Abstract
Introduction: Trace metals can negatively impact biological functions and brain development. Cognitive and neurobehavioral disorders in children are poorly documented in Haut-Katanga Province, an area with significant and multiple exposures to trace metals that can lead to the exacerbation of cognitive and behavioral disorders. Objective: This study aimed to characterize the behavior of schoolchildren linked to their cognitive performance in urban and rural environments. Methods: A cross-sectional pilot study was conducted on 52 schoolchildren aged 6 to 11 (22 from rural areas presumed less exposed to metals and 30 from Lubumbashi, DRC). This study employed NEPSY-II tests, the Strengths and Difficulties Questionnaire (SDQ-Tutor), ENA 2020 software and trace metal spectrometry assays. Statistical tests were carried out with SPSS-20 and Stata-18. Results: Our findings revealed a correlation between children’s malnutrition and low mother’s education. The “total difficulties score” was more prevalent in rural areas (73%) compared to urban settings (37%) p < 0.05), in contrast to the “negative impact of difficulties” (59% versus 57%, p > 0.05). Urban children demonstrated superior cognitive performance, particularly in “facial affect recognition” (8 versus 4, p = 0.013) and “inhibitory control” (6.5 versus 3, p = 0.032). As-U(urine), Cd-B(blood), Hg-B, Mo-U, Ni-U, Pb-U, Pb-B and Sb-U were elevated compared to references. In general, urban areas had higher metal levels than rural areas. Blood and urine metals showed a complex and significant relationship with behavioral difficulties or cognitive performance. Conclusions: The observed behavioral issues, cognitive performance deficits and their association with nutritional deficiencies and trace metal exposure suggest a multifactorial neurodevelopmental origin. These findings highlight the need for further research in the region.
1. Introduction
The Haut-Katanga province is part of the Copperbelt region, located on the border between northern Zambia and the southern Democratic Republic of Congo (DRC). This region is globally known for its copper and cobalt mining industries. However, the poor regulation of mining activities has been identified as a major source of environmental pollution and human exposure to trace metals (TMs) in the area [1]. Acute and chronic exposures to trace metals can negatively impact biological functions and brain development, leading to adverse effects on the nervous, skeletal, endocrine, immune and circulatory systems [2].
Epidemiological studies conducted in Lubumbashi [3,4] have reported nutritional and cognitive impairments among children exposed to trace metals. Similarly, research in the former Katanga province has identified contaminated vegetables, fish and dust ingestion as the primary sources of trace metals in children [5]. Several factors contribute to children’s vulnerability, including their tendency to play on the ground and put objects in their mouths, their higher food intake per unit of body weight and their faster absorption rates compared to adults. Additionally, their nervous and immune systems are still maturing [6,7].
Approximately 5% to 15% of children worldwide are affected by developmental disorders [8]. A study conducted in six sub-Saharan African countries, including Uganda, Nigeria, South Africa, Ethiopia, the DRC and Kenya, reported a prevalence of behavioral disorders in children ranging from 12% to 33% [9]. In the nervous system, trace metal accumulation disrupts metabolism, causing behavioral, socio-emotional and cognitive disorders [2]. For example, exposure to lead has been shown to impair cognitive function and contribute to various neurodevelopmental disorders [10]. In the DRC, studies have described children’s behavioral disorders associated with lead [11], zinc and copper [12] and cognitive impairment related to exposure to multiple TMs in the Haut-Katanga province [4].
Environmental exposure to metals and neurotoxicants, as well as malnutrition, is likely to affect children’s behavior before cognitive disorders manifest. Given the reported cognitive impairments in school-aged children in Haut-Katanga, an area heavily impacted by metal pollution, it is important to investigate whether behavioral disorders are associated with these cognitive deficits.
This study aimed to assess the socio-emotional, behavior and cognitive performances of school-aged children in metal-polluted areas of Haut-Katanga.
