Abstract
Background: The impact of perinatal pharmacologic agents on spontaneous intestinal perforation (SIP) in extremely low-birthweight (ELBW, <1000 g) preterm infants remains inconclusive based on findings from retrospective cohort or case–control studies. This study aims to address this uncertainty by using propensity score matching (PSM) to reduce bias. Methods: We retrospectively reviewed ELBW infants in our unit between 2014 and 2023 to identify SIP cases. Confirmed through medical notes, surgical consultation, and author review, each SIP case was matched at a 1:3 ratio using propensity scores on factors including the gestational age (GA), birthweight, gender, and birth year. Pharmacologic agents commonly given antenatally and postnatally were analyzed. Only medications that were started 24 h before the onset of SIP or the corresponding age (PSM-controls) were included. Results: A total of 858 ELBW infants were reviewed, 28 SIP cases (GA 25.3 ± 2.1 weeks, BW 735 ± 167 g) were identified, and 84 PSM-controls were matched. The SIP cases received hydrocortisone (25% (7/28) vs. 9.5% (8/84), p = 0.037) and combined inotropic agents (17.9% (5/28) vs. 2.4% (2/84), p = 0.020) to a significantly greater extent. No differences were observed in the use of other medications. In logistic regression, the use of hydrocortisone and combined inotropes remained independent risks for SIP, with ORs (95% CIs) of 3.4 (1.1–10.9) and 2.1 (1.2–3.8), respectively. Conclusions: This first PSM-based study supported postnatal hydrocortisone and combined inotrope use as independent risks for SIP in ELBW infants. Clinicians should be aware of these risks and remain vigilant for SIP when administering hydrocortisone and inotropes.
1. Introduction
Spontaneous intestinal perforation (SIP) refers to an idiopathic condition that typically involves the distal ileum and occurs without focal signs of inflammation or necrosis in the intestinal tissue. SIP is a severe complication that is often observed in extremely low-birthweight (ELBW, birthweight <1000 g) preterm infants. Although emergent surgery may not always be necessary, the affected infants usually present with significant abdominal illness, delayed enteral feeding, respiratory distress, intra-abdominal infection, sepsis, or mortality [1]. Given the potential for SIP to become the most prevalent form of severe bowel disease in ELBW infants [2], despite its unclear pathophysiology, there is a need to identify those at risk for this condition. Several perinatal factors are linked to SIP, including lower gestational age (GA), decreased birthweight, and male sex; however, these factors remain nonspecific.
In addition to demographic factors, various pharmacologic agents have been studied, but no conclusive results have been obtained. Recent studies to identify treatment-associated risk factors that include SIP as the primary outcome are summarized in Table 1 [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. For example, antenatal steroids (ANS), which are the standard care provided to stimulate fetal lung maturation, showed no significant difference in most studies, except for one study that reported an increased risk of SIP when ANS is used in combination with postnatal prophylactic indomethacin [13]. Magnesium sulfate (MgSO4) is given for neuroprotection, but concerns have been raised about the potential effects on intestinal motility and atony [7,16]. Indomethacin is a tocolytic agent that has been associated with intestinal hypoperfusion [15,16]. However, no definite conclusions regarding prenatal medication and the development of SIP have been reached.

Table 1.
Summary of current studies aimed to identify pharmacologic treatment-related risk factors for SIP ¶.
The associations between SIP and postnatal pharmacologic regimens have been widely discussed. Postnatal steroids, including hydrocortisone and dexamethasone, are applied in extremely preterm infants to prevent bronchopulmonary dysplasia. Although these therapies target the lungs, they also affect other organ systems, including ileal crypt cells, which can result in alterations in the intestinal mucosal alignment and motility [3]. Some studies have suggested a possible link between early systemic steroid use and a greater risk of SIP [3,4,6,8,12,16]. Patent ductus arteriosus (PDA) is prevalent among ELBW infants, and pharmacologic interventions are typically employed to close the ductus. Prostaglandin inhibitors, such as indomethacin (in short supply in Taiwan since 2010) and ibuprofen, are traditionally the drugs of choice; both have been linked to SIP in retrospective studies. Whereas more than half of all studies suggested that indomethacin is a risk factor [4,5,6,8,10,14,16], only one of the three previously referenced studies reported an association [8]. An emerging agent for PDA, acetaminophen (paracetamol) has not yet been studied in relation to SIP. Systemic hypotension and intestinal hypoperfusion, which are often managed with inotropes (dopamine, dobutamine, or epinephrine), have also been found to be associated with SIP [5,11,12].
