Diagnosis, Treatment, and Follow-Up of Tracheo/Bronchomalacia in Children: The Italian Multicenter Experience
Abstract
1. Introduction
2. The Clinical History and the Typical Two-Tone/Biphasic Cough of Patients with Tracheomalacia (TM)
- “Does it seem to you that your child has a different tone of cough than his/her peers?”
- “Would you be able to recognize your child’s cough in different contexts?”
3. Diagnostic Tests
3.1. Imaging Techniques
- Chest radiography (CXR): It is not useful for the diagnosis of TBM, although it can visualize the tracheal lumen, which may be focally slightly lateralized in cases of extrinsic compression from the aortic arch.
- Tracheobronchography–fluoroscopy (TBG-TBF): This is often performed concurrently with endoscopy in the operating room under general anesthesia. Iso-osmolar CM is injected through the working channel of the bronchoscope, obtaining an immediate and panoramic evaluation of the airways. Data are obtained regarding airway dimensions (even downstream from stenosis), morphology (normal or abnormal bronchial bifurcation, vs. isomerisms), as well as changes in tracheal lumen during different phases of respiration. TBG is also very useful as a guide for subsequent interventional procedures, favoring precise luminal localization of devices (balloon tracheoplasty, cutting balloon, bioabsorbable stent) [13,14,15].
- Esophagography–fluoroscopy (EG-EF): This is performed for searching tracheoesophageal fistulas (TEF), often present in esophageal atresia (EA) and frequently associated with TM. Diagnostic accuracy for some authors is >80%, while for others this is not an effective technique to reveal the fistula [16]. CT can demonstrate the presence of the TEF, but definitive diagnosis on patency or closure of the fistula is certainly entrusted to endoscopy with direct injection of CM into the fistula through the working channel of the endoscope.
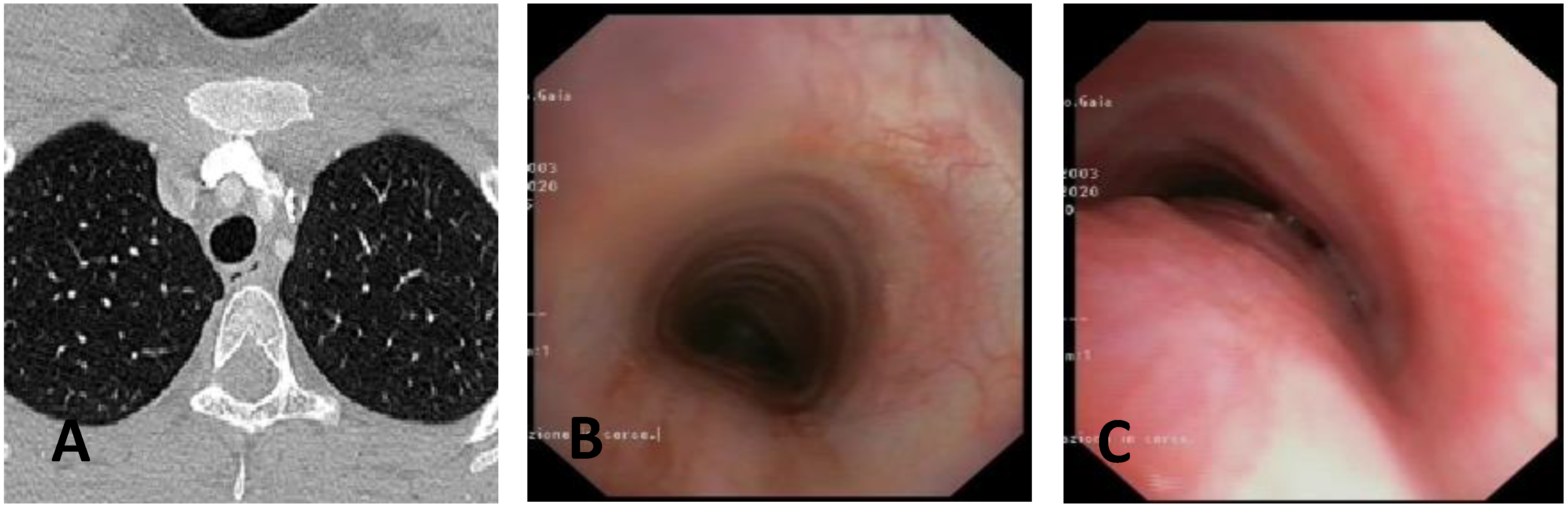
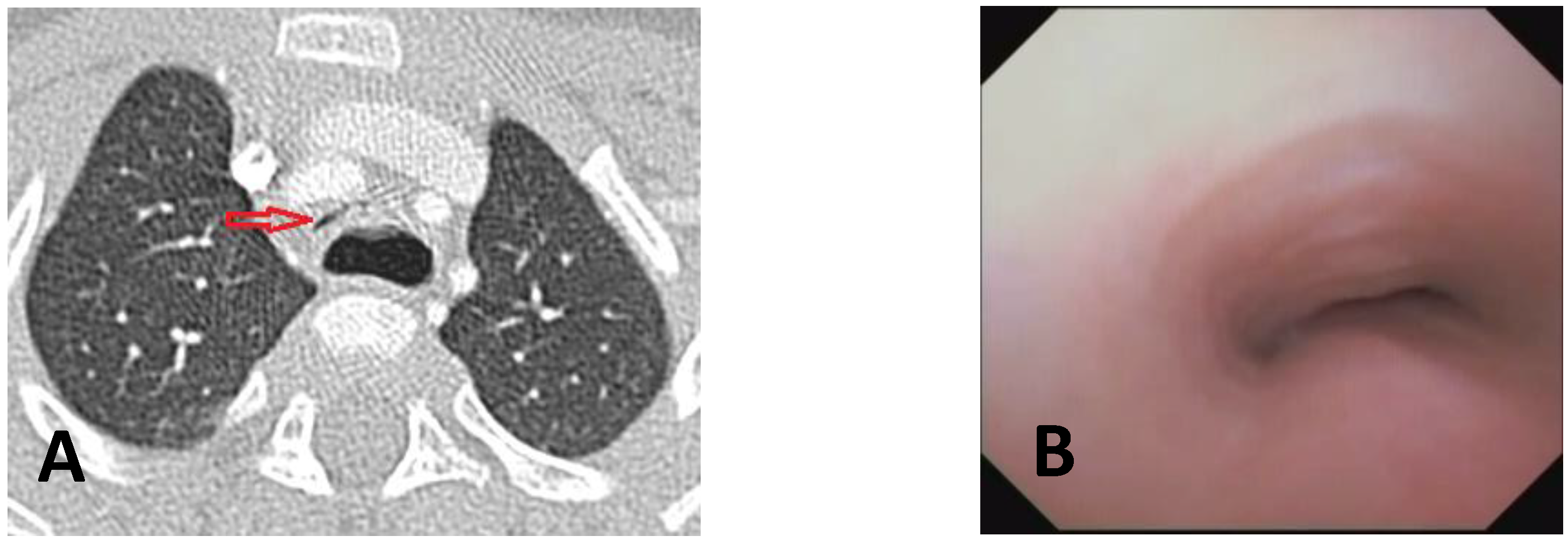
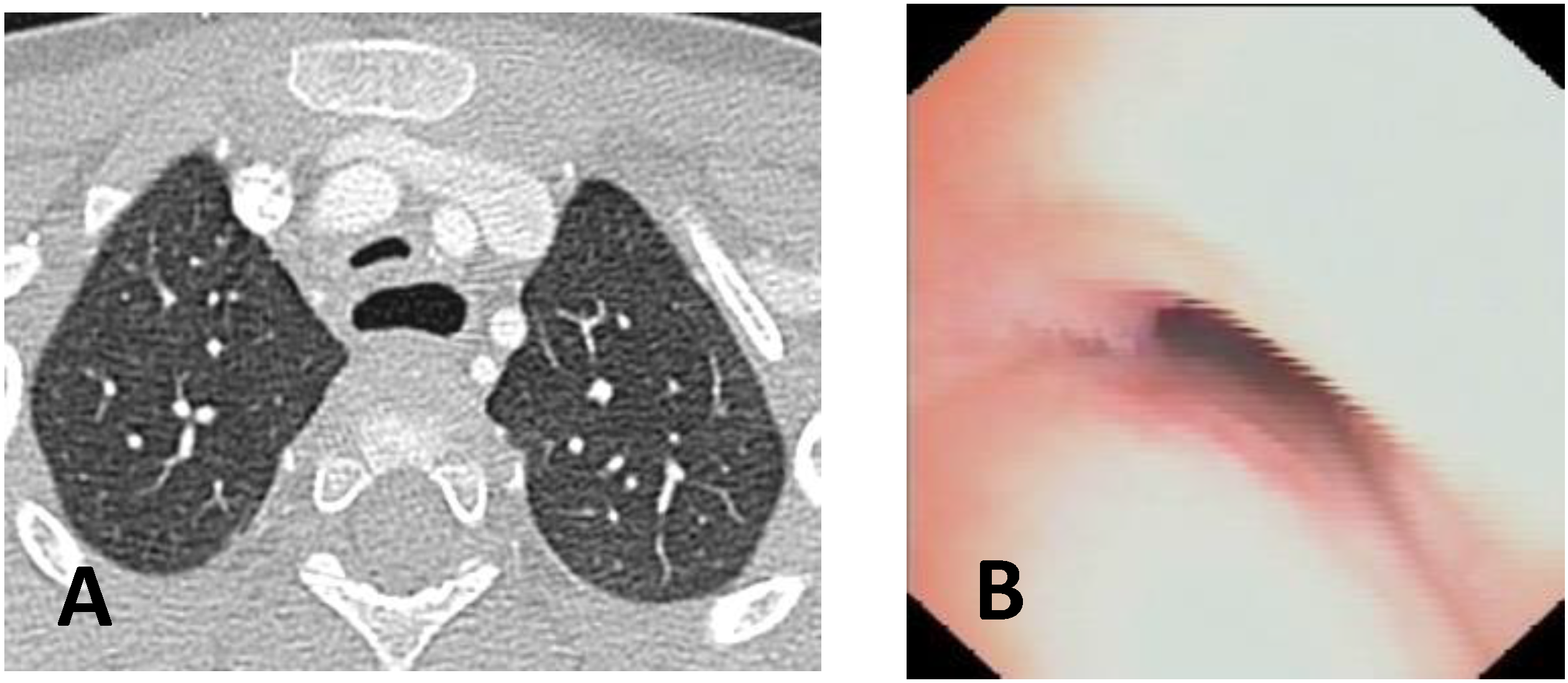
- Computed Tomography (CT): This is a continuously evolving imaging technique, rapid and non-invasive, providing an excellent overall view, independent of body size, with high spatial/temporal resolution. It allows multiplanar and volumetric reconstructions (MPR, MinPR, MipPR, and Volumetric 3D). It can be performed on children of all ages; anesthesia/sedation may be necessary under 5 years of age. Flash Monophasic Technique performed with a single scan, after intravenous injection of CM, provides information on airway morphology (but not dynamics), visualizing airways even distal to the site of obstructions and on mediastinal vessels exerting compression on the trachea or bronchi, highlighting any mediastinal pathology. CT shows cardiovascular anomalies compressing the airway, such as right aortic arch, complete/incomplete double aortic arch, pulmonary sling, and aberrant IA, all causing more or less severe TBM [16]. TM is very frequently associated with EA; CT can demonstrate malacia and extrinsic tracheal compression with a significant reduction in the tracheal ADP at the point of intersection with IA. CT can also demonstrate irreversible lung damage, such as bronchiectasis formation, caused by chronic recurrent lung infections resulting from reduced mucociliary clearance in TBM. Skeletal anomalies (e.g., pectus excavatum and scoliosis) that can cause airway compression and consequent TBM are also demonstrated. CT also evaluates tracheal compressions caused by space-occupying mediastinal lesions. Virtual bronchoscopy obtained with 3D airway reconstruction on CT images has not been very sensitive (<75%) in detecting TBM [17,18].