To our knowledge, this is the first study in the region to combine behavioral, socio-emotional and cognitive assessments with the biomonitoring of trace metals, thereby providing a unique perspective on children’s health outcomes. This study further strengthens its originality by comparing two distinct groups of school-aged children—those from metal-polluted areas and those from less exposed areas—allowing for a more robust evaluation of the impact of environmental exposure on child development.
2. Methods
2.1. Study Design and Setting
This cross-sectional pilot study, conducted between April and July 2019, included school-aged children (6 to 11 years old) who were selected through convenience sampling from two areas: Lubumbashi, an urban area known for high levels of pollution (urban area, UA), and Kasongo village, a rural control area located approximately 50 km from Lubumbashi (rural area, RA).
Children were eligible if they had attended the same school for at least two years before the survey, to ensure their long stay in that environment. This was convenience sampling. The rural comparison group was recruited using the same criteria as the urban group, based on maternal and paternal age at childbirth, as well as maternal education level, to be considered as potential confounding factors in interpretation of results. Written consent was obtained from parents, and oral assent was obtained from children.
Due to logistical constraints and the high cost of analysis, consent was obtained for 32 children from the urban area and 25 from the rural area. However, three children from the rural area and two from the urban area were excluded due to a history of epilepsy, resulting in a final sample of 52 participants (30 from the urban area and 22 from the rural area) (Figure 1).
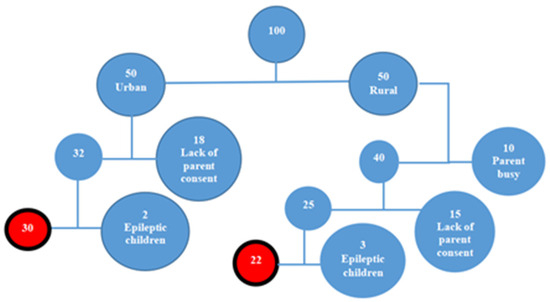
Figure 1.
Schematic conception of child recruitment.
2.2. Data Collection
2.2.1. Socio-Demographic Characteristics
A standardized questionnaire was used to collect socio-demographic information for all children and their parents. Each child’s nutritional status was also assessed using ENA 2020 for SMART [13], which focuses on age, weight and height.
2.2.2. Behavioral Assessment
We administered the tutor version of the Strengths and Difficulties Questionnaire (SDQ-Tutor) to assess emotional symptoms, conduct problems, hyperactivity, peer relationship issues and prosocial behavior [14]. The SDQ contains 25 items divided into five scales, each scored on a 0–10 scale. Based on these scores, behavior was classified as “normal,” “borderline” or “abnormal.” This study dichotomized the results into “normal” and “behavioral problems” to facilitate analysis. The latter combined both borderline and abnormal categories.
2.2.3. Neurocognitive Assessment
We used the French version of the NEPSY-II [15] to assess children’s cognitive performance across six domains: (1) attention and executive function, (2) memory and learning, (3) language, (4) visuospatial processing, (5) sensorimotor function and (6) social perception. Multiple scores were calculated for each test, and calibrated scores were obtained for each child, as described in the NEPSY-II documentation [16].
We acknowledged that NEPSY-II norms are not adapted to the Congolese context, which may influence cross-group comparisons.
2.2.4. Sample Collection and Analysis
Urine samples were collected in 40 mL polystyrene bottles. Blood samples (4 mL) were taken from the brachial vein using BD Vacutainer® K2EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA). The toxicology laboratory team from the University of Lubumbashi collected samples, kept them frozen at −20 °C and then transferred them to Leuven, Belgium, where they were stored at −80 °C until analysis.
All samples were analyzed at the Leuven Centre for Applied Toxicology and Pharmacology using inductively coupled plasma mass spectrometry (ICP-MS) for metal detection. Eight elements were measured in blood [17], and twenty-five elements were measured in urine [18].
International pediatric reference values from Belgium and Canada were used due to lack of local norms [19,20].
2.3. Statistical Analysis
Initial analyses included comparisons of socio-demographic, behavioral and cognitive characteristics between the two study groups using Student’s t-tests, Mann–Whitney U tests, median and geometric mean. Spearman’s correlation coefficients were calculated to assess the relationships between age, nutritional status and behavioral and cognitive scores. Statistical significance was set at p ≤ 0.05.