As demonstrated in Table 1, the associations between these medications and SIP remain inconclusive. A significant limitation of previous studies is their designs. All existing reports in which SIP is the primary outcome have been either retrospective cohort studies or matched case-control studies, making them susceptible to selection bias. Propensity score matching (PSM) is a statistical method that minimizes bias related to patient characteristics. It helps investigators to reduce the likelihood of confounding when analyzing retrospective nonrandomized observational data [17]. Consequently, this approach provides a more robust estimate of treatment effects. In this study, we aimed to utilize PSM to reduce confounding bias in the examination of pharmacologic risk factors for SIP in ELBW preterm infants.
2. Materials and Methods
2.1. SIP Cases
This study was conducted in the neonatal unit at Chang Gung Memorial Hospital, Linkou, a referral center in northern Taiwan with 50 intensive care beds and 54 sick-baby nursery beds. We reviewed the unit’s database between January 2014 and December 2023 to identify ELBW preterm infants and locate SIP cases based on the following criteria: diagnosis documented by the attending physician, consultation sheet from the surgeon, and verification by the authors through medical record review. Each SIP case was confirmed by evidence of an abrupt onset of radiographic pneumoperitoneum, along with physical signs of acute abdominal distension or bluish-black discoloration. Infants with suspected necrotizing enterocolitis, meconium ileus, or gastrointestinal malformations were excluded.
2.2. Propensity Score Matching
Using the cohort of ELBW preterm infants from the same period, the propensity scores for SIP were calculated based on a logistic regression model, incorporating variables including GA, birthweight, gender, and birth year. A greedy PSM strategy was employed to find cases with the closest estimated propensity scores, utilizing either nearest neighbor matching or nearest neighbor within caliper ≤0.2. Each SIP case was matched with 3 infants (1:3) to create a PSM-control group, ensuring an absolute standardized mean difference (ASMD) of less than 0.1.
2.3. Risk Factors
In addition to examining individual demographics, maternal history and conditions that may relate to neonatal stress, we focused on analyzing pharmacologic agents that were applied antenatally and postnatally. The antenatal medications of interest included ANS to promote fetal lung maturation, MgSO4 for fetal neuroprotection, and indomethacin as a tocolytic agent. Postnatal pharmacologic agents of interest included surfactants for treating respiratory distress syndrome, dexamethasone and hydrocortisone for the prevention of bronchopulmonary dysplasia (BPD), ibuprofen (intravenous or oral) and acetaminophen (oral) for ductus arteriosus closure, caffeine for apnea of prematurity, milrinone to treat pulmonary hypertension and inotropes (dopamine, dobutamine, and epinephrine) to treat hypotension (defined as the mean blood pressure less than postmenstrual age (PMA) in weeks). Our unit typically followed a stepwise protocol for inotropes: dopamine as the first-line treatment, dobutamine as the second-line, and epinephrine as the third-line for severe cases, often in combination with dopamine and dobutamine. Only the medications that given at least 24 h before the onset of SIP or the corresponding age (PSM-controls) were adopted.
2.4. Statistics
PSM was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), whereas the remaining statistical analyses were conducted via SPSS version 24 (IBM, Armonk, NY, USA). ASMD was used to compare SIP cases with the cohort and PSM-controls. Continuous variables were analyzed using independent-samples t tests, whereas categorical variables were analyzed via the χ2 test or Fisher’s exact test. Significant risk factors identified between SIP cases and PSM-controls were subsequently analyzed via multivariable logistic regression, with the results expressed as odds ratios (ORs) and 95% confidence intervals (CIs). A p value < 0.05 was considered statistically significant.