- Dynamic CT (DCT) allows visualization of the entire airway in a single gantry rotation using a dynamic volumetric scanning technique. Images can be acquired over one or two respiratory cycles with a total scan time of less than 2 s, while the child is breathing at tidal volume during this rapid acquisition. Anesthesia is not necessary, making the exam comfortable and drastically reducing patient discomfort. Dynamic imaging throughout the respiratory cycle allows accurate determination of end-inspiration/end-expiration phases in three dimensions (3D), with accurate determination of luminal collapse degree. The disadvantage of this technique is increased radiation dose to the patient, so it is performed only in selected cases [19,20].
- Magnetic Resonance Imaging (MRI) is the exam of choice for studying cervical or thoracic masses compressing or displacing the trachea in pediatric age (cervical lymphangioma, venous vascular malformation, neuroblastoma, esophageal duplication, lymphoma, and teratoma). The main advantage of MRI is the lack of exposure to ionizing radiation, while the disadvantages are the long acquisition times and the lower spatial resolution than CT. Dynamic MRI for tracheomalacia, using three high-field Tesla (3.0 Tesla or 3T MRI) scanners and dedicated coils, is still limited to the research field rather than clinical application. The time-resolved sequence can demonstrate the dynamic aspect of the airway during forced expiration [1,21].
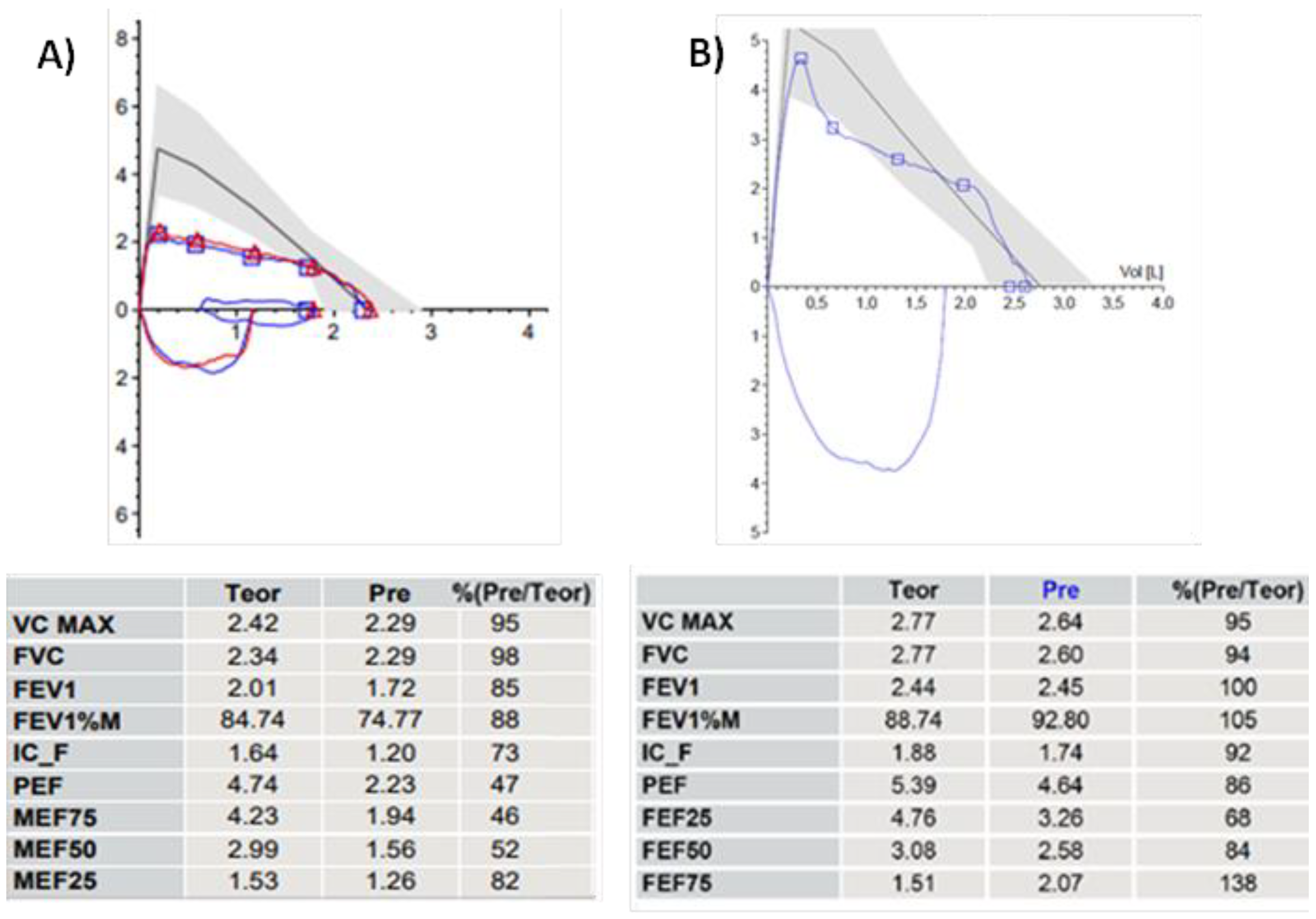
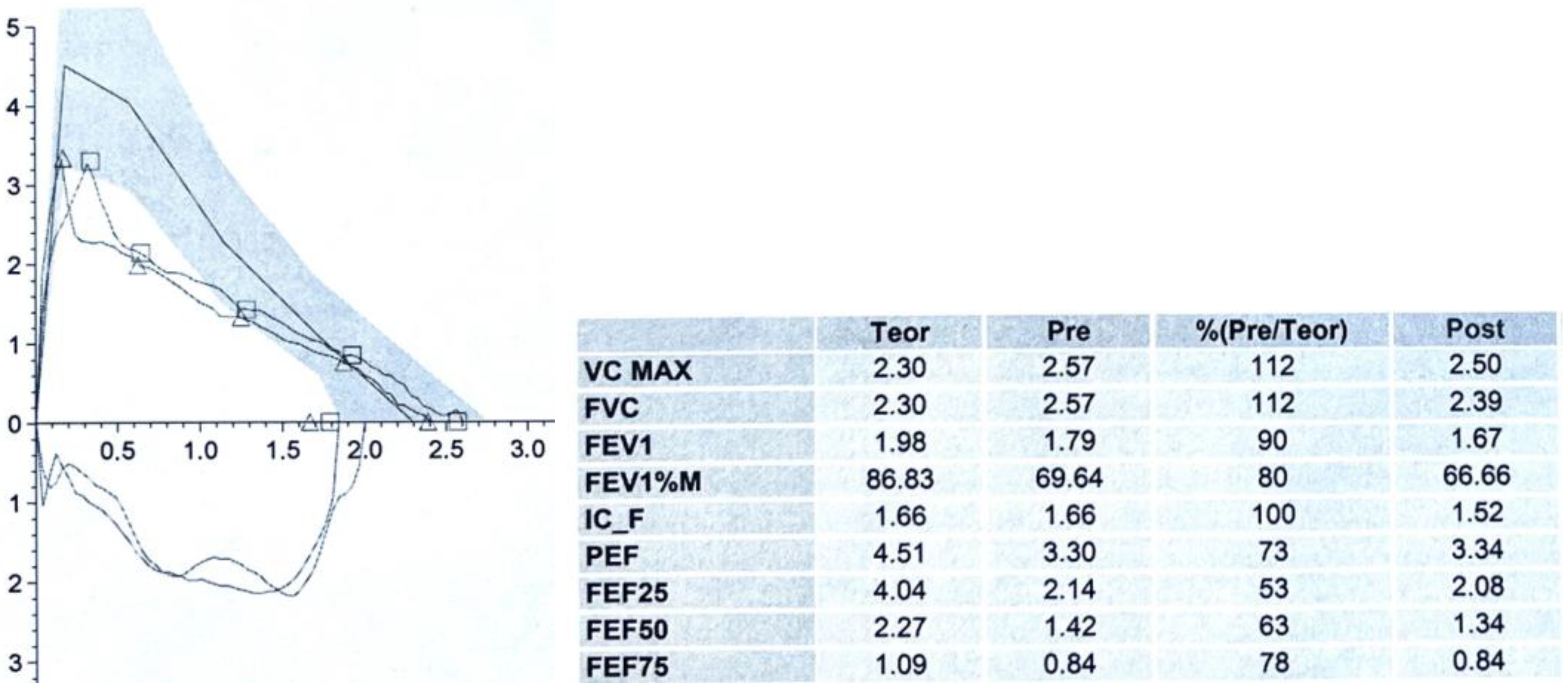
3.2. Spirometry
3.3. Static and Dynamic VBS (S/DVBS): Endoscopic Pictures in Clinical Cases
- 1.
- Primary malacia: This is due to intrinsic alteration of the cartilage of the respiratory tract walls, and it is a less common condition compared to the following:
- 2.
- Secondary malacia: This is the normally shaped cartilage that is deformed by extrinsic compression, usually of vascular origin, on the tracheal wall.
- 3.
- Mild TM: reduction between 50–75%;
- Moderate TM: reduction between 75–90%;
- Severe TM: reduction is >90%.