Effect sizes and 95% confidence intervals for the main comparisons were used to improve interpretation, but this study did not adjust for multiple testing due to its pilot nature, and as such all associations should be considered exploratory.
2.4. Ethical Considerations
The study protocol was approved by the Medical Ethics Committee of the University of Lubumbashi (UNILU), under approval number UNILU/CEM/036/2019.
3. Results
3.1. General Characteristics (Table 1)

Table 1.
Socio-demographic characteristics.
Among the children in the urban area (UA), girls accounted for 57%, while in the rural area (RA) the sex ratio was 1:1. The children’s ages ranged from 6 to 11 years, with a mean (±SD) of 9.0 ± 1.6 years for urban children and 8.6 ± 1.5 years for rural children. The reported birth weight of children in the rural area was significantly higher than that of urban children (p = 0.001). But rural children had a statistically significant worse nutritional status (HAZ and WAZ) compared to those living in urban areas [13]. In addition, children in the urban area had mothers with higher levels of education, compared with mothers of children in the rural area (Table 1).
3.2. Biological Monitoring of Metals (Table 2)

Table 2.
Biological monitoring of trace metal elements.
The concentrations of several trace metals (arsenic As-U, cadmium Cd-B and mercury Hg-B for Canadian inquiry, rural molybdenum Mo-U, nickel Ni-U, lead Pb-U, lead Pb-B, Sb-U), were elevated when compared with reference values obtained in industrially developed countries [19,20]. The Cd-B study values were higher only for urban children, compared with the references of Bohn et al. In contrast, the latter reference of Hg-B was higher than the study’s urban and rural values. For several trace metals, concentrations were, as expected, higher in blood or urine among urban children than rural children. In blood, exposed children had higher geometric mean concentrations than controls for cobalt (p < 0.001), cadmium (p < 0.0001) and mercury (p < 0.0001). In urine, geometric mean concentrations with creatinine corrections were higher for lithium (p < 0.001) among the urban area (UA) compared to the rural area (RA). In the urine of rural children, the molybdenum (p < 0.008) and nickel (p < 0.025) values were higher than the urban ones.
3.3. Behavioral Characteristics and Cognitive Assessment
Table 3 shows no statistically significant differences between the groups in the conduct problems score, relationship score, impact score, or prosocial score. However, children in the rural area (RA) had higher scores, closer to abnormal values, and significant differences compared to those in the urban area (UA) for hyperactivity (3 vs. 5; p = 0.018) and total difficulties (11.5 vs. 16.5; p = 0.018). A difference was also observed in the emotional score (2 vs. 4; p = 0.021).

Table 3.
Behavioral and cognitive characteristics.
UA values were statistically higher than the RA values for inhibition (IND: 6.5 vs. 3; p = 0.032), facial affect recognition (FRA: 8 vs. 4; p = 0.013), delayed word list memory (DLWLM: 6 vs. 3; p < 0.05), categorization (“CA Total”: 14 vs. 10.5; p = 0.008) and list interference (“IM total”: 12 vs. 4; p = 0.002).
Table 4, with SDQ-Tutor, shows a statistically significant (p = 0.01) difference for the rate of behavioral problems only in total difficulties (UA 37% vs. RA 73%; p = 0.010). Without a statistically significant difference compared with the UA (Table 3 SDQ-Tutor), the RA had higher rates of other behavioral problems: relationship problems (68% vs. 57%), Impact (59% vs. 57%) and emotional disorders (55% vs. 30%). Overall, the lower rate of behavioral problems was 37% in the UA, and the higher rate was 73% in the RA.

Table 4.
Behavioral problem rates with SDQ-Tutor.
3.4. Relationship Between Behavioral Problems, Sex, Age and Nutritional Status
There were no differences between the scores of girls and boys, so the values for both sexes were combined.
