From Overgrowth to Complex Malformations: A Novel EZH2 Variant Reveals the Expanding Clinical Spectrum of Weaver Syndrome
Abstract
1. Introduction
2. Case Presentation
2.1. Patient Demographics and Initial Presentation
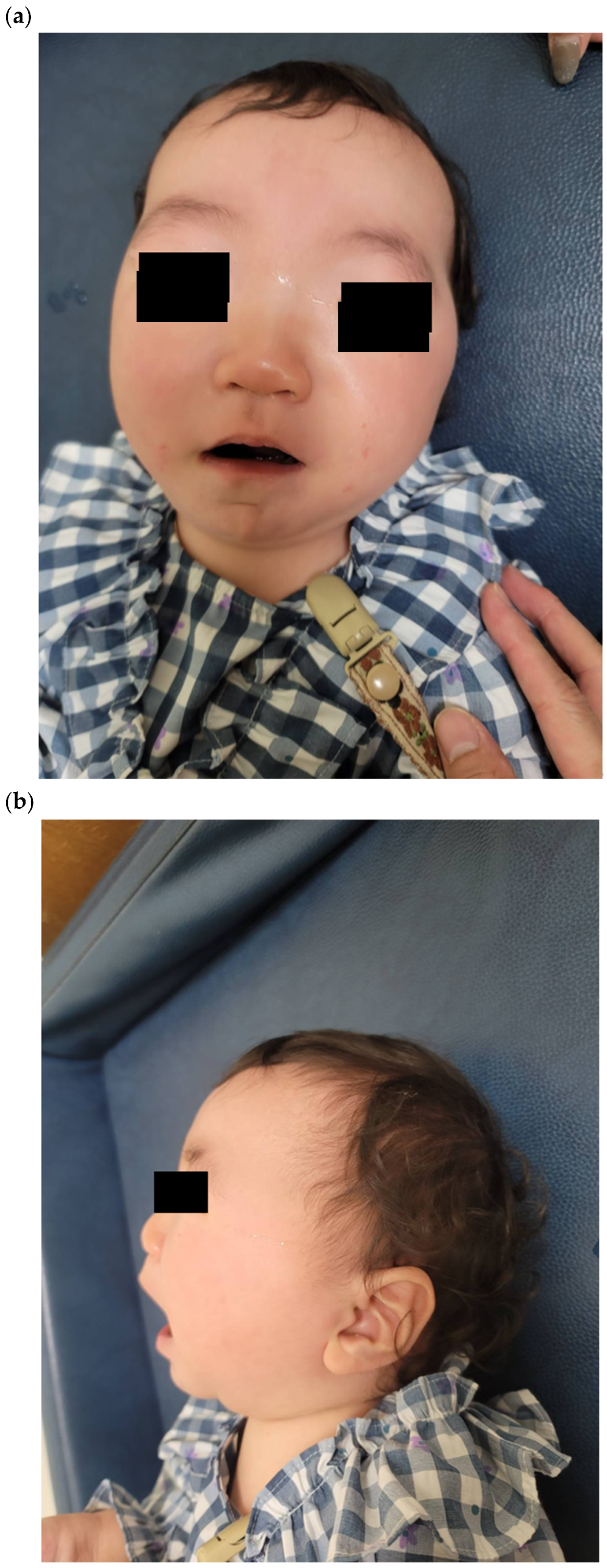
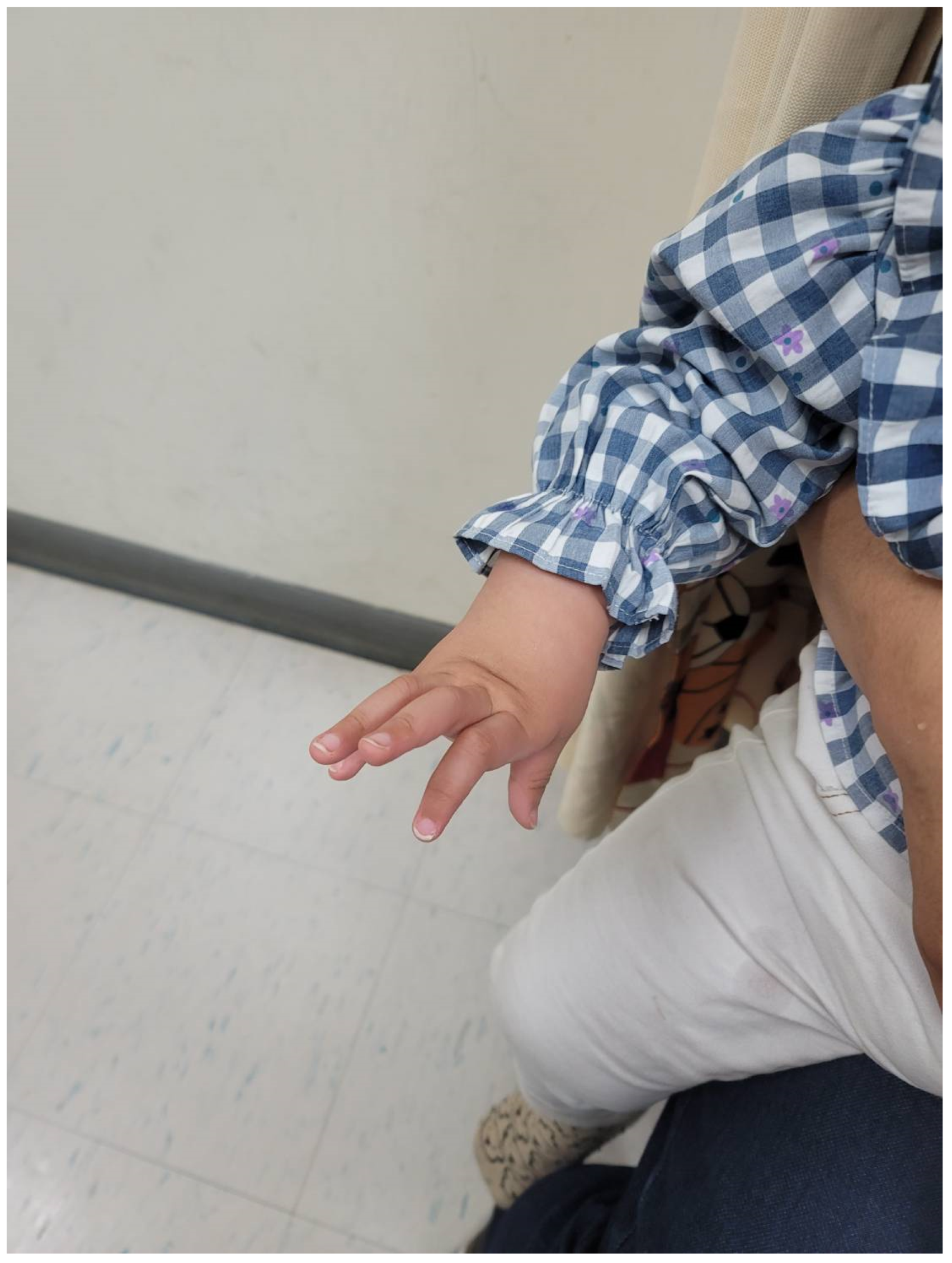
2.2. Clinical Features and Physical Examination
2.3. Developmental Assessment
2.4. Neuroimaging Findings
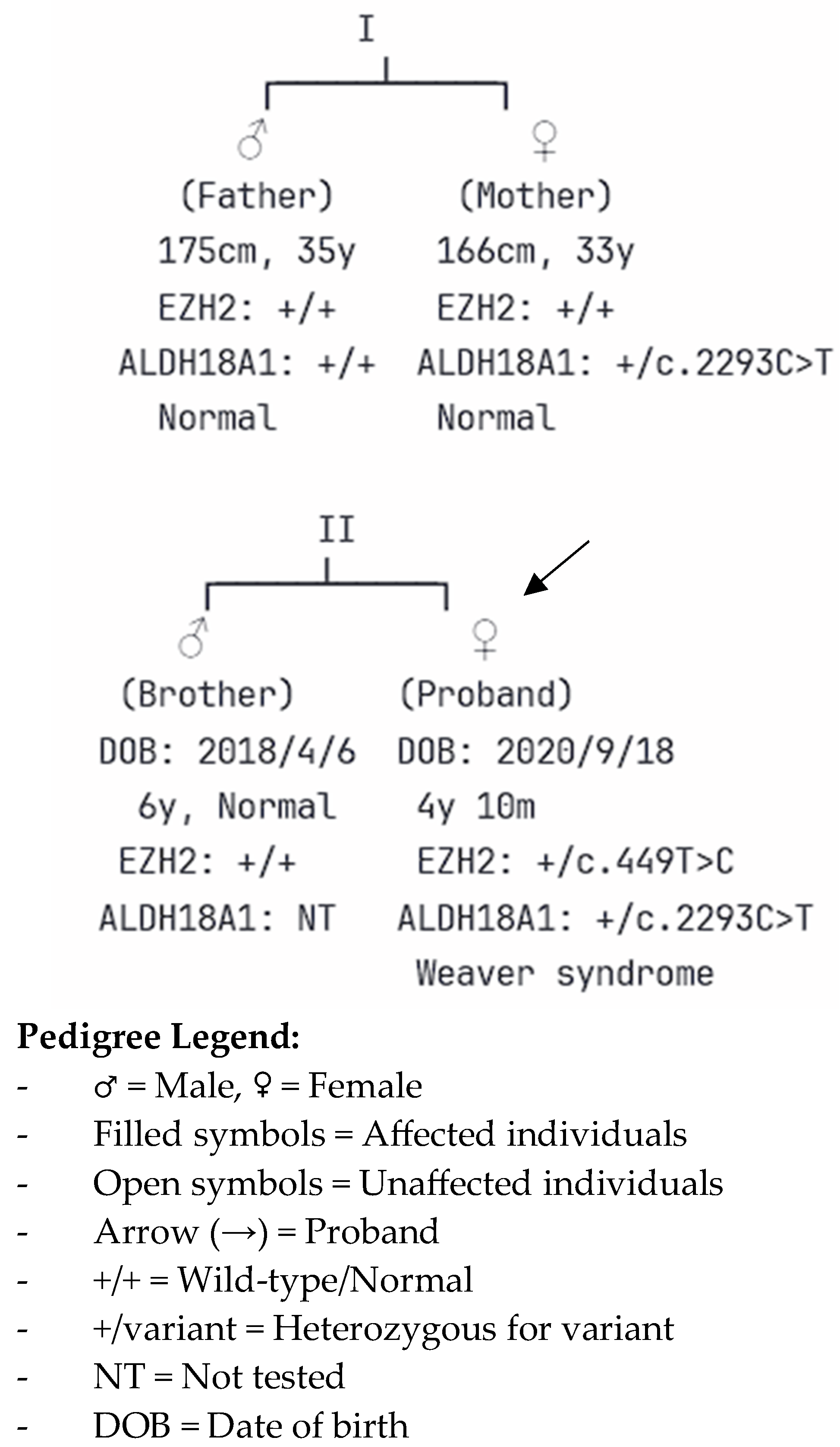
2.5. Genetic Testing and Molecular Diagnosis
2.6. Treatment and Management
3. Discussion
3.1. Genotype–Phenotype Correlation and Domain-Specific Effects
3.2. Expansion of the Phenotypic Spectrum
3.3. Clinical Implications and Diagnostic Considerations
3.4. Population-Specific Considerations
3.5. Management Considerations
3.6. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tatton-Brown, K.; Murray, A.; Hanks, S.; Douglas, J.; Armstrong, R.; Banka, S.; Bird, L.M.; Clericuzio, C.L.; Cormier-Daire, V.; Cushing, T.; et al. Weaver syndrome and EZH2 mutations: Clarifying the clinical phenotype. Am. J. Med. Genet. A 2013, 161A, 2972–2980. [Google Scholar] [CrossRef]
- Weaver, D.D.; Graham, C.B.; Thomas, I.T.; Smith, D.W. A new overgrowth syndrome with accelerated skeletal maturation, unusual facies, and camptodactyly. J. Pediatr. 1974, 84, 547–552. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Hanks, S.; Ruark, E.; Zachariou, A.; Duarte Sdel, V.; Ramsay, E.; Snape, K.; Murray, A.; Perdeaux, E.R.; Seal, S.; et al. Germline mutations in the oncogene EZH2 cause Weaver syndrome and increased human height. Oncotarget 2011, 2, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.T.; Hood, R.L.; Zhan, S.H.; Bulman, D.E.; Fejes, A.P.; Moore, R.; Mungall, A.J.; Eydoux, P.; Babul-Hirji, R.; An, J.; et al. Mutations in EZH2 cause Weaver syndrome. Am. J. Hum. Genet. 2012, 90, 110–118. [Google Scholar] [CrossRef]
- Cohen, A.S.; Yap, D.B.; Lewis, M.E.; Chijiwa, C.; Ramos-Arroyo, M.A.; Tkachenko, N.; Milano, V.; Fradin, M.; McKinnon, M.L.; Townsend, K.N.; et al. Weaver Syndrome-Associated EZH2 Protein Variants Show Impaired Histone Methyltransferase Function In Vitro. Hum. Mutat. 2016, 37, 301–307. [Google Scholar] [CrossRef]