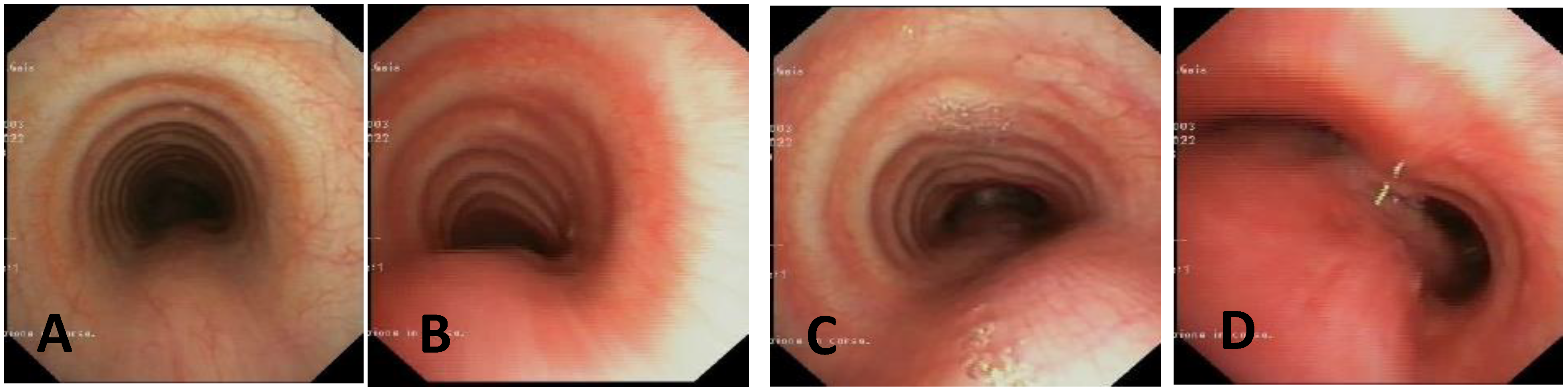
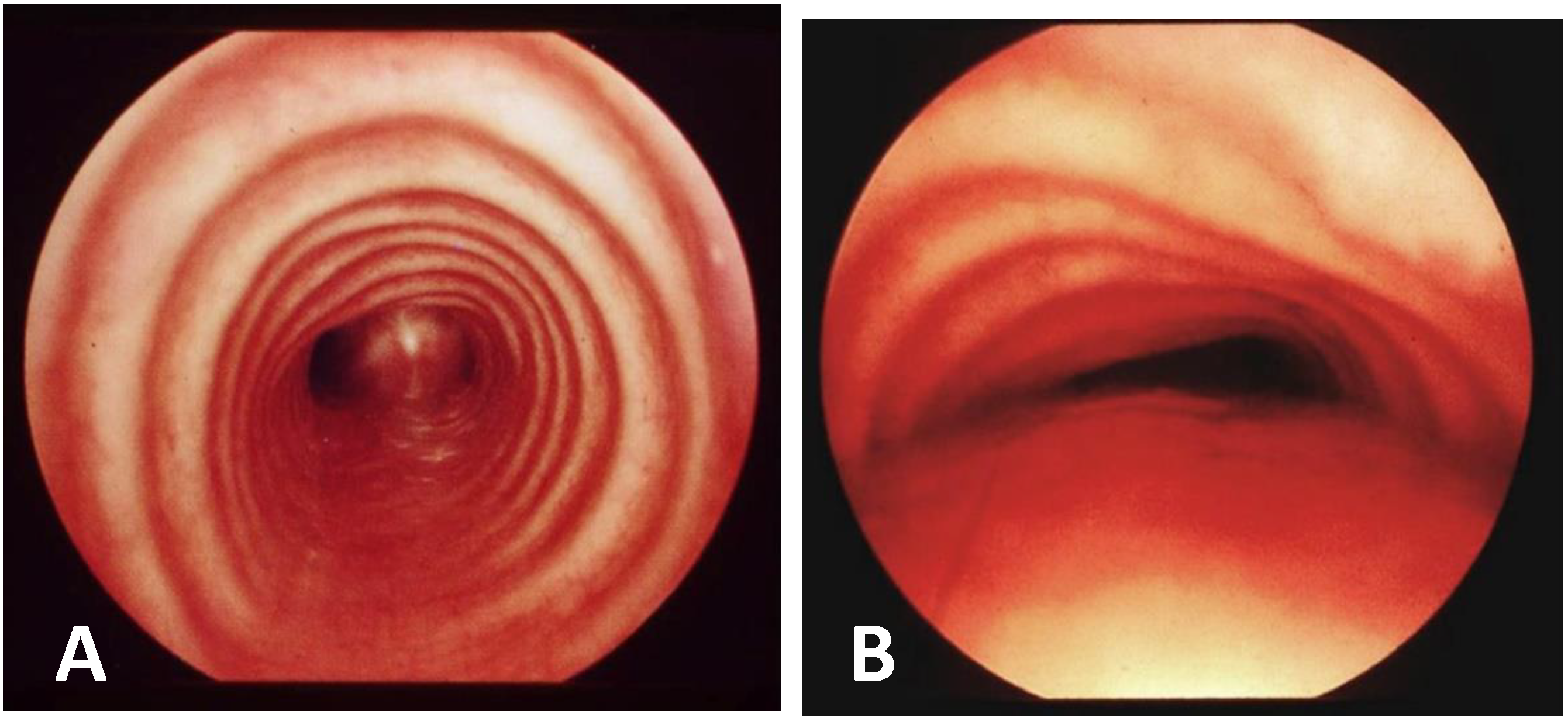
- Static VBS (SVBS) is the phase in which the tracheal lumen is evaluated during spontaneous, quiet breathing, but there should also be a phase of:
- Dynamic VBS (DVBS), in which the patient (while sedation, as it is at the end of the examination, becomes increasingly superficial), is stimulated to cough by contact of the endoscope with the carina and/or main bronchial walls. The patient, performing abdominal straining to attempt to cough, increases the intrathoracic pressure similarly to what happens when the patient is awake. The increasing pressure, if there is malacia/extrinsic compression or hypermobility of the pars membranacea, causes a pathological (i.e., greater than 50%) decrease in tracheal lumen, as can happen in everyday life [27,28].
3.4. Indications for Surgical Treatment
- 4.
- The degree of clinical severity: biphasic cough, stridor, wheezing, recurrent respiratory infections, dyspnea, and respiratory failure.
- 5.
- The degree of TM severity according to radiological imaging (Chest CT with CM) and endoscopic criteria: S/DVBS.
- TM Clinical Score (TMCS) was formulated in accordance with the experience of Gaslini TT, characterized by the absence, with a score = 0, or presence, with a score = 1, of certain symptoms (each symptom contributes with its score to the final sum score), stratified by age groups. Symptoms must be present even in substantial well-being, outside of acute infectious events.
| 0–2 years |
|
| 2–6 years |
|
| >6 years |
|
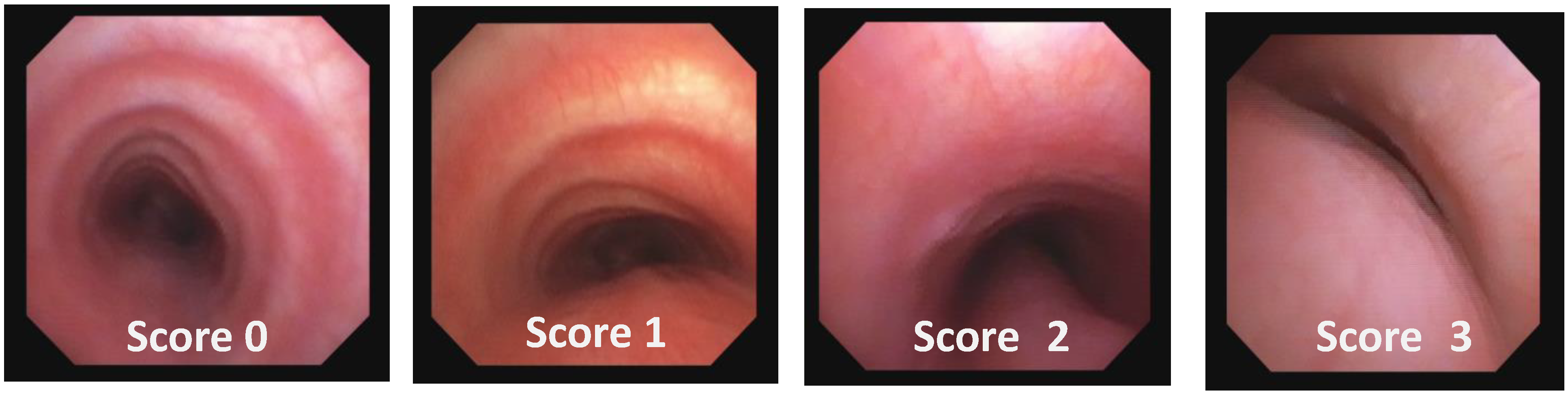