In the urban area (UA), statistically significant negative correlations were found between age and total behavioral difficulties scores (r = −0.549; p = 0.002), emotional scores (r = −0.494; p = 0.006), hyperactivity scores (r = −0.538; p = 0.002), relational scores (r = −0.410; p = 0.024) and the prosocial behavior score (r = −0.443; p = 0.014). In contrast, no statistical correlations were observed for these variables in the rural area (RA) group.
Overall, total behavioral difficulties scores correlated negatively with the HAZ nutritional status (r = −0.284; p = 0.042). Prosocial behavior scores correlated positively with the HAZ (r = 0.28; p = 0.045) and WAZ (r = 0.283; p = 0.042).
3.5. Relationship Between Behavioral Problems and Cognitive Performances
In Table 5 we present the statistically significant results. In the RA group, we found a negative correlation between the impact of difficulties (impact scores) and the inhibition scores (IND). Also, impact scores were positively correlated to TPRC (digital dexterity performance) scores.

Table 5.
Spearman correlation coefficients between cognitive performance and behavioral SDQ scores.
In the UA group, negative correlations were also found between facial recognition scores (FRA) and total difficulties scores and hyperactivity scores. On the other hand, positive correlations were observed between the comprehension of instruction (CC) and conduct problems, emotional scores and their impact score. The emotional score was negatively correlated with the inhibition score (IND) and word list interference (IM). Also, the total difficulties score was negatively correlated with the inhibition score (IND).
Finally, high scores in conduct problems and hyperactivity were negatively associated with digital dexterity performance (TPRC).
3.6. Relationship Between Behavioral Problems and Trace Metals Exposition
Overall, Table 6 shows that total behavioral difficulties scores correlated positively with corrected urinary levels of aluminum (Al-U) and molybdenum (Mo-U) and negatively with blood levels of cadmium (Cd-B) and mercury (Hg-B).

Table 6.
Spearman correlation between blood or urine metal concentrations and SDQ scores.
Emotional scores negatively correlated with blood levels of Hg-B and Cd-B, as well as with corrected urinary levels of titanium (Ti-U).
Hyperactivity scores positively correlated with corrected urinary levels of Mo-U, Al-U and Ti-U.
Conduct problems scores correlated positively with corrected urinary aluminum levels (Al-U), manganese (Mn-U and Ti-U).
Relationship scores were negatively correlated with Hg-B, selenium (Se-U) and chromium (Cr-U).
Prosocial scores correlated positively with blood levels of cobalt (Co-B) and cadmium (Cd-B) and corrected urinary levels of titanium (Ti-U) then negatively with copper (Cu-U).
Finally, the total impact score correlated positively with blood lead levels (Pb-B) and then negatively with Hg-B and Co-U.
Some of these correlations remain significant when considering only the UA group, as most of these metals were detected at higher levels in the UA compared to the RA.
4. Discussion
This study aimed to characterize children’s behavior in relation to cognitive performance and exposure to trace metals in two neighborhoods: the urban area with high metal exposure and the rural area presumed to have a lower exposure. We focused on behavioral characteristics, cognitive performance, the relationship between behavioral characteristics and cognitive performance and then the association between behavioral characteristics and exposure to trace metals.
Compared to urban children, rural children had higher birth weights but a lower HAZ, WAZ and mother’s education. The DRC DHS (2023–2024) found a low HAZ and low WAZ in rural settings [21].
4.1. Behavioral Characteristics
This high frequency of behavioral problems, observed in this study using SDQ-Tutor (37 to 73% overall), is in line with what was found in Latvia in a metropolitan area among children aged 2 to 17, with frequencies of 60.2% for relationship problems, 49.3% for emotional disorders and 47.9% for social conduct disorders [22].
In our study, the significantly higher frequency of problems relating to “total difficulties” of rural children (73%) compared with urban children (37%) could be explained by the poorer nutritional status in rural areas [21]. In our study, total behavioral difficulties scores correlated negatively with the HAZ nutritional status. Malnutrition is a known risk factor for behavioral problems in children [23].