- Turkkahraman, D.; Sakarya, A.N.P.; Randa, N.C. A novel EZH2 gene variant in a case of Weaver syndrome with postaxial polydactyly. Am. J. Med. Genet. A 2021, 185, 2234–2237. [Google Scholar] [CrossRef]
- Smigiel, R.; Biernacka, A.; Biela, M.; Murcia-Pienkowski, V.; Szmida, E.; Gasperowicz, P.; Kosinska, J.; Kostrzewa, G.; Koppolu, A.A.; Walczak, A.; et al. Novel de novo mutation affecting two adjacent aminoacids in the EED gene in a patient with Weaver syndrome. J. Hum. Genet. 2018, 63, 517–520. [Google Scholar] [CrossRef]
- Lui, J.C. Growth disorders caused by variants in epigenetic regulators: Progress and prospects. Front. Endocrinol. 2024, 15, 1327378. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.T.; Asokumar, D.; Sohn, M.; Naskar, S.; Elisha, L.; Stevenson, P.; Lee, D.R.; Zhang, Y.; Rocha, P.P.; Dale, R.K.; et al. Loss of Ezh2 in the medial ganglionic eminence alters interneuron fate, cell morphology and gene expression profiles. Front. Cell. Neurosci. 2024, 18, 1334244. [Google Scholar] [CrossRef]
- Husmann, D.; Gozani, O. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Bansal, A. Weaver syndrome: A report of a rare genetic syndrome. Indian J. Hum. Genet. 2009, 15, 36–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griffiths, S.; Loveday, C.; Zachariou, A.; Behan, L.A.; Chandler, K.; Cole, T.; D’Arrigo, S.; Dieckmann, A.; Foster, A.; Gibney, J.; et al. EED and EZH2 constitutive variants: A study to expand the Cohen-Gibson syndrome phenotype and contrast it with Weaver syndrome. Am. J. Med. Genet. A 2019, 179, 588–594. [Google Scholar][Green Version]
- Szczupak, D.; Kossmann Ferraz, M.; Gemal, L.; Oliveira-Szejnfeld, P.S.; Monteiro, M.; Bramati, I.; Vargas, F.R.; IRC5 Consortium; Lent, R.; Silva, A.C.; et al. Corpus callosum dysgenesis causes novel patterns of structural and functional brain connectivity. Brain Commun. 2021, 3, fcab057. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.J.; Fenlon, L.R.; Dean, R.J.; Bunt, J.; IRC5 Consortium; Sherr, E.H.; Richards, L.J. Altered structural connectivity networks in a mouse model of complete and partial dysgenesis of the corpus callosum. Neuroimage 2020, 217, 116868. [Google Scholar] [PubMed]
- Szczupak, D.; Lent, R.; Tovar-Moll, F.; Silva, A.C. Heterotopic connectivity of callosal dysgenesis in mice and humans. Front. Neurosci. 2023, 17, 1191859. [Google Scholar] [CrossRef]