- TM Endoscopic Score (TMES) tracks the indications of the 2019 ERS statement on TBM in children [1]. The S/DVBS leads to the assignment of the following scores:
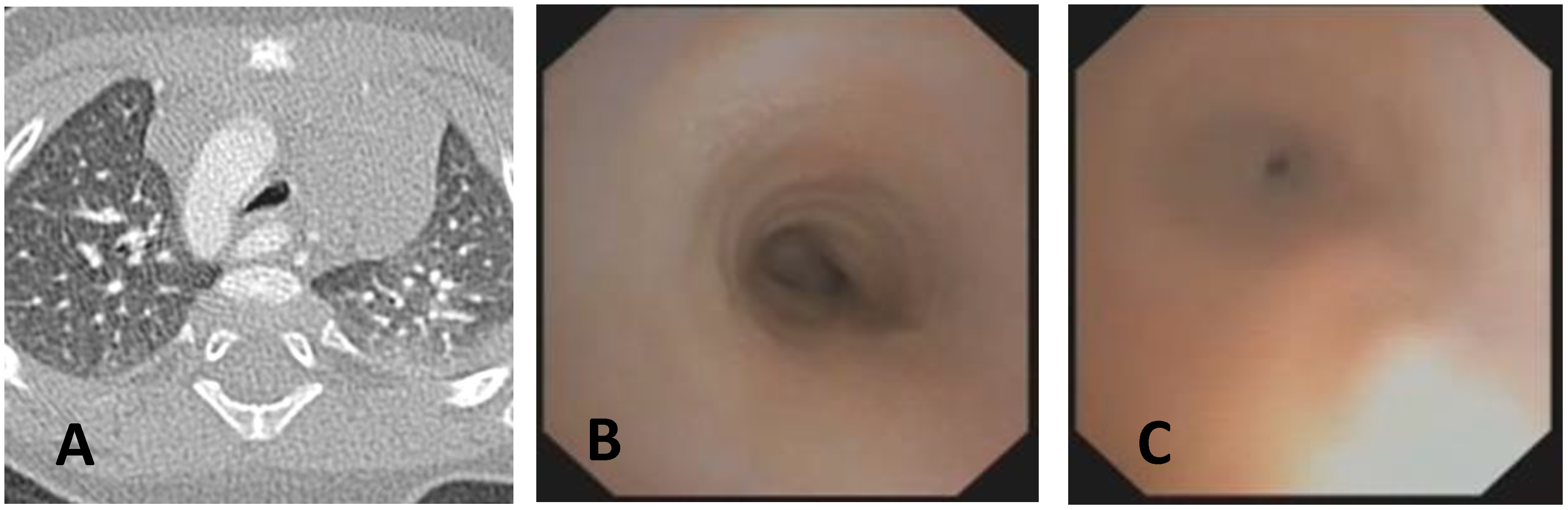
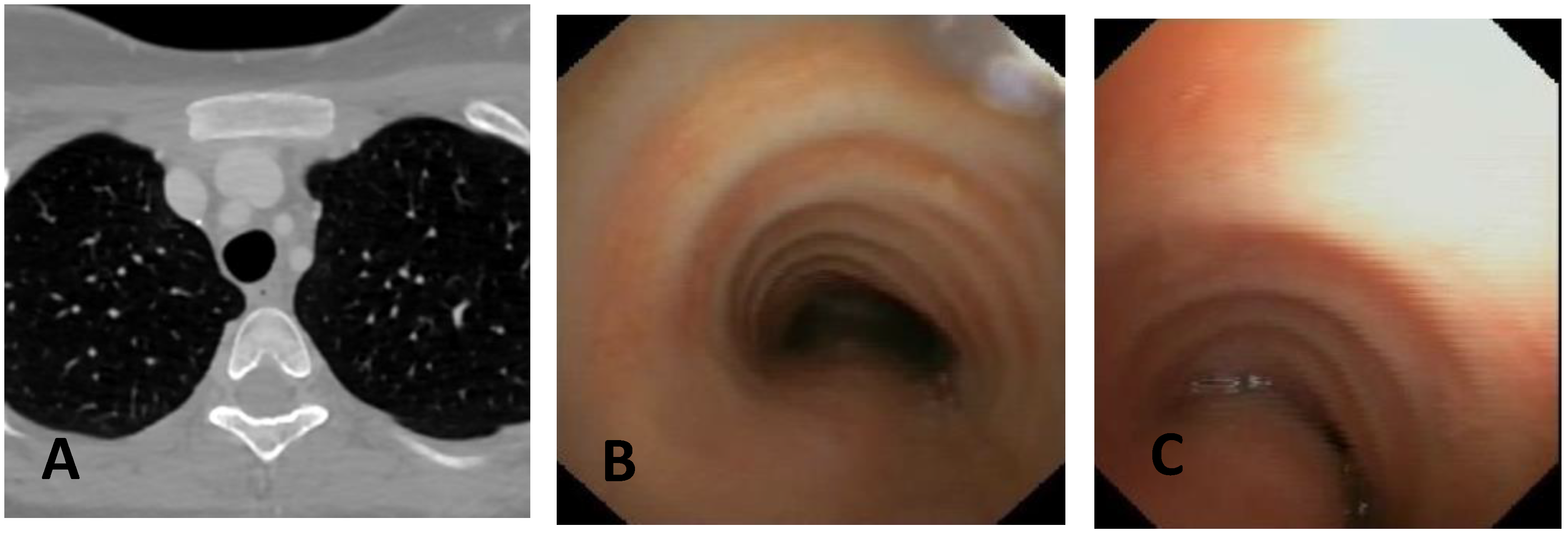
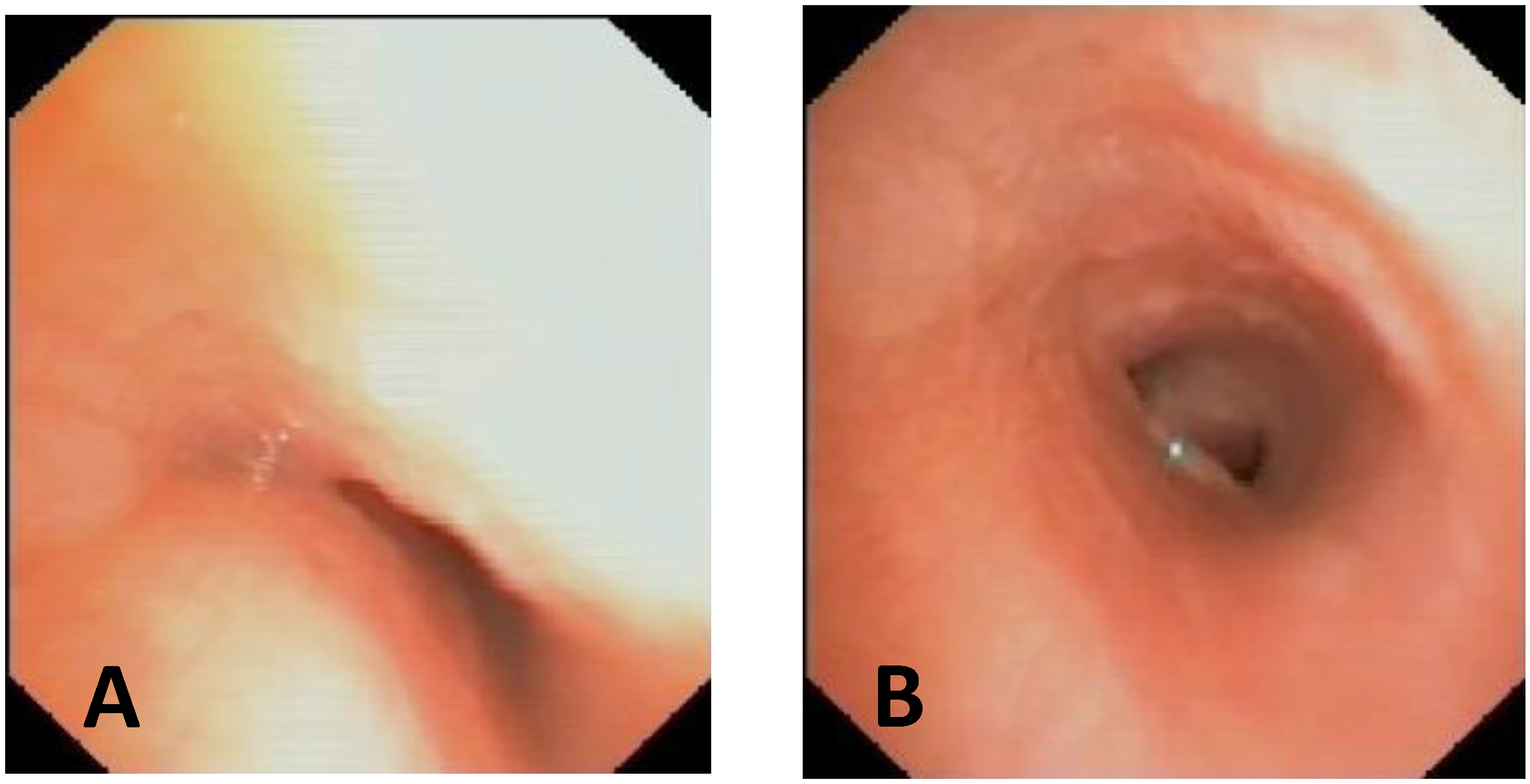
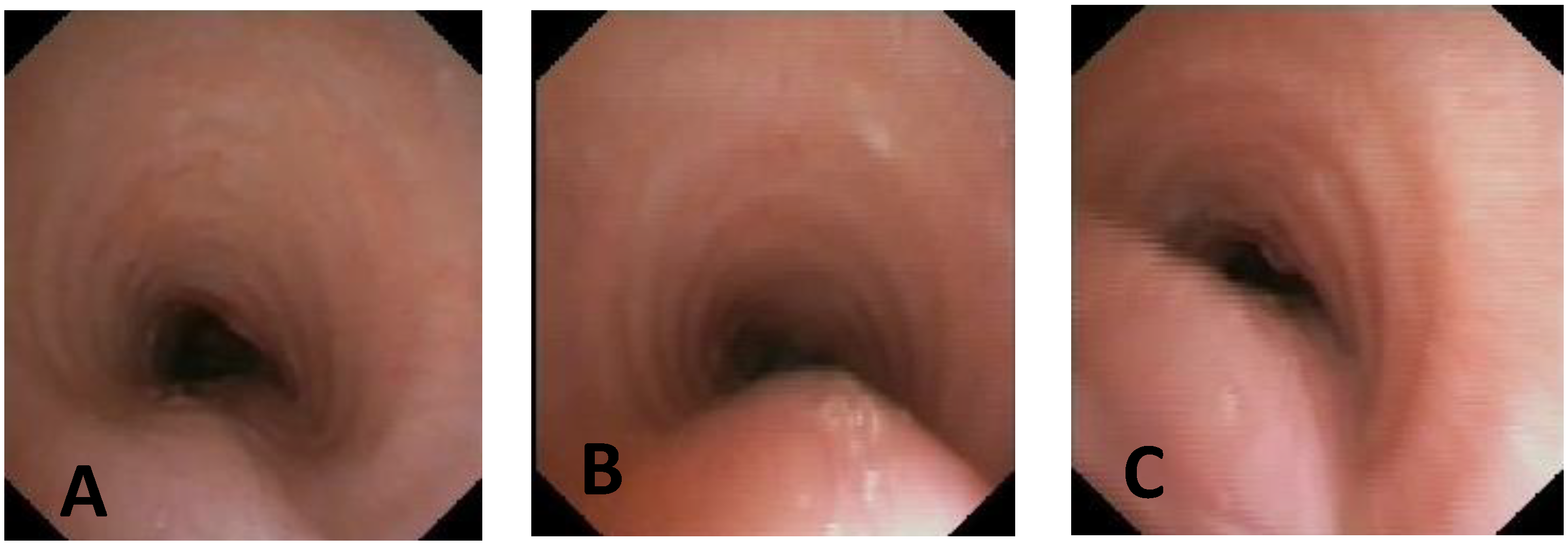
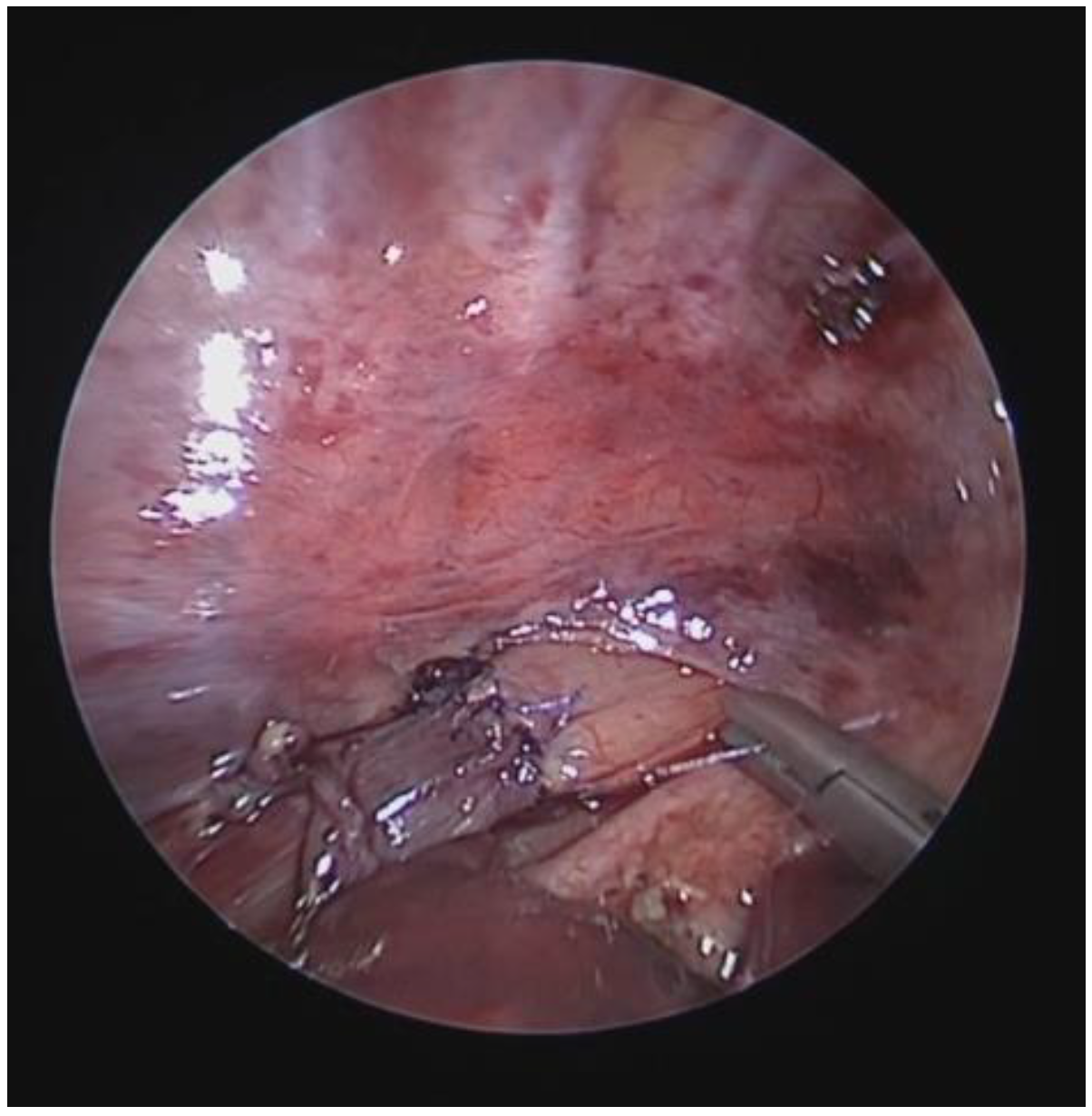
| Score: 0 | Pulsating extrinsic deformation on the anterior tracheal wall, reduction in the APD of the trachea of less than 50% compared to the suprastenotic tract, even during expiration. Good representation of the cartilaginous rings. Figure 9, score 0 |
| Score: 1 | Reduction in the tracheal APD between 50% and 75% compared to the suprastenotic tract, with an absence of contact between the anterior tracheal walls and the pars membranacea, even when the patient performs abdominal straining. Good representation of the cartilaginous rings. Figure 9, Score 1 |
| Score: 2 | Reduction in the APD of the trachea between 75% and 90% compared to the suprastenotic tract and/or anterior tracheal wall and pars membranacea, tending to touch, without complete closure of the lumen, even when the patient performs abdominal straining, with poor representation of the cartilaginous rings. Figure 1, score 2 |
| Score: 3 | Reduction in the APD of the trachea is already greater than 90% during the expiratory phase, when the tracheal lumen completely closes. Figure 9, score 3 |
4. Surgical Treatments
4.1. Introduction
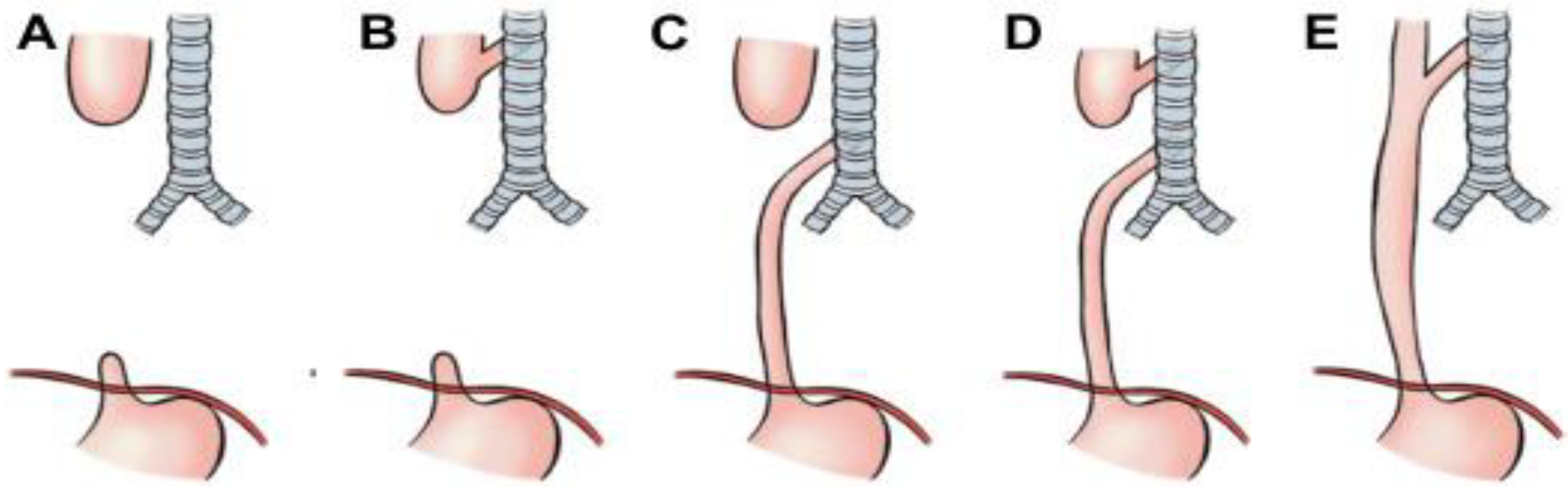
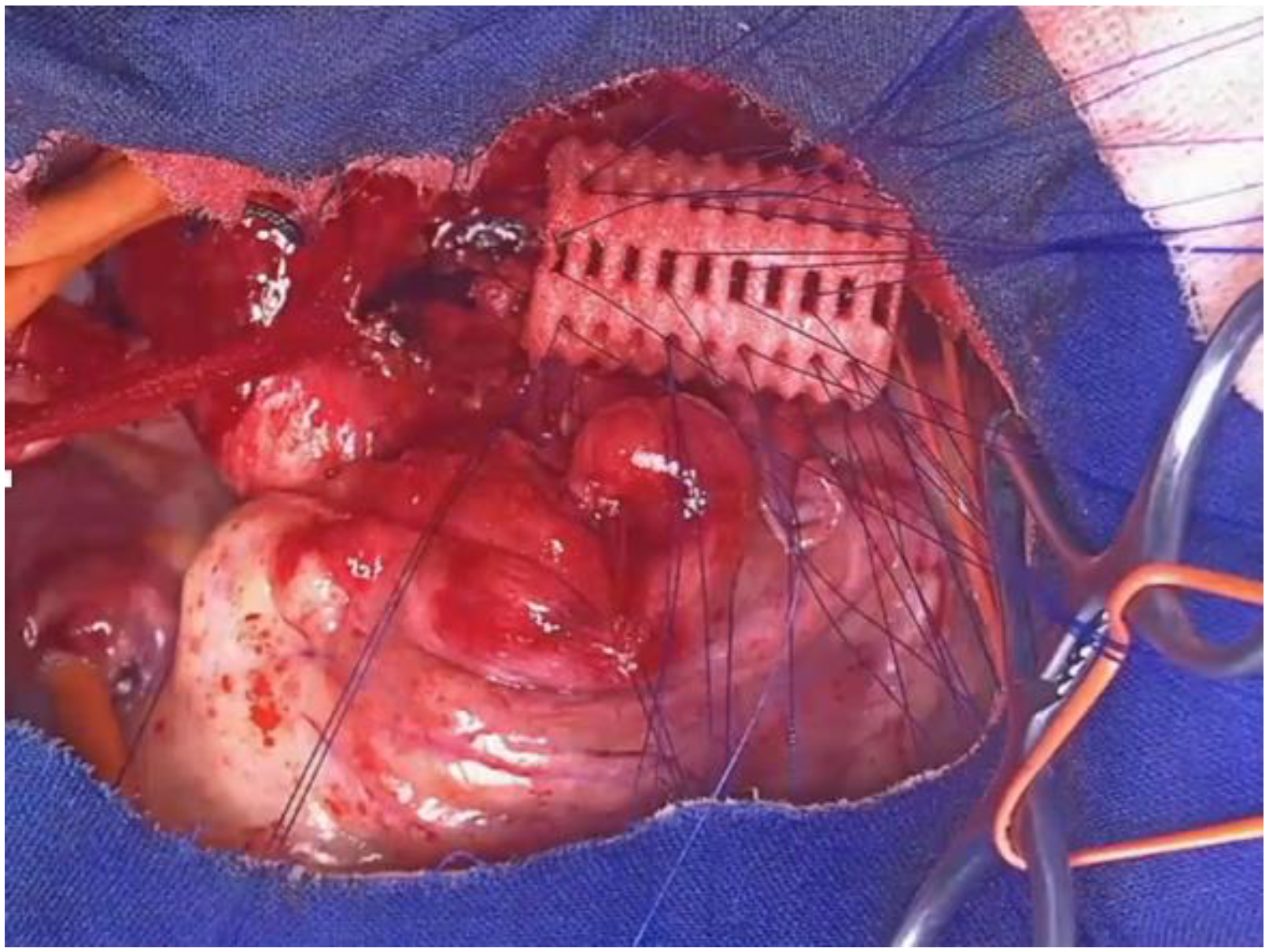