4.2. Cognitive Performances
Table 2 showed that children in the urban environment performed cognitively (p < 0.05) better than those in the rural environment in terms of attention and executive functions measured by categorization and the degree of inhibition, social perception assessed by facial recognition of affect and learning memory in relation to word list interference and delayed word list memory. The difference in cognitive scores between the two environments could be explained by the negative consequences of malnutrition on cognition [24]. In this study, the children in the rural environment showed deteriorated nutritional status in terms of statural and weight development and growth. The parents’ level of education is a determinant of the cognitive scores assessed with NEPSY-II [24]. Several studies support the theory that a high level of parental education has an impact on children’s cognitive level, with a particular emphasis on the mother’s level of education [25].
4.3. Cognitive Performance and Behavioral Characteristics
The present study revealed several correlations between cognitive performance and the behavioral characteristics of children. Facial affect recognition scores were negatively correlated with total difficulty scores. This aligns with the existing literature, which indicates that emotional and behavioral disorders, as assessed by the Strengths and Difficulties Questionnaire—Teacher Version (SDQ-T), are associated with poorer performance in facial affect recognition [26].
Lower inhibition performance was associated with higher emotional behavior scores among urban children and with a greater negative impact of behavioral difficulties among rural children. These findings underscore the interconnectedness of attention, executive functions and emotional and behavioral regulation [27].
Relational difficulties and hyperactivity scores were negatively correlated with social cognition and facial affect recognition, highlighting the critical role of social cognition and the theory of mind in interpersonal relationships and social behavior [28].
Furthermore, higher scores for social conduct problems, hyperactivity and the negative impact of behavioral difficulties were significantly associated with poorer performance on digital dexterity tasks (TPRC), suggesting potential prefrontal dysfunction in children exhibiting hyperactivity and conduct disorders [29].
4.4. Behavioral Characteristics and Exposure to Trace Metals
The presence of metals that have no biological role in the human body in the urine and blood of children proves the exposure to trace metals in Haut-Katanga province, which requires further in-depth study (Pb, As, Cd, Hg, Ti) [1,6].
As-U; urban Cd-B (references of Bohn et al.); Hg-B (for Canadian inquiry); and rural Mo-U, Ni-U, Pb-U, Pb-B and Sb-U were elevated when compared to the reference values reported by Bohn et al. [19,20].
Moreover, it was found that blood levels of cobalt, cadmium and mercury and corrected urine levels of lithium were higher in the UA than in the RA. Contrarily, Mo-U and Ni-U in the RA were higher than in the UA, as reported elsewhere [30].
Table 6 reveals that prosocial behavior in the UA was associated positively with Co-B, Cd-B and Ti-U but negatively with Cu-U. Urban relational scores and the negative impact of behavioral difficulties decreased with higher Hg-B levels, while Pb-B levels increased with the negative impact of behavioral difficulties. Our data showed that the exposure of children to trace metals in both rural and urban environments was associated with behavioral problems in a complex way (total difficulties, emotional, hyperactivity, relationship, conduct problems, prosocial and impact scores), as reported elsewhere [31].
Al-U levels were associated with higher scores of hyperactivity and conduct problems. Indeed, aluminum accumulation in the body, even at low doses, has long-term neurotoxic effects for neurobehavioral expression [32]. It is important to note that all trace metals in excess in the children’s blood (Cd, Pb, Co) impact behavioral scores. The negative correlation between Hg-B and behavioral scores may reflect complex inter-metal interactions rather than a protective effect. The urinary elimination of cobalt and chromium reduced the negative impact scores for behavioral difficulties, which probably reflects a behavioral neurotoxicity of these metals. It is known that urinary concentrations of cobalt and chromium are reliable indicators of exposure to these metals [33]. The presence of titanium in children’s urine with emotional and behavioral disorders suggests exposure to this metal, which has no biological role in the body.
In the context of multiple exposures, which implies interactions between different metals, this relationship with behavioral problems should be investigated in future studies. Each development activity (e.g., mining) or health intervention (promotional, preventive, curative, etc.) must consider our results.
