrations and Its Impact on Metabolic Bone Disease in Preterm Infants: A Retrospective Single-Centre Study
Abstract
1. Introduction
Objectives
2. Materials and Methods
- No MBDP: normal density with white line at metaphyseal region
- Grade 1: loss of dense white line at metaphyses and thinning of cortex (Figure 1a)
- Grade 2: changes of grade 1 plus irregularity and fraying of metaphyses, with splaying and cupping (Figure 1b)
- Grade 3: bone changes seen in grade 1 and grade 2 in addition to fracture (Figure 1c)
Statistics
3. Results
3.1. Participants
3.2. Radiologic MBDP
3.3. Biomarkers at MBDP Screening
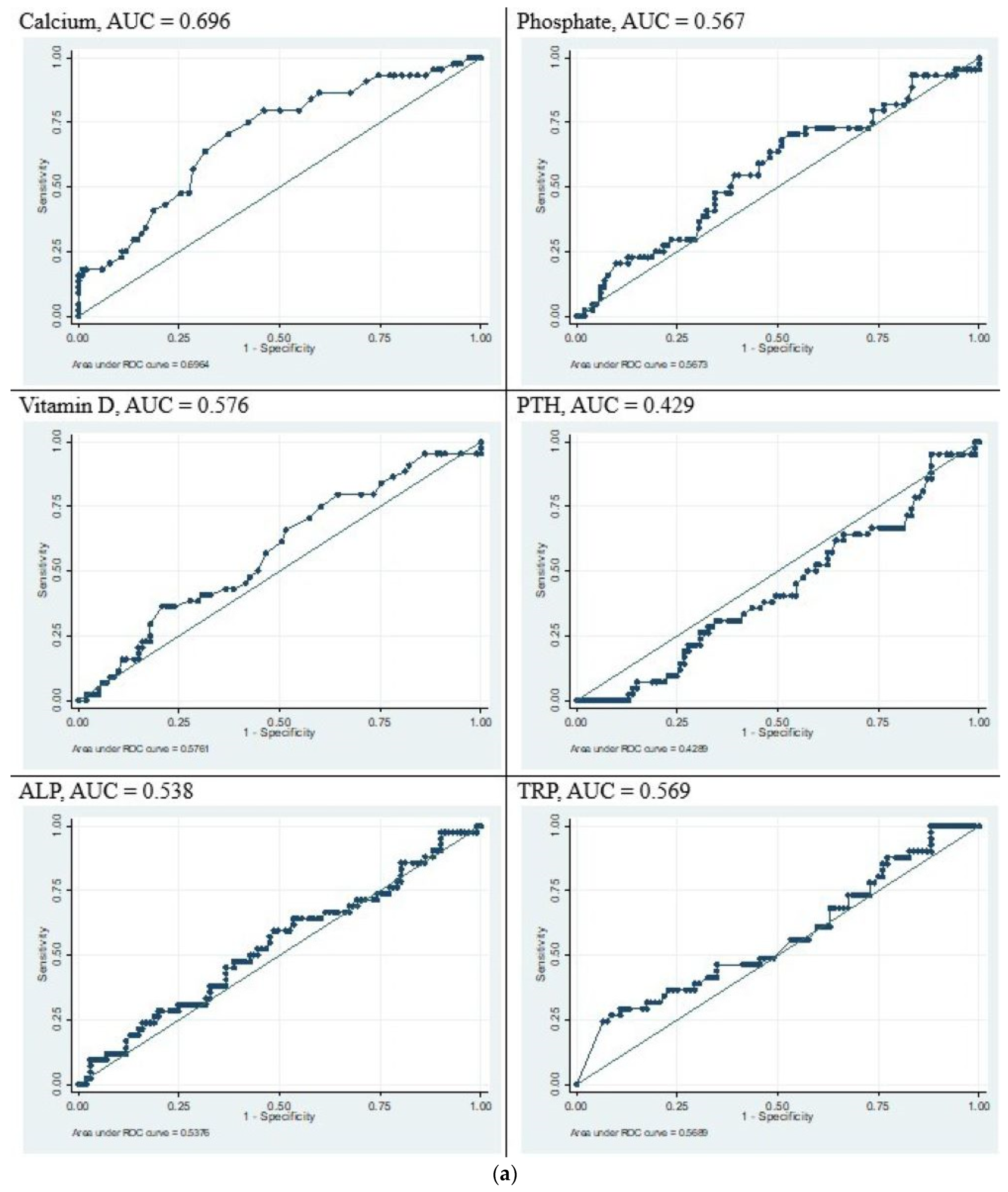
3.4. Sensitivity and Specificity Analysis
3.5. Biochemical Parameters When Receiving PN Formulation
4. Discussion
4.1. Limitations
4.2. Strengths
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rustico, S.E.; Calabria, A.C.; Garber, S.J. Metabolic bone disease of prematurity. J. Clin. Transl. Endocrinol. 2014, 1, 85–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chacham, S.; Pasi, R.; Chegondi, M.; Ahmad, N.; Mohanty, S.B. Metabolic Bone Disease in Premature Neonates: An Unmet Challenge. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.V.; Wagner, C.L. History, epidemiology and prevalence of neonatal bone mineral metabolic disorders. Semin. Fetal Neonatal Med. 2020, 25, 101069. [Google Scholar] [CrossRef] [PubMed]
- Backström, M.C.; Kuusela, A.-L.; Mäki, R. Metabolic Bone Disease of Prematurity. Ann. Med. 1996, 28, 275–282. [Google Scholar] [CrossRef]
- Done, S.L. Fetal and neonatal bone health: Update on bone growth and manifestations in health and disease. Pediatr. Radiol. 2012, 42 (Suppl. S1), 158–176. [Google Scholar] [CrossRef]
- Viswanathan, S.; Khasawneh, W.; McNelis, K.; Dykstra, C.; Amstadt, R.; Super, D.M.; Groh-Wargo, S.; Kumar, D. Metabolic bone disease: A continued challenge in extremely low birth weight infants. J. Parenter. Enter. Nutr. 2014, 38, 982–990. [Google Scholar] [CrossRef]
- Pohlandt, F. Bone mineral deficiency as the main factor of dolichocephalic head flattening in very-low-birth-weight infants. Pediatr. Res. 1994, 35, 701–703. [Google Scholar] [CrossRef]