4.2. Open Anterior Aortopexy (OAA)
- A total of 131 patients;
- Age: 5.1 ± 4.2 years (range 0.2–16.39 years);
- Female: 32%, Male: 68%;
- TEF: 38 patients (29%);
- Comorbidities: cardiopathy, chromosomal abnormalities, autism, etc.: 37 patients (28.2%);
- A total of 107 patients (82%): Upper ministernotomy (split sternum);
- A total of 24 patients (18%): Right or left anterior thoracotomy.
- Mortality: zero;
- One major bleeding;
- Two pericardial effusions (percutaneous drainage);
- Three cases of chronic pericarditis;
- Two transient peripheral nerve injuries (phrenic nerve).
- A total of 109/131 patients (83%);
- Average follow-up months: 29.75 ± 27.17 (range 3.3–130.66).
- A total of 52% were asymptomatic;
- A total of 35% were mildly symptomatic;
- 1A total of 3% were still symptomatic.
- Increase in tracheal APD and disappearance of pulsatility: 85%;
- Unchanged diameters and pulsatility: 15%;
- Requiring second-stage treatment with PT: 7 patients (6.4%).
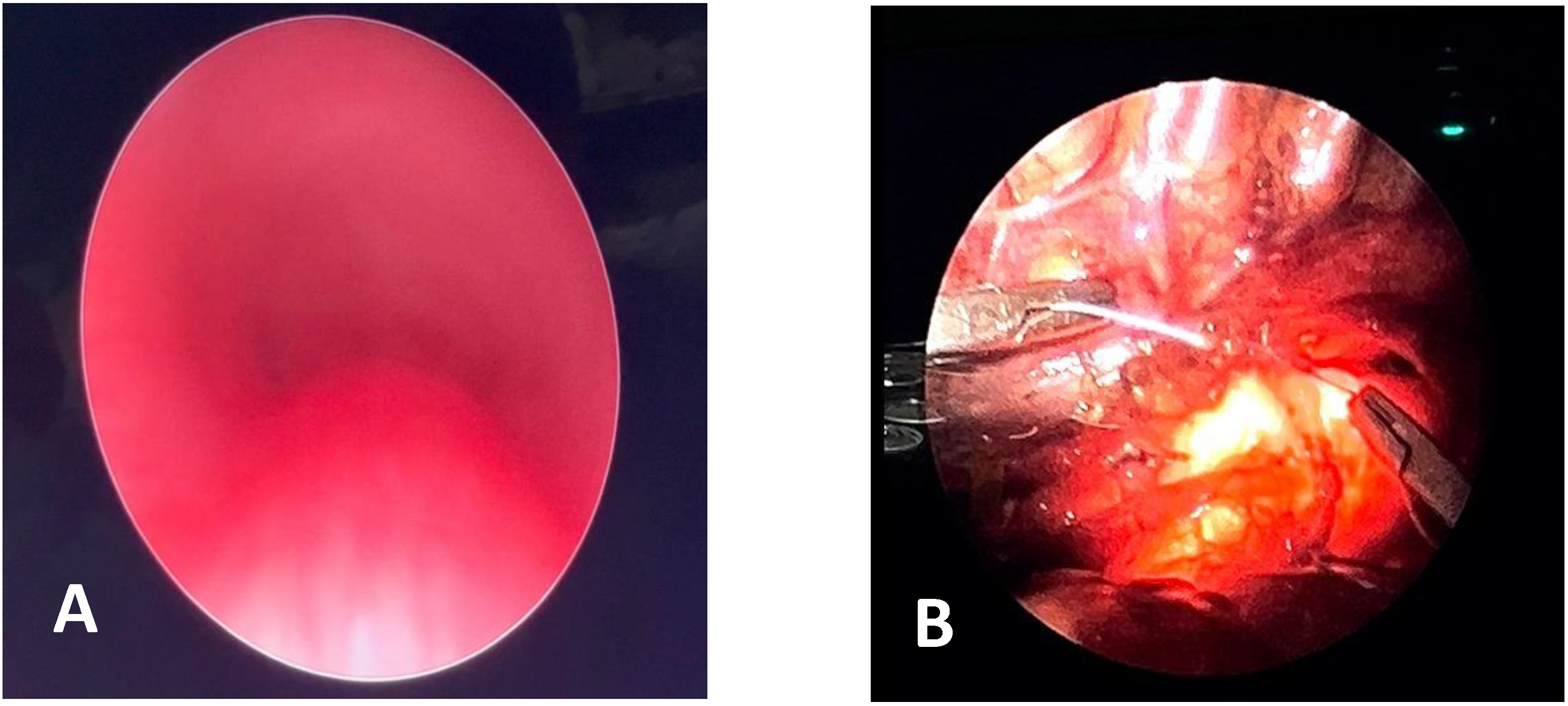
4.3. Thoracoscopic Anterior Aortopexy (TAA)
- Preliminary VBS;
- Supine position with left flank and shoulder elevated by 30°;
- Three trocars: 3 mm for patients <1 year; 5 mm for children >1 year;
- Opening of the mediastinal pleura;
- Thymectomy (possibly total);
- Isolation and lifting of the IA;
- Pericardiotomy;
- Non-absorbable sutures with Dacron pledgets on the aorta and IA;
- Pericardial flap;
- Retrieval of trans sternal sutures with “Suture Passer”;
- Retrosternal scarification;
- Tension and closure of the sutures subcutaneously;
- No drainage;
- VBS postoperative control.