- Paul, L.K.; Brown, W.S.; Adolphs, R.; Tyszka, J.M.; Richards, L.J.; Mukherjee, P.; Sherr, E.H. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007, 8, 287–299. [Google Scholar] [CrossRef]
- Mancuso, L.; Uddin, L.Q.; Nani, A.; Costa, T.; Cauda, F. Brain functional connectivity in individuals with callosotomy and agenesis of the corpus callosum: A systematic review. Neurosci. Biobehav. Rev. 2019, 105, 231–248. [Google Scholar] [CrossRef]
- Baumgartner, M.R.; Hu, C.A.; Almashanu, S.; Steel, G.; Obie, C.; Aral, B.; Rabier, D.; Kamoun, P.; Saudubray, J.M.; Valle, D. Hyperammonemia with Reduced Ornithine, Citrulline, Arginine and Proline: A New Inborn Error Caused by a Mutation in the Gene Encoding Delta(1)-Pyrroline-5-Carboxylate Synthase. Hum. Mol. Genet. 2000, 9, 2853–2858. [Google Scholar]
- Fischer-Zirnsak, B.; Escande-Beillard, N.; Ganesh, J.; Tan, Y.X.; Al Bughaili, M.; Lin, A.E.; Sahai, I.; Bahena, P.; Reichert, S.L.; Loh, A.; et al. Recurrent De Novo Mutations Affecting Residue Arg138 of Pyrroline-5-Carboxylate Synthase Cause a Progeroid Form of Autosomal-Dominant Cutis Laxa. Am. J. Hum. Genet. 2015, 97, 483–492. [Google Scholar]
- Reversade, B.; Escande-Beillard, N.; Dimopoulou, A.; Fischer, B.; Chng, S.C.; Li, Y.; Shboul, M.; Tham, P.Y.; Kayserili, H.; Al-Gazali, L.; et al. Mutations in PYCR1 Cause Cutis Laxa with Progeroid Features. Nat. Genet. 2009, 41, 1016–1021. [Google Scholar] [CrossRef]
- Coutelier, M.; Goizet, C.; Durr, A.; Habarou, F.; Morais, S.; Dionne-Laporte, A.; Tao, F.; Konop, J.; Stoll, M.; Charles, P.; et al. Alteration of Ornithine Metabolism Leads to Dominant and Recessive Hereditary Spastic Paraplegia. Brain 2015, 138, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Barr, E.K.; Applebaum, M.A. Genetic Predisposition to Neuroblastoma. Children 2018, 5, 119. [Google Scholar] [CrossRef]
- Weaver, T.M.; Liu, J.; Connelly, K.E.; Coble, C.; Varzavand, K.; Dykhuizen, E.C.; Musselman, C.A. The EZH2 SANT1 domain is a histone reader providing sensitivity to the modification state of the H4 tail. Sci. Rep. 2019, 9, 987. [Google Scholar] [CrossRef]