- Berger, C.; Goltzman, D.; Langsetmo, L.; Joseph, L.; Jackson, S.; Kreiger, N.; Tenenhouse, A.; Davison, K.S.; Josse, R.G.; Prior, J.C.; et al. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J. Bone Miner. Res. 2010, 25, 1948–1957. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Cole, T.J.; Bishop, N.J.; Lucas, A. Neonatal factors predicting childhood height in preterm infants: Evidence for a persisting effect of early metabolic bone disease? J. Pediatr. 2000, 137, 668–673. [Google Scholar] [CrossRef]
- Wood, C.L.; Wood, A.M.; Harker, C.; Embleton, N.D. Bone mineral density and osteoporosis after preterm birth: The role of early life factors and nutrition. Int. J. Endocrinol. 2013, 2013, 902513. [Google Scholar] [CrossRef]
- Abrams, S.A. Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics 2013, 131, e1676–e1683. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ashraf, A.P.; Bowden, S.A.; Calabria, A.; Diaz-Thomas, A.; Krishnan, S.; Miller, J.L.; Robinson, M.-E.; DiMeglio, L.A. Invited Mini Review Metabolic Bone Disease of Prematurity: Overview and Practice Recommendations. Horm. Res. Paediatr. 2024, 1–11. [Google Scholar] [CrossRef]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. S2), S1–S4. [Google Scholar] [PubMed]
- Pereira-da-Silva, L.; Costa, A.; Pereira, L.; Filipe, A.; Virella, D.; Leal, E.; Moreira, A.; Rosa, M.; Mendes, L.; Serelha, M. Early High Calcium and Phosphorus Intake by Parenteral Nutrition Prevents Short-term Bone Strength Decline in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.M.; Gibson, A.T. Osteopenia in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F272–F275. [Google Scholar] [CrossRef]
- Kleinman, R.E.; Greer, F.R. Pediatric Nutrition; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2014. [Google Scholar]
- Bolisetty, S.; Osborn, D.; Schindler, T.; Sinn, J.; Deshpande, G.; Wong, C.S.; Jacobs, S.E.; Phad, N.; Pharande, P.; Tobiansky, R.; et al. Standardised neonatal parenteral nutrition Formulations- Australasian neonatal parenteral nutrition consensus update 2017. BMC Pediatr. 2020, 20, 59. [Google Scholar] [CrossRef]