- Pneumothorax and/or pleural effusion: 3%;
- Pulmonary atelectasis: 2.5%;
- Pericardial effusion: 2%;
- Phrenic nerve paralysis: 1.3%;
- Hemorrhage: 1%;
- Recurrence: 1%;
- Death: 6%.
4.4. Posterior Tracheopexy (PT)
- A total of 18 patients: 11 males, 7 females;
- Average age: 10.50 ± 5.57 years (range: 3.33–22.89 years);
- A total of 6 patients (33.3%): EA/TEF;
- A total of 1 patient: congenital diaphragmatic hernia (CDH);
- A total of 6 patients (33.3%) had previously undergone AA;
- A total of 1 patient: AA was performed simultaneously with the PT procedure;
- Average postoperative hospital stay for patients undergoing RATS was 8 ± 11 days (range: 3–16 days);
- Average follow-up of 10.23 ± 11.62 months (range: 1.41–40.62 months).
- Two patients: thoracic duct injury;
- One patient: dysphagia;
- One patient: esophageal perforation, treated with stent placement.
- Complete resolution of respiratory symptoms in 50% of cases (8/16);
- Clinical improvement in 43.75% (7/16);
- Stability in 6.25% (1/16);
- Overall benefit in 93.75%.
5. The Patient with Esophageal Atresia (EA) with/Without Tracheoesophageal Fistula (TEF)
5.1. The Severe TM in Patients with EA with/Without TEF
5.2. Indications for Consensual PT During Esophageal Recanalization in EA Patients
5.3. Endoscopic Treatment of Recurrent/Persistent TEF
- Endoscopic treatment for the closure of recurrent TEF is reliable and has few complications.
- The method with laser edge abrasion, followed by two local applications of TCA, has proven to be the most effective. The application time of TCA to the abraded edges of the TEF is variable and operator-dependent; we maintain a total time (two applications) of about 100 s.
- Treatment limitations include patient weight (not less than 4 kg) and the possibility of using a rigid bronchoscope of at least 3.5 mm diameter.
- Repeated applications (an average of four in the literature) are necessary to achieve closure; we wait about 20 days between sessions.
- So far, no limit has been set beyond which the treatment is considered ineffective.
6. Follow-Up of the Patient with TM
- Improvement of airway patency;
- Enhancement of mucociliary clearance;
- Prevention of recurrent respiratory infections and PBB;
- Reduction in the risk of lung damage;
- Improvement of long-term respiratory prognosis.
- VBS with bronchoalveolar lavage (BAL);
- Chest CT with or without CM;
- PFT: baseline and post-salbutamol;
- Exercise tests (walk test/cardiopulmonary exercise test);
- Nocturnal oximetry;
- Microbiological examination of sputum and/or BAL.
7. Respiratory Assistance in Patients with TM
7.1. The Tracheal Splint
7.2. The Tracheal Stent
- AA, in case of extrinsic vascular compression;
- PT, in case of hypermobility of the pars mebranacea;
- Positioning of external tracheal splint;
- Positioning of endoluminal stents, which we will talk about.
- Dislocation: to prevent it, the stent diameter must be about 2 mm larger than the trachea’s diameter.
- Growth of granulation tissue and/or scar tissue at the edges of the stent, which occurs due to (1) stagnation of secretions triggering chronic inflammatory phenomena and (2) chronic ischemic damage related to the larger diameter of the stent at its edges (about 1–2 mm compared to the stent central section).
- Stagnation of catarrhal secretions inside the stent: this occurs mainly with silicone- and nitinol-coated stents because the stent, covering the tracheal mucosa along its entire length, cancels ciliary activity/motility, causing catarrhal stagnation inside it. With resorbable PDS stents, this problem occurs less frequently as they are open-mesh devices that do not completely abolish ciliary activity. It is important to promote expectoration, keep the airways humid, and keep the secretions well hydrated.
- Nitinol Stent: Self-expanding, made of a thermoplastic metal alloy coated in silicone that is not covered by the tracheal mucosa, so the stent is not incorporated into the tracheal wall. It needs to be periodically replaced, according to the child’s growth curve.
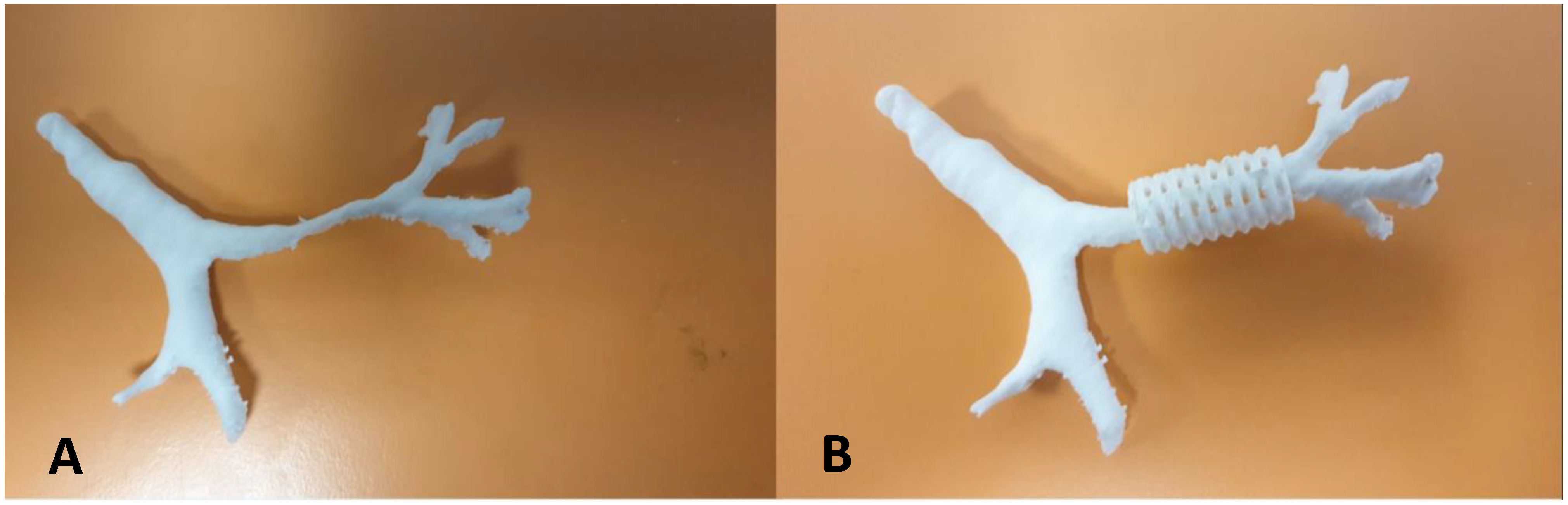
- Dumon Silicone Stent: Also self-expanding, but with a lower radial pressure than the nitinol stent. Currently, it is possible to produce a custom-made stent using a 3D printer, modeled after the measurement of the patient’s airways by CT scan.
- Stainless Steel Stent (BEMS): Usually pre-mounted on a balloon that needs to be inflated to dilate the device to its nominal size. This stent is not indicated for the treatment of TM because, while it is covered by the mucosa at the bronchial level and is no longer visible endoscopically after 4–6 months, it cannot be incorporated into the tracheal wall, and the high pressures to which the trachea is subjected can cause ovalization and, in the worst case, stent fracture/breakage.
- PDS (Polydioxanone) Stent entered the market. Being resorbable, they revolutionized tracheal stenting, replacing other types of stents [81,82]. Like the steel one, the PDS stent is a self-expanding open-mesh device, positioned with its own kit. It exerts radial support pressure for about 6–8 weeks, after which the resorption phase begins, lasting about another 8 weeks, occurring partly by lysis and partly by fragmentation. Therefore, it does not need to be removed, which is a great advantage as it avoids a potentially traumatic maneuver for the trachea, and the follow-up has been simplified and shortened in time. Another advantage is that, during the period of in situ permanence for about 8 + 8 weeks, it seems to help consolidate the tracheal wall with which it is in contact. PDS stents have a cost of up to about 3000 Euros, and this must be taken into account when planning conservative treatment. In conclusion, tracheal stenting, performed in centers specialized in advanced airway management, is a valid conservative treatment in the management of TM.
7.3. Indication for Decannulation in Tracheostomized Patients
- Identify the presence of other obstructive sites to take any further therapeutic measures.
- Evaluate the motility of the vocal cords (VC) and larynx.
- Assess the obstructive tracheal pathology and its resolution.
- The progressive reduction in the tracheal cannula’s diameter and temporary closure during the day under the caregiver’s close supervision;
- Respiratory monitoring by oximetry and polysomnography with the cannula closed even during sleep, which strongly indicates the possible removal of the tracheal cannula;
- Endoscopic re-evaluation of the airway, looking for any residual obstructive sites;
- The final multidisciplinary decision to decannulate the patient.
7.4. Indications for Prescribing N-Invasive Ventilation (NIV) in Patients with TM
8. Conclusions
- Each patient must be evaluated individually, in their own specificity.
- The patient must be taken care of by a multidisciplinary team: a Tracheal Team, which must include pulmonologists, thoracic surgeons, cardiologists, otolaryngologists, radiologists, gastroenterologists, and anesthesiologists for case-by-case therapeutic decisions, all experts in the care of TM patients.
- The clinical history and CT scan with CM contribute to the overall evaluation of the individual patient, and the role of DVBS is essential: the endoscopy must also include a phase in which the patient twists their abdomen and tries to cough, with a consequent increase in intrathoracic pressure. It can thus be verified whether the main pathology, causing tracheal malacia, affects the anterior or posterior tracheal wall; it is therefore possible to decide whether the patient will benefit from the anterior aortopexy operation, in case of extrinsic compression from an arterial vessel, or posterior tracheopexy, if there is an abnormal hypermobility of the pars membranacea.
- When TM is present and severe, but the patient cannot undergo surgical treatment, it is also essential that the patient can benefit from all other respiratory assistance aids: insertion of tracheal splint and stent, placement of tracheostomy, and, hopefully, once the surgical therapeutic process is completed and the obstruction at the lower airways level is resolved, address the decannulation process.