- Lui, J.C.; Barnes, K.M.; Dong, L.; Yue, S.; Graber, E.; Rapaport, R.; Dauber, A.; Nilsson, O.; Baron, J. Ezh2 Mutations Found in the Weaver Overgrowth Syndrome Cause a Partial Loss of H3K27 Histone Methyltransferase Activity. J. Clin. Endocrinol. Metab. 2018, 103, 1470–1478. [Google Scholar]
- Kendir-Demirkol, Y.; Yeter, B.; Jenny, L.A. Expanding the Phenotypic and Genotypic Spectrum of Weaver Syndrome: A Missense Variant of the EZH2 Gene. Mol. Syndromol. 2024, 15, 161–166. [Google Scholar]
- Usemann, J.; Ernst, T.; Schäfer, V.; Lehmberg, K.; Seeger, K. EZH2 Mutation in an Adolescent with Weaver Syndrome Developing Acute Myeloid Leukemia and Secondary Hemophagocytic Lymphohistiocytosis. Am. J. Med. Genet. A 2016, 170A, 1274–1277. [Google Scholar]
- Al-Salem, A.; Alshammari, M.J.; Hassan, H.; Alazami, A.M.; Alkuraya, F.S. Weaver syndrome and defective cortical development: A rare association. Am. J. Med. Genet. A 2013, 161A, 225–227. [Google Scholar]
- Wang, A.M.Q.; Kim, M.; Ho, E.S.; Davidge, K.M. Surgery and Conservative Management of Camptodactyly in Pediatric Patients: A Systematic Review. Hand 2020, 15, 761–770. [Google Scholar] [PubMed]
- Edwards, T.J.; Sherr, E.H.; Barkovich, A.J.; Richards, L.J. Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 2014, 137, 1579–1613. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, F.; Wasiewski, W.; McCabe, E.R. Weaver syndrome: The changing phenotype in an adult. Am. J. Med. Genet. 1989, 33, 127–129. [Google Scholar]
- Villani, A.; Greer, M.C.; Kalish, J.M.; Nakagawara, A.; Nathanson, K.L.; Pajtler, K.W.; Pfister, S.M.; Walsh, M.F.; Wasserman, J.D.; Zelley, K.; et al. Recommendations for Cancer Surveillance in Individuals with RASopathies and Other Rare Genetic Conditions with Increased Cancer Risk. Clin. Cancer Res. 2017, 23, e83–e90. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, L.; Jiang, X.; Wang, H.; Li, Y.; Ren, X. Clinical and genetic characterization of Weaver syndrome: A case report of an EZH2 mutation and review of the literature. Medicine 2025, 104, e44080. [Google Scholar] [CrossRef]
- Yuan, X.; Chu, S.; Wang, D.; Liu, Q.; Gu, W. Case report of Weaver syndrome caused by EZH2 gene mutation. Chin. J. Appl. Clin. Pediatr. 2021, 36, 380–382. [Google Scholar]