- Bolisetty, S.; Osborn, D.; Sinn, J.; Lui, K. Standardised neonatal parenteral nutrition formulations—An Australasian group consensus 2012. BMC Pediatr. 2014, 14, 48. [Google Scholar] [CrossRef]
- Koo, W.W.; Gupta, J.M.; Nayanar, V.V.; Wilkinson, M.; Posen, S. Skeletal changes in preterm infants. Arch. Dis. Child. 1982, 57, 447–452. [Google Scholar] [CrossRef]
- Mulla, S.S.S.; Cowey, S.; Close, R.; Pullan, S.; Howe, R.; Radbone, L.; Clarke, P. Severe hypercalcaemia and hypophosphataemia with an optimised preterm parenteral nutrition formulation in two epochs of differing phosphate supplementation. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F451–F455. [Google Scholar] [CrossRef] [PubMed]
- Motokura, K.; Tomotaki, S.; Hanaoka, S.; Yamauchi, T.; Tomotaki, H.; Iwanaga, K.; Niwa, F.; Takita, J.; Kawai, M. Appropriate Phosphorus Intake by Parenteral Nutrition Prevents Metabolic Bone Disease of Prematurity in Extremely Low-Birth-Weight Infants. J. Parenter. Enter. Nutr. 2021, 45, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020, 12, 1–215. [Google Scholar]
- Fewtrell, M.S.; Williams, J.E.; Singhal, A.; Murgatroyd, P.R.; Fuller, N.; Lucas, A. Early diet and peak bone mass: 20 year follow-up of a randomized trial of early diet in infants born preterm. Bone 2009, 45, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Aloy, J.; Álvarez-Domínguez, E.; Pérez-Fernández, J.M.; Moretones-Suñol, G.; Vidal-Sicart, S.; Botet-Mussons, F. Metabolic bone disease and bone mineral density in very preterm infants. J. Pediatr. 2014, 164, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Motte-Signoret, E.; Jlassi, M.; Lecoq, L.; Wachter, P.Y.; Durandy, A.; Boileau, P. Early elevated alkaline phosphatase as a surrogate biomarker of ongoing metabolic bone disease of prematurity. Eur. J. Pediatr. 2023, 182, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Backström, M.C.; Kouri, T.; Kuusela, A.L.; Sievänen, H.; Koivisto, A.M.; Ikonen, R.S.; Mäki, M. Bone isoenzyme of serum alkalinephosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr. 2000, 89, 867–873. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Q.; Piao, M.; Chang, Y.; Zhang, J.; Tong, X.; Han, T. Screening of Serum Alkaline Phosphatase and Phosphate Helps Early Detection of Metabolic Bone Disease in Extremely Low Birth Weight Infants. Front. Pediatr. 2021, 9, 642158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faerk, J.; Peitersen, B.; Petersen, S.; Michaelsen, K.F. Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F133–F136. [Google Scholar] [CrossRef]
- Mimouni, F. Vitamin D in the Newborn, Part II: Bases for Current Dietary Recommendations in Term and Preterm Neonates. NeoReviews 2014, 15, e193–e198. [Google Scholar] [CrossRef]
- Moreira, A.F.M.; Geary, C. Parathyroid hormone levels in neonates with suspected osteopenia. J. Paediatr. Child. Health 2013, 49, E12–E16. [Google Scholar] [CrossRef] [PubMed]

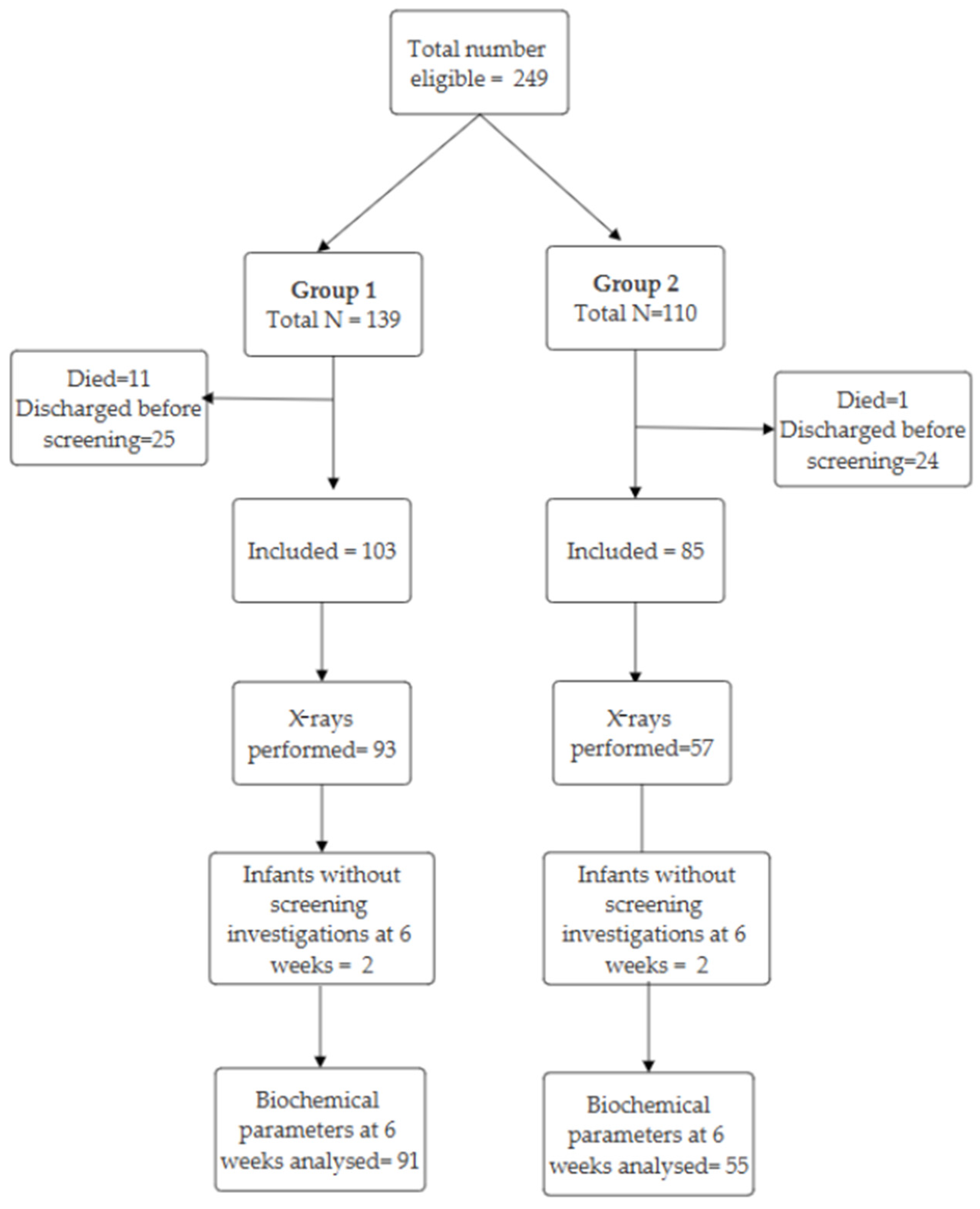

| Characteristic | Group 1 (n = 103) | Group 2 (n = 85) | p |
|---|---|---|---|
| Gestational age (weeks) | 26 (25–27.5) † | 26 (25–27) † | 0.90 |
| Birthweight (g) | 898 (755–1040) † | 940 (763–1040) † | 0.36 |
| Birthweight (percentile) | 60 (33.5–77) † | 63 (39–80) † | 0.48 |
| Ethnicity | |||
| White | 59 (57%) | 41 (48%) | 0.24 |
| Asian | 29 (28%) | 34 (40%) | |
| Other | 15 (15%) | 10 (12%) | |
| Abnormal Dopplers | 11 (15%) | 9 (12%) | 0.81 |
| Antenatal steroids received | |||
| None | 7 (7%) | 6 (7%) | |
| Incomplete | 42 (41%) | 24 (28%) | |
| Complete | 29 (28%) | 42 (49%) | 0.02 |
| More than 7 days | 25 (24%) | 13 (15%) | |
| Multiple gestation | |||
| Singleton | 73 (71%) | 58 (68%) | 0.21 |
| Twins | 20 (29%) | 24 (28%) | |
| Triplets | 0 (0%) | 3 (4%) | |
| Maternal pregnancy induced hypertension (PIH) | 26 (25%) | 17 (20%) | 0.49 |
| Chronic lung disease | 56 (54%) | 41 (48%) | 0.49 |
| Necrotising enterocolitis (NEC) | 11 (11%) | 7 (8%) | 0.92 |
| Patent ductus arteriosus | 93 (90%) | 76 (89%) | 1.00 |