- We must also consider how, in recent decades, the increasingly widespread use of non-invasive ventilation (NIV) has made it increasingly less necessary to resort to tracheostomy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wallis, C.; Alexopoulou, E.; Antón-Pacheco, J.L.; Bhatt, J.M.; Bush, A.; Chang, A.B.; Charatsi, A.-M.; Coleman, C.; Depiazzi, J.; Douros, K.; et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur. Respir. J. 2019, 54, 1900382. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, R.; Huijsmans, S.H.; Pijnenburg, M.W.; Tiddens, H.A.; de Jongste, J.C.; Merkus, P.J. Tracheomalacia and bronchomalacia in children: Incidence and patient characteristics. Chest 2005, 128, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H.; Millqvist, E.; Bieksiene, K.; Birring, S.S.; Dicpinigaitis, P.; Domingo Ribas, C.; Hilton Boon, M.; Kantar, A.; Lai, K.; McGarvey, L.; et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 2020, 55, 1901136. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Silvestri, M.; Sacco, O.; Panigada, S.; Girosi, D.; Magnano, G.M.; Rossi, G.A. Mild tracheal compression by aberrant innominate artery and chronic dry cough in children. Pediatr. Pulmonol. 2016, 51, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Oppenheimer, J.J.; Irwin, R.S.; CHEST Expert Cough Panel. Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Marchant, J.M.; Masters, I.B.; Taylor, S.M.; Cox, N.C.; Seymour, G.J.; Chang, A.B. Evaluation and outcome of young children with chronic cough. Chest 2006, 129, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Robertson, C.F.; Van Asperen, P.P.; Glasgow, N.J.; Mellis, C.M.; Masters, I.B. A multicenter study on chronic cough in children: Burden and etiologies based on a standardized management pathway. Chest 2012, 142, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kompare, M.; Weinberger, M. Protracted bacterial bronchitis in young children: Association with airway malacia. J. Pediatr. 2012, 160, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wurzel, D.F.; Marchant, J.M.; Yerkovich, S.T.; Upham, J.W.; Mackay, I.M.; Brent Masters, I.; Chang, A.B. Prospective Characterization of Protracted Bacterial Bronchitis in Children. Chest 2015, 145, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santiago-Burruchaga, M.; Zalacain-Jorge, R.; Vazquez-Cordero, C. Are airways structural abnormalities more frequent in children with recurrent lower respiratory tract infections? Respir. Med. 2014, 108, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Keng, L.T.; Chang, C.J. All that wheezes is not asthma: Adult tracheomalacia resulting from innominate artery compression. Postgrad. Med. J. 2017, 93, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, J.C.; Zhao, Y.D.; Willis, E.B. Bronchoscopy findings in recurrent croup: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; D’Auria, E.; Farolfi, A.; Calcaterra, V.; Zenga, A.; De Silvestri, A.; Pelizzo, G.; Zuccotti, G.V. Airway Malacia: Clinical Features and Surgical Related Issues, a Ten-Year Experience from a Tertiary Pediatric Hospital. Children 2021, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro, N.; Sacco, O.; Torre, M.; Moscatelli, A.; Marasini, M.; Guerriero, V.; Gagliardi, L.; Sambuceti, V.; Rizzo, F. Tracheobronchography for pediatric airway disease is still a valuable technique? Minerva Pediatr. 2025, 77, 121–129. [Google Scholar] [CrossRef]
- Little, A.F.; Phelan, E.M.; Boldt, D.W.; Brown, T.C. Paediatric tracheobronchomalacia and its assessment by tracheobronchography. Australas. Radiol. 1996, 40, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, E. Hali, M. Radiologic diagnosis of tracheoesophageal fistula in children. Curr. Chall Thorac. Surg. 2022, 4, 25. [Google Scholar] [CrossRef]
- Douros, K.; Kremmydas, G.; Grammeniatis, V.; Papadopoulos, M.; Priftis, K.N.; Alexopoulou, E. Helical multi-detector CT scan as a tool for diagnosing tracheomalacia in children. Pediatr. Pulmonol. 2019, 54, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Masters, I.B.; Buntain, H.; Frawley, K.; Sarikwal, A.; Watson, D.; Ware, F.; Wuth, J.; Chang, A.B. A comparison of virtual bronchoscopy versus flexible bronchoscopy in the diagnosis of tracheobronchomalacia in children. Pediatr. Pulmonol. 2017, 52, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Kobayashi, T.; Morikawa, A.; Nakano, T.; Matsuda, S.; Sakai, T.; Shikada, M. Utility of virtual bronchoscopy in congenital tracheomalacia. J. Exp. Clin. Med. 2007, 32, 67–69. [Google Scholar] [PubMed]
- Koenigs, M.; Young, C.; Lillis, A.; Morrison, J.; Kelly, N.; Elmaraghy, C.; Krishnamurthy, R.; Chiang, T. Dynamic Volumetric Computed Tomography Angiography is an Effective Method to Evaluate Tracheomalacia in Children. Laryngoscope 2023, 133, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.P.; Ali, S.; Agarwal, A.; Matlock, D.N.; Sharma, M. Dynamic computed tomography for evaluation of tracheobronchomalacia in premature infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2023, 58, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Hysinger, E.B.; Bates, A.J.; Higano, N.S.; Benscoter, D.; Fleck, R.J.; Hart, C.K.; Burg, G.; De Alarcon, A.; Kingma, P.S.; Woods, J.C. Ultrashort Echo-Time MRI for the Assessment of Tracheomalacia in Neonates. Chest 2020, 157, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Boonjindasup, W.; Marchant, J.M.; McElrea, M.S.; Yerkovich, S.T.; Thomas, R.J.; Masters, I.B.; Chang, A.B. Pulmonary function of children with tracheomalacia and associated clinical factors. Pediatr. Pulmonol. 2022, 57, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Kusak, B.; Cichocka-Jarosz, E.; Jedynak-Wąsowicz, U.; Lis, G. Pulmonary function tests leading to the diagnosis of vascular malformations in school-aged children. Adv. Respir. Med. 2017, 85, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Shieh, H.F.; Smithers, C.J.; Hamilton, T.E.; Zurakowski, D.; Rhein, L.M.; Manfredi, M.A.; Baird, C.W.; Jennings, R.W. Posterior tracheopexy for severe tracheomalacia. J. Pediatr. Surg. 2017, 52, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, L.; Wine, T.; Prager, J.; Masaracchia, M.; Janosy, N.; Polaner, D.; DeBoer, E.; Somme, S. Thoracoscopic Posterior Tracheopexy Is a Feasible and Effective Treatment for Tracheomalacia. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Snijders, D.; Barbato, A. An Update on Diagnosis of Tracheomalacia in Children. Eur. J. Pediatr. Surg. 2015, 25, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Gaurav, K.; Sanchez, J.M.; Berger, R.L.; Folch, E.; Fernandez-Bussy, S.; Ernst, A.; Gangadharan, S.P. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy. A pilot study. Ann. Am. Thorac. Soc. 2014, 11, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Foran, A.; Phinizy, P.; Biko, D.M.; Piccione, J.C.; Rapp, J.B. Dynamic airway computed tomography and flexible bronchoscopy for diagnosis of tracheomalacia in children: A comparison study. Pediatr. Pulmonol. 2024, 59, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Mastouri, M.; Boboli, H.; Demarche, M.; Brandt, H.; Moonen, V.; Seghaye, M.C.; Kempeneers, C. Treatment of tracheo (broncho) malacia in children. Rev. Med. Liege 2021, 76, 145–151. [Google Scholar] [PubMed]
- Mukharesh, L.; Krone, K.A.; Hamilton, T.E.; Shieh, H.F.; Smithers, C.J.; Winthrop, Z.A.; Muise, E.D.; Jennings, R.W.; Mohammed, S.; Demehri, F.R.; et al. Outcomes of surgical treatment of tracheobronchomalacia in children. Pediatr. Pulmonol. 2024, 59, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.E. Arterial Malformations which Cause Compression of the Trachea or Esophagus. Circulation 1955, 11, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Rijnberg, F.M.; Butler, C.R.; Bieli, C.; Kumar, S.; Nouraei, R.; Asto, J.; McKavanagh, E.; de Coppi, P.; Muthialu, N.; Elliott, M.J.; et al. Aortopexy for the treatment of tracheobronchomalacia in 100 children: A 10-year single-centre experience. Eur. J. Cardiothorac. Surg. 2018, 54, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Santoro, F.; Ribera, E.; Magnano, G.M.; Rossi, G.A. Short-length ligamentum arteriosum as a cause of congenital narrowing of the left main stem bronchus. Pediatr. Pulmonol. 2016, 51, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Kamran, A.; Jennings, R.W. Tracheomalacia and Tracheobronchomalacia in Pediatrics: An Overview of Evaluation, Medical Management, and Surgical Treatment. Front. Pediatr. 2019, 7, 512. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, D.C.; Bax, N.M.A. Thoracoscopic tracheoaortopexia for the treatment of life-threatening events in tracheomalacia. Surg. Endosc. 2007, 21, 2024–2025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perger, L.; Kim, H.B.; Jaksic, T.; Jennings, R.W.; Linden, B.C. Thoracoscopic aortopexy for treatment of tracheomalacia in infants and children. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19 (Suppl. S1), S249–S254. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.; Maughan, E.; Pianosi, K.; Jama, G.; Rouhani, M.J.; Hewitt, R.; Muthialu, N.; Butler, C.; De Coppi, P. Open and Thoracoscopic Aortopexy for Airway Malacia in Children: 15 Year Single Centre Experience. J. Pediatr. Surg. 2024, 59, 197–201. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, D.C.; Straver, M. Thoracoscopic aortopexy for tracheomalacia. World J. Surg. 2015, 39, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lawal, T.A.; Gosemann, J.H.; Kuebler, J.F.; Glüer, S.; Ure, B.M. Thoracoscopy versus thoracotomy improves midterm musculoskeletal status and cosmesis in infants and children. Ann. Thorac. Surg. 2009, 87, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Bastard, F.; Bonnard, A.; Rousseau, V.; Gelas, T.; Michaud, L.; Irtan, S.; Piolat, C.; Ranke-Chrétien, A.; Becmeur, F.; Dariel, A.; et al. Thoracic skeletal anomalies following surgical treatment of esophageal atresia. Lessons from a national cohort. J. Pediatr. Surg. 2018, 53, 605–609. [Google Scholar] [CrossRef]
- Macchini, F.; Leva, E.; Gentilino, V.; Morandi, A.; Rothenberg, S.S. Mentoring in Pediatric Thoracoscopy: From Theory to Practice. Front. Pediatr. 2021, 9, 630518. [Google Scholar] [CrossRef]
- Jennings, R.W.; Hamilton, T.E.; Smithers, C.J.; Ngerncham, M.; Feins, N.; Foker, J.E. Surgical approaches to aortopexy for severe tracheomalacia. J. Pediatr. Surg. 2014, 49, 66–70; discussion 70–71. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Carlucci, M.; Speggiorin, S.; Elliott, M.J. Aortopexy for the treatment of tracheomalacia in children: Review of the literature. Ital. J. Pediatr. 2012, 38, 62. [Google Scholar] [CrossRef] [PubMed]
- Polites, S.F.; Kotagal, M.; Wilcox, L.J.; de Alarcon, A.; Benscoter, D.T.; von Allmen, D. Thoracoscopic posterior tracheopexy for tracheomalacia: A minimally invasive technique. J. Pediatr. Surg. 2018, 53, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Durkin, N.; De Coppi, P. Anatomy and embryology of tracheo-esophageal fistula. Semin. Pediatr. Surg. 2022, 31, 151231. [Google Scholar] [CrossRef] [PubMed]