3. Results
Over a 10-year study period, 858 ELBW infants (mean ± SD GA 26.2 ± 2.2, BW 770 ± 147) were admitted to our unit. Among the cohort, 28 SIP cases were identified, yielding an annual incidence ranging 1.3% to 6.1% and an overall prevalence rate of 3.3%. The GA and BW of the SIP cases were 25.3 ± 2.1 weeks and 735 ± 167 g, respectively, which were significantly more immature than the cohort (GA p = 0.043). Nineteen (67.9%) of these 28 patients were male. The diagnosis of SIP was made at an average chronological age of 8.5 ± 4.7 days or a PMA of 26.5 ± 2.0 weeks. Of them, four infants developed SIP early (age 0–3 days), and they were more mature than infants with SIP after the fourth day of life (GA 27.8 ± 2.5 vs. 24.9 ± 1.7 weeks, p = 0.008). No difference was seen in other demographic characteristics or risk factors.
After PSM, 84 preterm infants were selected for the control group. The characteristics of the SIP cases, the cohort, and PSM-controls are presented in Table 2, which shows minimal mean differences between the SIP cases and PSM-controls. The enrollment flow diagram is illustrated in Figure 1.

Table 2.
Demographic data for SIP cases and the control before (cohort) vs. after PSM (PSM-controls).
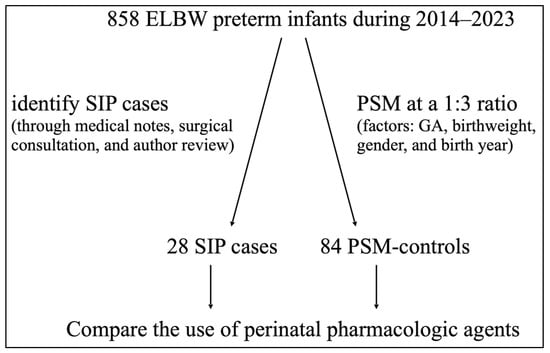
Figure 1.
Enrollment flow diagram. ELBW: extremely low birthweight; SIP: spontaneous intestinal perforation; PSM: propensity score matching; GA: gestational age.
The comparisons between SIP cases and PSM-controls are presented in Table 3. There were no significant differences in demographics or maternal conditions between the two groups, though fewer infants in the SIP group initiating feeding. However, there was a significantly greater usage of postnatal hydrocortisone to prevent BPD in the SIP group (25% (7/28) vs. 9.5% (8/84), p = 0.037). The SIP group also used more inotropic agents. Specifically, compared with PSM-controls (17.9% (5/28) vs. 2.4% (2/84); p = 0.016), a higher number of infants in the SIP group received a combination of three inotropes (dopamine + dobutamine + epinephrine). No significant differences were observed for other prenatal or postnatal pharmacologic agents. Furthermore, among infants who remained nil per os (NPO), the SIP group demonstrated higher usage of either hydrocortisone or inotropic agents. In multivariable logistic regression involving the use of hydrocortisone and combined inotropic agents, both risk factors remained independent for SIP, with ORs (95% CIs) of 3.4 (1.1–10.9) and 2.1 (1.2–3.8), respectively.

Table 3.
Comparison between SIP cases and PSM-controls.
4. Discussion
This study explored the associations between multiple pharmacologic treatments commonly used in ELBW preterm infants and the risk of SIP. The results showed that postnatal hydrocortisone and combined inotropic agents (dopamine, dobutamine, and epinephrine) were independent risk factors for SIP. The strength of this study lies in the use of PSM, which reduced the selection bias compared with previous cohort or matched case-control studies and provided a more reliable estimation of the relationship between pharmacologic treatments and SIP.
In our 10-year single center cohort, the overall prevalence of SIP in ELBW preterm infants was 3.3%, consistent with findings from a recent national cohort [1]. Subgroup analysis based on the onset age of SIP showed that infants who developed SIP at 0–3 days of life were more mature compared to those with a later onset. This trend in GA differences has also been observed in a large-scale dataset [18]. However, we found no significant differences in other patient characteristics or perinatal risk factors, as noted in a previous study. This finding may be limited by the small number of early-onset cases in our study. Although fewer infants in the SIP group initiating feeding, similar to the findings of Rayyan’s study [11], both our study and Rayyan et al. suggested that the lack of feeding most likely reflects the severity of illness, particularly since both hydrocortisone and inotropic agents remained risk factors among neonates who were NPO.