| Duration of parenteral nutrition (days) | 23.7 (15.4–34.7) † | 21.1 (15.8–28.4) † | 0.32 |
| Postnatal systemic steroids | 26 (25%) | 30 (35%) | |
| Inhaled steroids | 6 (6%) | 18 (21%) | 0.01 |
| Duration of loop diuretic therapy (days) | 1 (0–4) † | 2 (0–5) † | 0.23 |
| Placenta histology | |||
| Normal | 12 (13%) | 21 (23%) | 0.07 |
| Chorioamnionitis | 60 (65%) | 46 (58%) | |
| Ischemia | 20 (22%) | 12 (15%) | |
| Sex | |||
| Male | 46 (45%) | 45 (53%) | 0.31 |
| Days to full fortification * | 25 (18–35) † | 24 (19–31) † | 0.48 |
| MBDP Severity | Group 1 (n = 93) | Group 2 (n = 57) | Total Cohort |
|---|---|---|---|
| No MBDP | 16 (17%) | 30 (53%) | 46 (31%) |
| Grade 1 | 63 (68%) | 27 (47%) | 90 (60%) |
| Grade 2 | 13 (14%) | 0 (0%) | 13 (9%) |
| Grade 3 | 1 (1%) | 0 (0%) | 1 (0.67%) |
| Test | Number of Infants with Data Available | No MBDP (n = 44) † | Grade 1 MBDP (n = 88) † | Grade 2/3 MBDP (n = 14) † | p |
|---|---|---|---|---|---|
| Calcium (mmol/L) | 146/146 | 2.68 (2.64–2.78) | 2.62 (2.56–2.70) | 2.60 (2.47–2.66) | <0.01 |
| Phosphate (mmol/L) | 146/146 | 1.83 (1.46–2.04) | 1.80 (1.53–2.02) | 1.34 (1.18–1.47) | <0.01 |
| Vitamin D (nmol/L) | 145/146 | 46 (40–62) | 44 (37–56) | 41 (31–48) | 0.16 |
| PTH (pmol/L) | 140/146 | 7.15 (3.9– 11.4) | 8.00 (5.50–13.95) | 9.45 (4.60–17.30) | 0.37 |
| ALP (U/L) | 146/146 | 426 (332–567) | 384 (329–506) | 553 (484–817) | <0.01 |
| TRP (%) | 122/146 | 95 (91–100) | 95 (91–98) | 95 (88–100) | 0.44 |
| Test | Reference Values |
|---|---|
| Serum corrected Calcium | 1.95–2.8 mmol/L |
| Serum phosphate | 1.45–2.5 mmol/L |
| Alkaline Phosphatase (ALP) | 120–650 U/L |
| Parathyroid Hormone (PTH) | 1.6–7.5 pmol/L |
| 25-Hydroxy(OH) Vitamin D | >50 nmol/L |
| Tubular reabsorption of phosphate (TRP) | >95% |
| Radiograph of the wrist/knee joint | Radiologist review for MBDP |
| Renal ultrasound for nephrocalcinosis | Radiologist report |
| Group 1 (n = 103) | Group 2 (n = 85) | p | |
|---|---|---|---|
| Calcium | |||
| Low | 9 (9%) | 0 (0%) | <0.01 |
| Normal | 74 (72%) | 53 (62%) | |
| High | 20 (19%) | 32 (38%) | |
| Phosphate | |||