- Brosens, E.; Ploeg, M.; van Bever, Y.; Koopmans, A.E.; IJsselstijn, H.; Rottier, R.J.; Wijnen, R.; Tibboel, D.; de Klein, A. Clinical and etiological heterogeneity in patients with tracheo-esophageal malformations and associated anomalies. Eur. J. Med. Genet. 2014, 57, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Triglia, J.M.; Guys, J.M.; Louis-Borrione, C. Tracheomalacia caused by arterial compression in esophageal atresia. Ann. Otol. Rhinol. Laryngol. 1994, 103, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Mirra, V.; Maglione, M.; Di Micco, L.L.; Montella, S.; Santamaria, F. Longitudinal Follow-up of Chronic Pulmonary Manifestations in Esophageal Atresia: A Clinical Algorithm and Review of the Literature. Pediatr. Neonatol. 2017, 58, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, S.; Sfeir, R.; Rousseau, V.; Bonnard, A.; Gelas, T.; Aumar, M.; Panait, N.; Rabattu, P.Y.; Irtan, S.; Fouquet, V.; et al. Esophageal Atresia and Respiratory Morbidity. Pediatrics 2021, 148, e2020049778. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.E.; Ladd, W.E. The Surgery of Infancy and Childhood: Its Principles and Techniques; WB Saunders: Philadelphia, PA, USA, 1953. [Google Scholar]
- Yang, S.; Yang, R.; Ma, X.; Yang, S.; Peng, Y.; Tao, Q.; Chen, K.; Tao, J.; Zhang, Y.; Du, J.; et al. Detail correction for Gross classification of esophageal atresia based on 434 cases in China. Chin. Med. J. 2022, 135, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, S.H.A.J.; van Herwaarden-Lindeboom, M.Y.A.; van Tuyll van Serooskerken, E.S.; van der Zee, D.C. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair: A new approach to prevent tracheomalacia complications. J. Pediatr. Surg. 2018, 53, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Kamran, A.; Izadi, S.; Visner, G.; Frain, L.; Demehri, F.R.; Shieh, H.F.; Jennings, R.W.; Smithers, C.J.; Zendejas, B. Primary Posterior Tracheopexy at Time of Esophageal Atresia Repair Significantly Reduces Respiratory Morbidity. J. Pediatr. Surg. 2024, 59, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Dong, J.; Li, B.; Li, M.; Zou, C.; Xiao, Y.; Xu, G.; Li, B. Effects of primary posterior tracheopexy in thoracoscopic repair of esophageal atresia. Heliyon 2023, 9, e15931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Tuyll van Serooskerken, E.S.; Tytgat, S.H.A.J.; Verweij, J.W.; Bittermann, A.J.N.; Coenraad, S.; Arets, H.G.M.; van der Zee, D.C.; Lindeboom, M.Y.A. Primary Posterior Tracheopexy in Esophageal Atresia Decreases Respiratory Tract Infections. Front. Pediatr. 2021, 9, 720618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shieh, H.F.; Smithers, C.J.; Hamilton, T.E.; Zurakowski, D.; Visner, G.A.; Manfredi, M.A.; Baird, C.W.; Jennings, R.W. Posterior Tracheopexy for Severe Tracheomalacia Associated with Esophageal Atresia (EA): Primary Treatment at the Time of Initial EA Repair versus Secondary Treatment. Front. Surg. 2018, 4, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasui, A.; Hinoki, A.; Amano, H.; Shirota, C.; Tainaka, T.; Sumida, W.; Yokota, K.; Makita, S.; Okamoto, M.; Takimoto, A.; et al. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair ameliorate tracheomalacia in neonates: A single-center retrospective comparative cohort study. BMC Surg. 2022, 22, 285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Samarrai, A.Y.; Jessen, K.; Haque, K. Endoscopic obliteration of a recurrent tracheoesophageal fistula. J. Pediatr. Surg. 1987, 22, 993. [Google Scholar] [CrossRef] [PubMed]
- Tobia, A.; Luque, C.G.; Leitmeyer, K.; Dorling, M.; Chadha, N.K. Endoscopic treatment in pediatric patients with recurrent and H-type tracheoesophageal fistulas—A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2023, 168, 111541. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.F.; Maltezeanu, A.; Laberge, J.M.; Kaspy, K.; Sant’Anna, A.; Broucqsault, H.; Fayoux, P.; Daniel, S.J. Endoscopic repair of tracheoesophageal fistulas: A contemporary multi-institutional case series and literature review. Int. J. Pediatr. Otorhinolaryngol. 2024, 181, 111960. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Valfré, L.; Aufiero, L.R.; Dall’Oglio, L.; De Angelis, P.; Villani, A.; Bagolan, P.; Bottero, S.; Cutrera, R. Respiratory problems in children with esophageal atresia and tracheoesophageal fistula. Ital. J. Pediatr. 2017, 43, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koumbourlis, A.C.; Belessis, Y.; Cataletto, M.; Cutrera, R.; DeBoer, E.; Kazachkov, M.; Laberge, S.; Popler, J.; Porcaro, F.; Kovesi, T. Care recommendations for the respiratory complications of esophageal atresia-tracheoesophageal fistula. Pediatr. Pulmonol. 2020, 55, 2713–2729. [Google Scholar] [CrossRef]
- Dingemann, C.; Eaton, S.; Aksnes, G.; Bagolan, P.; Cross, K.M.; De Coppi, P.; Fruithof, J.; Gamba, P.; Husby, S.; Koivusalo, A.; et al. ERNICA Consensus Conference on the Management of Patients with Esophageal Atresia and Tracheoesophageal Fistula: Follow-up and Framework. Eur. J. Pediatr. Surg. 2020, 30, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Farje, D.; Young, A.; Stein, E.; Eltayeb, O.M.; Ghadersohi, S.; Hazkani, I. Persistence of aerodigestive symptoms after vascular ring repair. Am. J. Otolaryngol. 2024, 45, 104147. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, N.; Secinaro, A.; Menchini, L.; Caggiano, S.; Verrillo, E.; Santangelo, T.P.; Cutrera, R.; Tomà, P. Dynamic expiratory CT: An effective non-invasive diagnostic exam for fragile children with suspected tracheo-bronchomalacia. Pediatr. Pulmonology 2018, 53, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Boonjindasup, W.; Marchant, J.M.; McElrea, M.S.; Yerkovich, S.T.; Thomas, R.J.; Masters, I.B.; Chang, A.B. The ‘knee’ pattern in spirometry flow-volume curves in children: Does it relate to tracheomalacia? Respir. Med. 2022, 204, 107029. [Google Scholar] [CrossRef] [PubMed]
- Chengli, Q.; Li, X. Preliminary Study of CPET in Patients with Central Airway Obstruction. Chest 2016, 150, 1116A. [Google Scholar] [CrossRef]
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, M.; Bush, A.; Chang, A.; Prifits, K.N.; Wallis, C.; Bhatt, J.M. The role of the pediatrician in caring for children with tracheobronchomalacia. Expert Rev. Respir. Med. 2020, 14, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Vasko, J.S.; Ahn, C. Surgical management of secondary tracheomalacia. Ann. Thorac. Surg. 1968, 6, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Filler, D.; Fritz, K.W.; Jander, R. Alloplastic tracheal prosthesis with biocarbone. A study of animal experiments. Langenbecks Arch. Chir. 1980, 350, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Nagase, Y.; Hasegawa, H.; Takahashi, Y. External stenting: A reliable technique to relieve airway obstruction in small children. J. Thorac. Cardiovasc. Surg. 2017, 153, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of Tracheobronchomalacia with 3D-Printed Personalized Medical Devices in Pediatric Patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [PubMed]
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Mahani, M.G.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.J.; Green, G.E. Department 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Flanagan, C.L.; Zopf, D.A.; Morrison, R.J.; Nasser, H.; Patel, J.J.; Ebramzadeh, E.; Sangiorgio, S.N.; Wheeler, M.B. Design control for clinical translation of 3D printed modular scaffolds. Ann. Biomed. Eng. 2015, 43, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Hong Tran, T.M.; Ghori, U.K.; Musani, A.I. Pediatric Interventional Pulmonology. Clin. Chest Med. 2018, 39, 229–238. [Google Scholar] [CrossRef]
- Varela, P.; Torre, M.; Schweiger, C.; Nakamura, H. Congenital tracheal malformations. Pediatr. Surg. Int. 2018, 34, 701–713. [Google Scholar] [CrossRef]
- Antón-Pacheco, J.L. Tracheobronchial stents in children. Semin. Pediatr. Surg. 2016, 25, 179–185. [Google Scholar] [CrossRef]
- Serio, P.; Fainardi, V.; Leone, R.; Baggi, R.; Grisotto, L.; Biggeri, A.; Mirabile, L. Tracheobronchial obstruction: Follow-up study of 100 children treated with airway stenting. Eur. J. Cardiothorac. Surg. 2014, 45, e100–e109. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Nelson, K.D.; Eberhart, R.C. Mechanical properties and in vitro degradation of bioresorbable fibers and expandable fiber-based stents. J. Biomed Mater. Res. B Appl. Biomater. 2005, 74, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Stramiello, J.A.; Mohammadzadeh, A.; Ryan, J.; Brigger, M.T. The role of bioresorbable intraluminal airway stents in pediatric tracheobronchial obstruction: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110405. [Google Scholar] [CrossRef] [PubMed]
- Barreto, C.G.; Rombaldi, M.C.; Holanda, F.C.; Lucena, I.S.; Isolan, P.M.S.; Jennings, R.; Fraga, J.C. Surgical treatment for severe pediatric tracheobronchomalacia: The 20-year experience of a single center. J. Pediatr. 2024, 100, 250–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawlor, C.; Smithers, C.J.; Hamilton, T.; Baird, C.; Rahbar, R.; Choi, S.; Jennings, R. Innovative management of severe tracheobronchomalacia using anterior and posterior tracheobronchopexy. Laryngoscope 2020, 130, E65–E74. [Google Scholar] [CrossRef] [PubMed]
- McNamara, V.M.; Crabbe, D.C.G. Tracheomalacia. Paediatr. Respir. Rev. 2004, 5, 147–154. [Google Scholar] [CrossRef]
- Ulrike, F.; Mark, M.; Heike, S. Dysphagic Patients with Tracheotomies: A Multidisciplinary Approach to Treatment and Decannulation Management. Dysphagia 2007, 22, 20–29. [Google Scholar] [CrossRef]
- Fiz, I.; Torre, M.; D’Agostino, R.; Rüller, K.; Fiz, F.; Sittel, C.; Burghartz, M. Übersichtsarbeit zur chirurgischen Behandlung des suprastomalen Kollapses bei tracheotomierten Kindern [Review of surgical treatment of suprastomal collapse in tracheostomised children]. HNO 2024, 72, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, M.D.; Peterson, J.; Kanotra, S.P. Ultrasound-Guided Suture Lateralization in Pediatric Bilateral Vocal Fold Immobility. Laryngoscope 2020, 130, E941–E944. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, C.; Installe, S.; Boudewyns, A.; Van Hoorenbeeck, K.; Verhulst, S.L. The Optiflow™ interface for chronic CPAP use in children. Sleep Med. 2018, 44, 1–3. [Google Scholar] [CrossRef]
- Piper, A.J.; Moran, F.M. Non-invasive ventilation and the physiotherapist: Current state and future trends. Phys. Ther. Rev. 2006, 11, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bedi, P.K.; Castro-Codesal, M.L.; Featherstone, R.; AlBalawi, M.M.; Alkhaledi, B.; Kozyrskyj, A.L.; Flores-Mir, C.; MacLean, J.E. Long-term Non-Invasive Ventilation in Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio, A.; Ghezzi, M.; Rizzo, F.; Del Greco, P.; Perri, K.; Antonelli, F.; Gallizia, A.; Santoro, F.; Ribera, E.; Macchini, F.; et al. Diagnosis, Treatment, and Follow-Up of Tracheo/Bronchomalacia in Children: The Italian Multicenter Experience. Children 2025, 12, 1511. https://doi.org/10.3390/children12111511
Florio A, Ghezzi M, Rizzo F, Del Greco P, Perri K, Antonelli F, Gallizia A, Santoro F, Ribera E, Macchini F, et al. Diagnosis, Treatment, and Follow-Up of Tracheo/Bronchomalacia in Children: The Italian Multicenter Experience. Children. 2025; 12(11):1511. https://doi.org/10.3390/children12111511
Chicago/Turabian StyleFlorio, Angelo, Michele Ghezzi, Francesca Rizzo, Paolo Del Greco, Katia Perri, Fabio Antonelli, Annalisa Gallizia, Francesco Santoro, Elena Ribera, Francesco Macchini, and et al. 2025. "Diagnosis, Treatment, and Follow-Up of Tracheo/Bronchomalacia in Children: The Italian Multicenter Experience" Children 12, no. 11: 1511. https://doi.org/10.3390/children12111511
APA StyleFlorio, A., Ghezzi, M., Rizzo, F., Del Greco, P., Perri, K., Antonelli, F., Gallizia, A., Santoro, F., Ribera, E., Macchini, F., Torre, M., Donati, F., Lena, F., Guerriero, V., Borgia, P., Gentilino, V., D’Agostino, R., Porcaro, F., Conte, A., ... Sacco, O. (2025). Diagnosis, Treatment, and Follow-Up of Tracheo/Bronchomalacia in Children: The Italian Multicenter Experience. Children, 12(11), 1511. https://doi.org/10.3390/children12111511