- Kloc, J.; Dzula, B.; Varga, I.; Klein, M.; Steno, B. Camptodactyly: From Embryological Basis to Surgical Treatment. Medicina 2023, 59, 966. [Google Scholar] [CrossRef]
- Evans, B.T.; Waters, P.M.; Bae, D.S. Early Results of Surgical Management of Camptodactyly. J. Pediatr. Orthop. 2017, 37, e317–e320. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Furtado, L.M.; Bernardes, H.M.; de Souza Félix Nunes, F.A.; Gonçalves, C.A.; Da Costa Val Filho, J.A.; de Miranda, A.S. The Role of Neuroplasticity in Improving the Decision-Making Quality of Individuals with Agenesis of the Corpus Callosum: A Systematic Review. Cureus 2022, 14, e26082. [Google Scholar] [CrossRef]
- Rahbari, R.; Wuster, A.; Lindsay, S.J.; Hardwick, R.J.; Alexandrov, L.B.; Turki, S.A.; Dominiczak, A.; Morris, A.; Porteous, D.; Smith, B.; et al. Timing, rates and spectra of human germline mutation. Nat. Genet. 2016, 48, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.M.; Stewart, J.R.; James, R.A.; Lupski, J.R.; Stankiewicz, P.; Olofsson, P.; Shaw, C.A. Parent of origin, mosaicism, and recurrence risk: Probabilistic modeling explains the broken symmetry of transmission genetics. Am. J. Hum. Genet. 2014, 95, 345–359. [Google Scholar] [CrossRef]
- Committee on Genetics and the Society for Maternal-Fetal Medicine. Committee Opinion No.682: Microarrays and next-generation sequencing technology: The use of advanced genetic diagnostic tools in obstetrics and gynecology. Obstet. Gynecol. 2016, 128, e262–e268. [Google Scholar] [CrossRef] [PubMed]
- Scriven, P.N.; Bossuyt, P.M. Diagnostic accuracy: Theoretical models for preimplantation genetic testing of a single nucleus using the fluorescence in situ hybridization technique. Hum. Reprod. 2010, 25, 2622–2628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biesecker, L.G.; Harrison, S.M.; ClinGen Sequence Variant Interpretation Working Group. The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet. Med. 2018, 20, 1687–1688. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.E.; Alford, B.A.; Abel, M. Cervical spine anomalies and tumors in Weaver syndrome. Am. J. Med. Genet. 2000, 95, 492–495. [Google Scholar] [CrossRef]