This study highlights the need for integrated actions addressing trace metal exposure, malnutrition and education in Haut-Katanga children. Future research should expand to larger, longitudinal studies to confirm these associations. Meanwhile, school-based nutritional programs, environmental monitoring and awareness campaigns are essential to reduce exposure and protect children’s neurodevelopment in the region.
5. Strengths and Limitations
The strengths of this study include its originality as the first epidemiological study to explore behavioral characteristics of children using validated behavioral and neurocognitive assessment tools and the characterization of trace metal exposure by blood and urinary biomonitoring. In this context, interventions related to trace metals, behavioral and cognitive aspects of school-age children will have to take into account our results. Nevertheless, we also acknowledge several limitations: firstly, the cross-sectional nature of the study; secondly the small sample size; thirdly, we were not able to determine cerebral morbidity, prenatal maternal stress and maternal depression; and fourthly, there was no predictor of negative impact. The results obtained are not entirely generalizable in another context without precautions.
6. Conclusions
The association of behavioral disorders with age, nutritional disorders, trace metals and cognitive performances in children in Haut-Katanga indicates the neurodevelopmental and multifactorial origin of these disorders, probably linked to multiple exposures to trace metals and the combined effect of malnutrition and metal exposure. Further studies are required to explore these complex relationships.
Author Contributions
A.M.W.M., L.R. and E.B.M.: conceptualization; A.M.W.M., L.R., D.O.L.E.-A., L.L. and E.B.M.: methodology; A.M.W.M. and C.M.M.: supervision of the field work; E.B.M., J.-P.N.M. and P.M.O.: investigations; E.B.M.: writing—original draft under supervision of D.O.L.E.-A., A.M.W.M., C.M.M., L.R., L.L. and E.B.M.; P.M.O., B.N., C.B.L.N. and V.H.: supervision of writing—review and editing; V.H., B.N. and C.B.L.N.: metal measurements; P.M.O., E.B.M. and D.O.L.E.-A. Data curation and formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This paper presents the results of research carried out as part of the project “Renforcement de la capacité opérationnelle et de la formation en neuropsychiatrie par l’étude des effets neurotoxiques de métaux dans la region minière du Katanga”, supported by ARES with funding from the Belgian Development Cooperation. The funders did not play any role in this study.
Institutional Review Board Statement
The study protocol was approved by the Medical Ethics Committee of the University of Lubumbashi (UNILU) on 19 July 2019, under approval number UNILU/CEM/036/2019.
Informed Consent Statement
The parents provided their informed consent after an explanation of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We thank the schoolchildren and their parents. We would also like to thank the psychologist MANYONGA TSHIBANGU Emmanuel for his help. We are also grateful to TSHALA KATUMBAY Desire (Oregon Health and Science University) and KOBA BORA Beatrice (University of Lubumbashi) for their advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Kabyla, B.I.; Luboya, O.N.; Ilunga, A.N.; Mutombo, A.M.; et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Musimwa, A.M.; Kanteng, G.W.; Kitoko, H.T.; Luboya, O.N. Eléments traces dans le sérum des enfants malnutris et bien nourris vivants à Lubumbashi et Kawama dans un contexte d’un environnement de pollution minière. Pan Afr. Med. J. 2016, 24, 11. [Google Scholar] [CrossRef]