This study revealed a significant association between postnatal hydrocortisone and a 3.4-fold increase in the risk of SIP. Our findings align with those of several studies that identified hydrocortisone as an independent risk factor for SIP, when SIP was used as the primary outcome measure [4,6,8,12,16]. In these studies, the use of postnatal hydrocortisone among SIP cases was reported from 7.3% to 31%. Although there were three other studies with different results [5,11,15], they had a relatively small number of SIP cases and may have had insufficient statistic power to disclose the association. In other large-scale prospective studies focused on preventing BPD in ELBW preterm infants, hydrocortisone-treated infants presented a higher incidence of gastrointestinal perforation, especially when the medication was used in combination with indomethacin [19]. Another individual patient data meta-analysis also showed that hydrocortisone combined with indomethacin increased the risk of SIP [20]. In a meta-analysis of randomized control trials that discussed the safety of systemic hydrocortisone for BPD prevention, a higher risk of bowel perforation was observed in infants who received hydrocortisone within the first postnatal week [21]. Because indomethacin was not available in Taiwan during this period, no infants received hydrocortisone in combination with postnatal indomethacin. However, we did not observe differences when hydrocortisone was used in combination with either intravenous or oral ibuprofen.
The proposed mechanism suggests that hydrocortisone affects insulin-like growth factors (IGFs), epidermal growth factor (EGF), transforming growth factor-alpha (TGF-α), and nitric oxide (NO) synthases [22,23]. In brief, steroids decrease the amount of IGFs in mesenchymal tissue and redistribute them to the villus and mucosa; they also deplete neuronal NO synthases, which influence the innervation of intestinal smooth muscle [22] and lower the level of TGF-α, an antiapoptotic factor, in the muscularis externa in relation to the inhibition of ileal smooth muscle proliferation [23]. These changes result in increased proliferation of the intestinal mucosa while compromising the submucosal thickness, potentially predisposing infants to SIP.
The study also revealed a significant association between the use of inotropes in combination (dopamine + dobutamine + epinephrine) and a 2.1-fold increased risk of SIP. Although previous studies showed an association between dopamine alone and SIP, our results indicated that inotropes used in combination were an independent risk factor for SIP. The possible mechanism involves systemic hypotension or epinephrine-related intestinal hypoperfusion and subsequent ischemia-reperfusion injury associated with SIP. Epinephrine is an endogenous catecholamine with β-1 and nonselective α effects and serves as both a potent inotrope to increase cardiac output and a vasopressor to increase systemic vascular resistance (SVR). In cases of hypotension and hypoperfusion, blood flow to the intestine is often compromised, increasing the vulnerability of intestinal circulation. Given that epinephrine is usually reserved for severe hypotension and is used in combination with other inotropes, our findings may reflect patients’ deteriorating conditions.
We must also consider the effect of vascular constriction caused by epinephrine. In one animal experiment, although systemic blood flow increased with epinephrine, it appeared to divert blood away from the gut, thereby decreasing intestinal microcirculation [24]. When hemodynamics improve, these initially low-perfusion areas may experience ischemia-reperfusion injury due to the induction of reactive oxygen species and proinflammatory factors, which negatively affect gut integrity.
Other perinatal medications that are commonly discussed were not associated with SIP in our study. No antenatal medication (ANS, indomethacin, or MgSO4) was significant in our study, which is consistent with those of the majority of previous studies. The lack of significance of postnatal dexamethasone corresponds with findings from other studies [4,11,12]. However, we have to cautiously interpret it, as only four infants in our cohort received postnatal dexamethasone. We did not find either intravenous or oral ibuprofen to be a risk factor, and the finding is consistent with those of a recent systematic review [25]. This study is the first to investigate whether acetaminophen is associated with SIP, and our negative finding aligns with those of another systematic review on the safety of acetaminophen for PDA in preterm infants [26]. However, there were only three such cases in both groups; hence, a large-scale study is warranted. Our study is also the first to clarify that there was no association of milrinone with SIP. Surfactant and caffeine were not associated with SIP.