- Zurynski, Y.; Deverell, M.; Dalkeith, T.; Johnson, S.; Christodoulou, J.; Leonard, H.; Elliott, E.J.; APSU Rare Diseases Impacts on Families Study Group. Australian children living with rare diseases: Experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J. Rare Dis. 2017, 12, 68. [Google Scholar] [CrossRef] [PubMed]
| Age | Height/Length (cm) | Height Z-Score | Weight (kg) | Weight Z-Score | Head Circumference (cm) | HC Z-Score |
|---|---|---|---|---|---|---|
| Birth | 56 | +3.5 | 4.46 | +3.2 | 35 | +2.8 |
| 15 months | 92 | +3.8 | 15.0 | +3.5 | 48 | +2.3 |
| 32 months | 103 | +3.6 | 19.0 | +3.4 | 49.9 | +2.2 |
| 45 months | 111 | +3.9 | 22.0 | +3.6 | 51.1 | +2.1 |
| 58 months | 123 | +4.1 | 26.0 | +3.7 | 52.0 | +2.1 |
| Gene | Nucleotide Change | Protein Change | Inheritance | Classification | Origin | Population Frequency |
|---|---|---|---|---|---|---|
| EZH2 | c.449T>C | p.Ile150Thr | Autosomal dominant | Pathogenic | De novo | Not reported |
| ALDH18A1 | c.2293C>T | p.Arg765Ter | Autosomal dominant/recessive | Likely pathogenic | Maternal | 0.0006 (East Asian) |
| Family Member | Relationship | EZH2 c.449T>C Status | ALDH18A1 c.2293C>T Status | Clinical Phenotype |
|---|---|---|---|---|
| Proband | - | Heterozygous (de novo) | Heterozygous (maternal) | Weaver syndrome with complex malformations |
| Father | Paternal | Wild-type | Wild-type | Normal |
| Mother | Maternal | Wild-type | Heterozygous | Normal |
| Brother | Sibling | Wild-type | Not tested | Normal development |
| Feature | Current Case (Present Study) | p.Pro132Leu [27] | SET Domain Mutations [1,3,5] |
|---|---|---|---|
| Genetic Information | |||
| Nucleotide change | c.449T>C | c.395C>T | Variable (clustered in SET) |
| Protein change | p.Ile150Thr | p.Pro132Leu | Variable |
| Domain location | SANT1 (residue 150) | SANT1 (residue 132) | SET domain (residues 612–726) |
| Inheritance | De novo | Germline | Predominantly de novo |
| Conservation | Highly conserved | Highly conserved | Highly conserved |
| Classical Weaver Features | |||
| Macrosomia | + (4460 g, >97th percentile) | + | + (>90% of cases) |
| Macrocephaly | + (>97th percentile throughout) | + | + (>90% of cases) |
| Advanced bone age | Not formally assessed | + | + (common feature) |
| Hypertelorism | + | + | + |
| Characteristic facies | + (including horizontal chin crease) | + | + |
| Developmental delay | + (global) | + | + (~80% of cases) |