- Bora, B.K.; Ramos-Crawford, A.L.; Sikorskii, A.; Boivin, M.J.; Lez, D.M.; Mumba-Ngoyi, D.; Mukalay Wa Mukalay, A.; Okitundu-Luwa, D.; Tshala-Katumbay, D. Concurrent exposure to heavy metals and cognition in school-age children in Congo-Kinshasa: A complex overdue research agenda. Brain Res. Bull. 2019, 145, 81–86. [Google Scholar] [CrossRef]
- Cheyns, K.; Banza Lubaba Nkulu, C.; Ngombe, L.K.; Asosa, J.N.; Haufroid, V.; De Putter, T.; Nawrot, T.; Kimpanga, C.M.; Numbi, O.L.; Ilunga, B.K.; et al. Pathways of human exposure to cobalt in Katanga, a mining area of the D.R. Congo. Sci. Total Environ. 2014, 490, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Cantoral, A.; Desai, G.; Halabicky, O.; Signes-Pastor, A.J.; Tellez-Rojo, M.M.; Peterson, K.E.; Karagas, M.R. Dietary Exposure to Toxic Elements and the Health of Young Children: Methodological Considerations and Data Needs. J. Nutr. 2022, 152, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.-C.; Huang, H.-Y.; Wang, S.-L.; Tseng, Y.-L.; Chang-Chien, J.; Tsai, H.-J.; Yao, T.-C. Association of exposure to environmental vanadium and manganese with lung function among young children: A population-based study. Ecotoxicol. Environ. Saf. 2023, 264, 115430. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Ssewamala, F.M.; Sensoy Bahar, O.; McKay, M.M.; Hoagwood, K.; Huang, K.-Y.; Pringle, B. Strengthening mental health and research training in Sub-Saharan Africa (SMART Africa): Uganda study protocol. Trials 2018, 19, 423. [Google Scholar] [CrossRef]
- Carlsson, T.; Molander, F.; Taylor, M.J.; Jonsson, U.; Bölte, S. Early environmental risk factors for neurodevelopmental disorders—A systematic review of twin and sibling studies. Dev. Psychopathol. 2021, 33, 1448–1495. [Google Scholar] [CrossRef]
- Kashala-Abotnes, E.; Mumbere, P.P.; Mishika, J.M.; Ndjukendi, A.O.; Mpaka, D.B.; Bumoko, M.-M.G.; Kayembe, T.K.; Tshala-Katumbay, D.; Kazadi, T.K.; Okitundu, D.L.E.-A. Lead exposure and early child neurodevelopment among children 12–24 months in Kinshasa, the Democratic Republic of Congo. Eur. Child Adolesc. Psychiatry 2016, 25, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Eunice, B.M.; Nanou, P.M.; Mabeguy, B.M.; John, K.M.; Daniel, O.L.; Desire, T.K. Zinc and copper serum levels in children and adolescents with neurodevelopmental disoders at the center for neuropsychopathology in Kinshasa: An exploratory study. J. Neurol. Sci. 2017, 381, 843. [Google Scholar] [CrossRef]
- Erhardt, J.; Golden, M.; Seaman, J.; Bilukha, O. ENA for SMART 2020. Emergency Nutrition Assessment. 2020. Available online: http://www.nutrisurvey.net/ena/ena.html (accessed on 15 September 2020).
- Goodman, A.; Goodman, R. Strengths and Difficulties Questionnaire scores and mental health in looked after children. Br. J. Psychiatry 2012, 200, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Korkman, M.; Kirk, U.; Kemp, S. Nepsy-II. Bilan Neuropsychologique de l’Enfant. De 5 ans à 16 ans 11 mois. Une Batterie Flexible pour une Évaluation sur Mesure. (Adaptation francaise ECPA), 2nd ed.; Pearson France-ECPA: Paris, France, 2012. [Google Scholar]
- Bachelier, D.; Roger-Kosiorowski, F.; Roch, D. Le Bilan Avec la Nepsy-II Enfants, Adolescents; Dunod: Malakoff, France, 2019. [Google Scholar]
- Hoet, P.; Jacquerye, C.; Deumer, G.; Lison, D.; Haufroid, V. Reference values of trace elements in blood and/or plasma in adults living in Belgium. Clin. Chem. Lab. Med. 2021, 59, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.; Jacquerye, C.; Deumer, G.; Lison, D.; Haufroid, V. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin. Chem. Lab. Med. 2013, 51, 839–849. [Google Scholar] [CrossRef]