This study’s limitations include the retrospective nature of the data collection, which may have led to missing or incomplete information. Additionally, the lack of predefined indications for certain medications might have affected the consistency of treatment decisions over the study period. Although PSM is effective in balancing cases with measurable confounders, it does not eliminate unmeasured or residual confounding factors. Furthermore, the dosage and duration of the aforementioned medications as well as the interval between medication initiation and the onset of SIP were not evaluated in this study.
5. Conclusions
This first PSM-based study supported the finding that postnatal hydrocortisone exposure and combined inotrope use are independent risk factors for SIP in ELBW infants. Clinicians should closely monitor extremely preterm infants receiving hydrocortisone or combined inotropes for an elevated risk of SIP.
Author Contributions
All authors conceived and designed the manuscript. W.-H.C. and L.-H.T. contributed to data acquisition, data analysis, and drafted the original manuscript. M.-C.C., Y.-N.C., W.-H.W. and K.-H.H. contributed to data analysis and reviewed the manuscript. K.-H.H. contributed to reviewing and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the of Chang Gung Medical Foundation (202401598B0, approved on 17 October 2024).
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Pin-Hsuan Huang and Yu-Ching Wang for the statistical consultation and wish to acknowledge the statistical and data analysis assistance and interpretation by the Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SIP | spontaneous intestinal perforation |
| ELBW | extremely low birthweight |
| PSM | propensity score matching |
| ANS | antenatal steroids |
References
- Elgendy, M.M.; Othman, H.F.; Heis, F.; Qattea, I.; Aly, H. Spontaneous intestinal perforation in premature infants: A national study. J. Perinatol. 2021, 41, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.R.; Hair, A.; Clark, R.H.; Gordon, P.V. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J. Perinatol. 2022, 42, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.; Friedlich, P.; Ramanathan, R.; Seri, I. Concurrent use of indomethacin and dexamethasone increases the risk of spontaneous intestinal perforation in very low birth weight neonates. J. Perinatol. 2006, 26, 486–492. [Google Scholar] [CrossRef][Green Version]
- Attridge, J.T.; Clark, R.; Walker, M.W.; Gordon, P.V. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J. Perinatol. 2006, 26, 93–99. [Google Scholar] [CrossRef]
- Ahmad, I.; Davis, K.F.; Emi, S.; Uy, C.; Sills, J. Risk Factors for Spontaneous Intestinal Perforation in Extremely Low Birth Weight Infants. Open Pediatr. Med. J. 2008, 2, 11–15. [Google Scholar]
- Wadhawan, R.; Oh, W.; Vohr, B.R.; Saha, S.; Das, A.; Bell, E.F.; Laptook, A.; Shankaran, S.; Stoll, B.J.; Walsh, M.C.; et al. Spontaneous intestinal perforation in extremely low birth weight infants: Association with indomethacin therapy and effects on neurodevelopmental outcomes at 18–22 months corrected age. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F127. [Google Scholar] [CrossRef] [PubMed]
- Rattray, B.N.; Kraus, D.M.; Drinker, L.R.; Goldberg, R.N.; Tanaka, D.T.; Cotten, C.M. Antenatal magnesium sulfate and spontaneous intestinal perforation in infants less than 25 weeks gestation. J. Perinatol. 2014, 34, 819–822. [Google Scholar] [CrossRef]
- Shah, J.; Singhal, N.; Da Silva, O.; Rouvinez-Bouali, N.; Seshia, M.; Lee, S.K.; Shah, P.S. Intestinal perforation in very preterm neonates: Risk factors and outcomes. J. Perinatol. 2015, 35, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, M.; Mohamed, A.; Lemyre, B.; Aziz, K.; Faucher, D.; Shah, P.S.; for the Canadian Neonatal Network Investigators. Antenatal Exposure to Magnesium Sulfate and Spontaneous Intestinal Perforation and Necrotizing Enterocolitis in Extremely Preterm Neonates. Am. J. Perinatol. 2017, 34, 1227–1233. [Google Scholar] [CrossRef]