| Low | 53 (51%) | 21 (25%) | <0.01 |
| Normal | 44 (43%) | 60 (71%) | |
| High | 6 (6%) | 4 (5%) |
| Group 1 (n = 103) | Group 2 (n = 85) | p | |
|---|---|---|---|
| Hypocalcaemia # | 9 (9%) | 0 (0%) | <0.01 |
| Hypercalcaemia # | 20 (19%) | 32 (38%) | <0.01 |
| Hypophosphataemia # | 53 (51%) | 21 (25%) | <0.01 |
| Nephrocalcinosis * | 22/95 (23%) | 20/71 (28%) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sureshchandra, S.; Maheshwari, R.; Nowland, T.; Elhindi, J.; Rundjan, L.; D'Cruz, D.; Luig, M.; Shah, D.; Lowe, G.; Baird, J.; et al. Implementation of Parenteral Nutrition Formulations with Increased Calcium and Phosphate Concentrations and Its Impact on Metabolic Bone Disease in Preterm Infants: A Retrospective Single-Centre Study. Children 2025, 12, 172. https://doi.org/10.3390/children12020172
Sureshchandra S, Maheshwari R, Nowland T, Elhindi J, Rundjan L, D'Cruz D, Luig M, Shah D, Lowe G, Baird J, et al. Implementation of Parenteral Nutrition Formulations with Increased Calcium and Phosphate Concentrations and Its Impact on Metabolic Bone Disease in Preterm Infants: A Retrospective Single-Centre Study. Children. 2025; 12(2):172. https://doi.org/10.3390/children12020172
Chicago/Turabian StyleSureshchandra, Sushma, Rajesh Maheshwari, Tamara Nowland, James Elhindi, Lily Rundjan, Daphne D'Cruz, Melissa Luig, Dharmesh Shah, Gemma Lowe, Jane Baird, and et al. 2025. "Implementation of Parenteral Nutrition Formulations with Increased Calcium and Phosphate Concentrations and Its Impact on Metabolic Bone Disease in Preterm Infants: A Retrospective Single-Centre Study" Children 12, no. 2: 172. https://doi.org/10.3390/children12020172
APA StyleSureshchandra, S., Maheshwari, R., Nowland, T., Elhindi, J., Rundjan, L., D'Cruz, D., Luig, M., Shah, D., Lowe, G., Baird, J., & Jani, P. R. (2025). Implementation of Parenteral Nutrition Formulations with Increased Calcium and Phosphate Concentrations and Its Impact on Metabolic Bone Disease in Preterm Infants: A Retrospective Single-Centre Study. Children, 12(2), 172. https://doi.org/10.3390/children12020172