- Bohn, M.K.; Nichols, M.; Yang, L.; Bhayana, V.; Macri, J.; Adeli, K. Pediatric Reference Value Profiling of Essential Trace and Toxic Elements in Healthy Children and Adolescents Using High-Resolution and Triple Quadrupole Inductively Coupled Plasma Mass Spectrometry. J. Appl. Lab. Med. 2023, 8, 674–688. [Google Scholar] [CrossRef]
- Health Canada. Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009); Minister of Health: Ottawa, ON, Canada, 2010. [Google Scholar]
- RDC; Rockville. RDC-Institut National de la Statistique, Ecole de Santé Publique de Kinshasa et ICF.2024. RDC, Enquete Démographique et de Santé 2023–2024: Rapport des Indicateurs Clés; ICF: Rockville, MD, USA, 2025. [Google Scholar]
- Bezborodovs, Ņ.; Kočāne, A.; Rancāns, E.; Villeruša, A. Clinical Utility of the Parent-Report Version of the Strengths and Difficulties Questionnaire (SDQ) in Latvian Child and Adolescent Psychiatry Practice. Medicina 2022, 58, 1599. [Google Scholar] [CrossRef]
- Lucarelli, L.; Sechi, C.; Cimino, S.; Chatoor, I. Avoidant/Restrictive Food Intake Disorder: A Longitudinal Study of Malnutrition and Psychopathological Risk Factors from 2 to 11 Years of Age. Front. Psychol. 2018, 9, 1608. [Google Scholar] [CrossRef]
- Brooks, B.L.; Sherman, E.M.S.; Iverson, G.L. Healthy children get low scores too: Prevalence of low scores on the NEPSY-II in preschoolers, children, and adolescents. Arch. Clin. Neuropsychol. 2010, 25, 182–190. [Google Scholar] [CrossRef]
- Cermakova, P.; Chlapečka, A.; Csajbók, Z.; Andrýsková, L.; Brázdil, M.; Marečková, K. Parental education, cognition and functional connectivity of the salience network. Sci. Rep. 2023, 13, 2761. [Google Scholar] [CrossRef]
- Bae, S.; Rhee, E.; Hwang, B.S.; Son, Y.D.; Bae, J.H.; Han, D.H. Correlations Between Psychological Status and Perception of Facial Expression. Psychiatry Investig. 2022, 19, 435–442. [Google Scholar] [CrossRef]
- Souissi, S.; Chamari, K.; Bellaj, T. Assessment of executive functions in school-aged children: A narrative review. Front. Psychol. 2022, 13, 991699. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-T.; Li, H.-J.; Tsai, C.-H.; Lin, C.-H.; Lai, S.-S.; Chen, K.-L. Cool Executive Function and Verbal Comprehension Mediate the Relation of Hot Executive Function and Theory of Mind in Children with Autism Spectrum Disorder. Autism Res. 2021, 14, 921–931. [Google Scholar] [CrossRef]
- Seol, J.; Lim, N.; Nagata, K.; Okura, T. Effects of home-based manual dexterity training on cognitive function among older adults: A randomized controlled trial. Eur. Rev. Aging Phys. Act. 2023, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Sánchez, A.; Pérez, R.; Pardo, O.; Yusà, V. Biomonitoring of 20 elements in urine of children. Levels and predictors of exposure. Chemosphere 2016, 144, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Karatela, S.; Ward, N.I.; Paterson, J.; Zeng, I.S. Environmental Influences on the Behavioural and Emotional Outcomes of Children: A Network Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8479. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Aluminium neurotoxicity: Neurobehavioural and oxidative aspects. Arch. Toxicol. 2009, 83, 965–978. [Google Scholar] [CrossRef]
- Caparros-Gonzalez, R.A.; Giménez-Asensio, M.J.; González-Alzaga, B.; Aguilar-Garduño, C.; Lorca-Marín, J.A.; Alguacil, J.; Gómez-Becerra, I.; Gómez-Ariza, J.L.; García-Barrera, T.; Hernandez, A.F.; et al. Childhood chromium exposure and neuropsychological development in children living in two polluted areas in southern Spain. Environ. Pollut. 2019, 252 Pt B, 1550–1560. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).