| Atypical/Severe Features | |||
| Camptodactyly | Severe bilateral with marked functional impairment | Not reported in detail | Mild to moderate (common) |
| CNS malformations | Corpus callosum dysgenesis (rostral agenesis, genu hypoplasia), bilateral frontal lobe hypoplasia, arachnoid cyst | Not reported in detail | Rare (polymicrogyria reported in some cases) |
| Malignancy | None (age 4 years) | AML + secondary HLH (age 16 years) | Neuroblastoma reported (~11% risk) |
| Skeletal abnormalities | Severe (thoracolumbar kyphoscoliosis, curved tibiae, equinovalgus foot deformity) | Not reported in detail | Mild to moderate (variable) |
| Functional Implications | |||
| Predicted effect | Impaired H4 tail binding [24] | Impaired H4 tail binding [24] | Reduced H3K27 methyltransferase activity [5] |
| Domain function | Histone reader (chromatin targeting) | Histone reader (chromatin targeting) | Catalytic activity (histone methylation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Chuang, C.-K.; Chiu, H.-C.; Chang, Y.-H.; Tu, Y.-R.; Lo, Y.-T.; Wu, J.-Y.; Lin, H.-Y.; Lin, S.-P. From Overgrowth to Complex Malformations: A Novel EZH2 Variant Reveals the Expanding Clinical Spectrum of Weaver Syndrome. Children 2025, 12, 1487. https://doi.org/10.3390/children12111487
Lee C-L, Chuang C-K, Chiu H-C, Chang Y-H, Tu Y-R, Lo Y-T, Wu J-Y, Lin H-Y, Lin S-P. From Overgrowth to Complex Malformations: A Novel EZH2 Variant Reveals the Expanding Clinical Spectrum of Weaver Syndrome. Children. 2025; 12(11):1487. https://doi.org/10.3390/children12111487
Chicago/Turabian StyleLee, Chung-Lin, Chih-Kuang Chuang, Huei-Ching Chiu, Ya-Hui Chang, Yuan-Rong Tu, Yun-Ting Lo, Jun-Yi Wu, Hsiang-Yu Lin, and Shuan-Pei Lin. 2025. "From Overgrowth to Complex Malformations: A Novel EZH2 Variant Reveals the Expanding Clinical Spectrum of Weaver Syndrome" Children 12, no. 11: 1487. https://doi.org/10.3390/children12111487
APA StyleLee, C.-L., Chuang, C.-K., Chiu, H.-C., Chang, Y.-H., Tu, Y.-R., Lo, Y.-T., Wu, J.-Y., Lin, H.-Y., & Lin, S.-P. (2025). From Overgrowth to Complex Malformations: A Novel EZH2 Variant Reveals the Expanding Clinical Spectrum of Weaver Syndrome. Children, 12(11), 1487. https://doi.org/10.3390/children12111487