- Stavel, M.; Wong, J.; Cieslak, Z.; Sherlock, R.; Claveau, M.; Shah, P.S.; for the Canadian Neonatal Network Investigators. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J. Perinatol. 2017, 37, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, M.; Myatchin, I.; Naulaers, G.; Ali Said, Y.; Allegaert, K.; Miserez, M. Risk factors for spontaneous localized intestinal perforation in the preterm infant. J. Matern. Fetal Neonatal Med. 2018, 31, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Arnautovic, T.I.; Longo, J.L.; Trail-Burns, E.J.; Tucker, R.; Keszler, M.; Laptook, A.R. Antenatal Risk Factors Associated with Spontaneous Intestinal Perforation in Preterm Infants Receiving Postnatal Indomethacin. J. Pediatr. 2021, 232, 59–64.e1. [Google Scholar] [CrossRef]
- Kandraju, H.; Kanungo, J.; Lee, K.-S.; Daspal, S.; Adie, M.A.; Dorling, J.; Ye, X.Y.; Lee, S.K.; Shah, P.S.; Beltempo, M.; et al. Association of Co-Exposure of Antenatal Steroid and Prophylactic Indomethacin with Spontaneous Intestinal Perforation. J. Pediatr. 2021, 235, 34–41.e1. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R.; Weydig, H.; Brion, L.P.; Wyckoff, M.H.; Arnautovic, T.I.; Younge, N.; Oh, W.; Chowdhury, D.; Keszler, M.; Das, A. Antenatal Steroids, Prophylactic Indomethacin, and the Risk of Spontaneous Intestinal Perforation. J. Pediatr. 2023, 259, 113457. [Google Scholar] [CrossRef]
- Mantle, A.; Yang, M.J.; Judkins, A.; Chanthavong, I.; Yoder, B.A.; Chan, B. Association of Intrapartum Drugs with Spontaneous Intestinal Perforation: A Single-Center Retrospective Review. Am. J. Perinatol. 2021, 41, 174–179. [Google Scholar] [CrossRef]
- Thakkar, P.V.; Sutton, K.F.; Detwiler, C.A.; Henegar, J.G.; Narayan, J.R.; Perez-Romero, M.; Strausser, C.M.; Clark, R.H.; Benjamin, D.K., Jr.; Zimmerman, K.O.; et al. Risk factors and epidemiology of spontaneous intestinal perforation among infants born at 22–24 weeks’ gestational age. J. Perinatol. 2024, 44, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Haukoos, J.S.; Lewis, R.J. The Propensity Score. JAMA 2015, 314, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Attridge, J.T.; Clark, R.; Walker, M.W.; Gordon, P.V. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J. Perinatol. 2006, 26, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Watterberg, K.L.; Gerdes, J.S.; Cole, C.H.; Aucott, S.W.; Thilo, E.H.; Mammel, M.C.; Couser, R.J.; Garland, J.S.; Rozycki, H.J.; Leach, C.L.; et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: A multicenter trial. Pediatrics 2004, 114, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, M.L.; Baud, O.; Lacaze-Masmonteil, T.; Peltoniemi, O.M.; Bonsante, F.; Watterberg, K.L. Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. J. Pediatr. 2019, 207, 136–142.e5. [Google Scholar] [CrossRef]
- Morris, I.P.; Goel, N.; Chakraborty, M. Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. Eur. J. Pediatr. 2019, 178, 1171–1184. [Google Scholar] [CrossRef]
- Gordon, P.V.; Herman, A.C.; Marcinkiewicz, M.; Gaston, B.M.; Laubach, V.E.; Aschner, J.L. A neonatal mouse model of intestinal perforation: Investigating the harmful synergism between glucocorticoids and indomethacin. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.V. Understanding Intestinal Vulnerability to Perforation in the Extremely Low Birth Weight Infant. Pediatr. Res. 2009, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Krejci, V.; Hiltebrand, L.B.; Sigurdsson, G.H. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit. Care Med. 2006, 34, 1456–1463. [Google Scholar] [CrossRef]
- Ohlsson, A.; Shah, S.S. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD004213. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Mitra, S.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2022, 12, CD010061. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).