Neural Network-Based Prediction of Post-Operative Visual Outcomes Following Secondary Pediatric Intraocular Lens Implantation
Abstract
Highlights
- A proof-of-concept neural network model was developed to predict visual outcomes after secondary intraocular lens (IOL) implantation in children with congenital cataracts.
- The model demonstrated encouraging predictive performance across training, validation, and test sets, suggesting feasibility despite the limited dataset.
- This work underscores the potential of machine learning to support clinical decision-making for secondary IOL implantation, an area currently lacking predictive tools.
- Broader, multi-center datasets and models restricted to preoperative variables will be essential to validate and translate this approach into clinical practice.
Abstract
1. Introduction
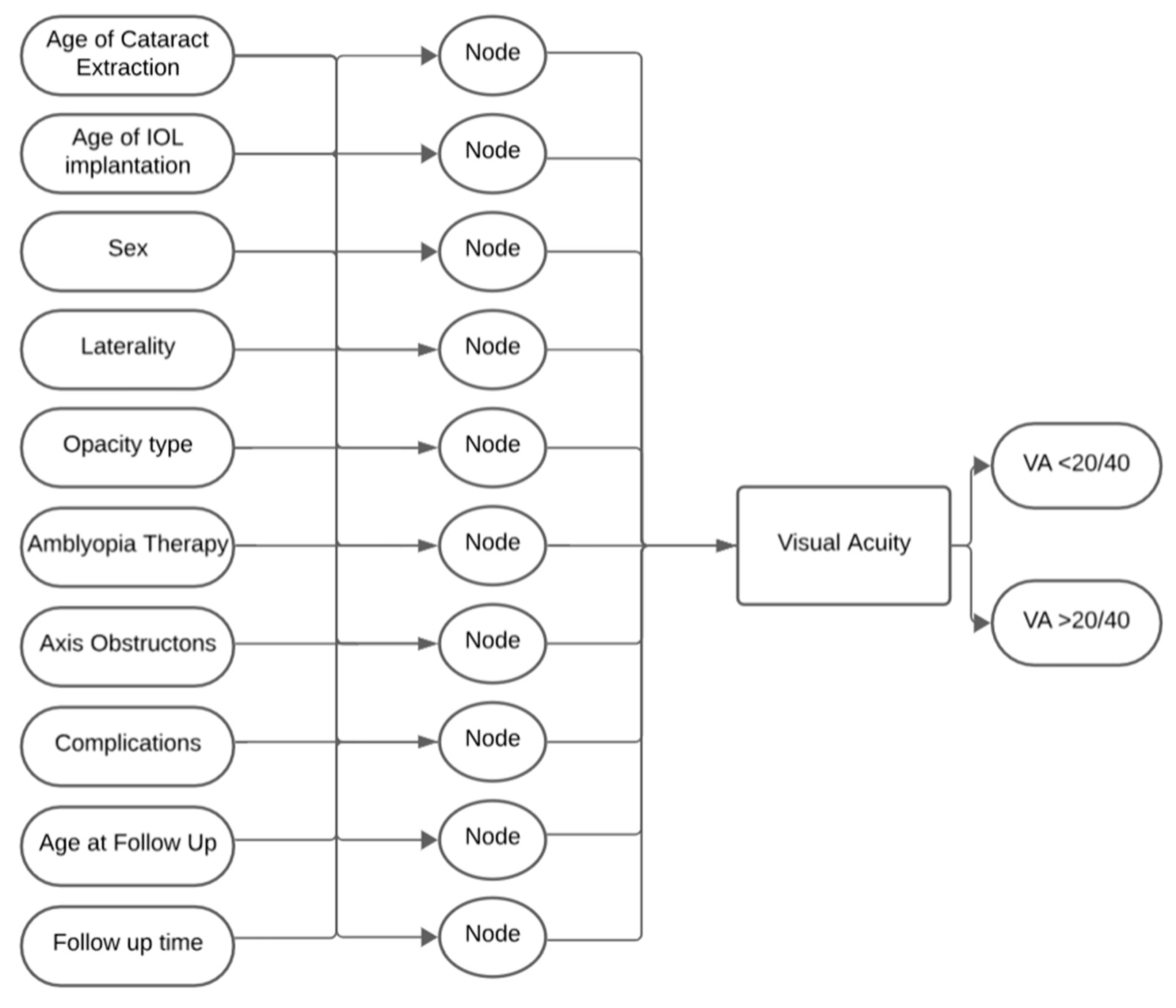
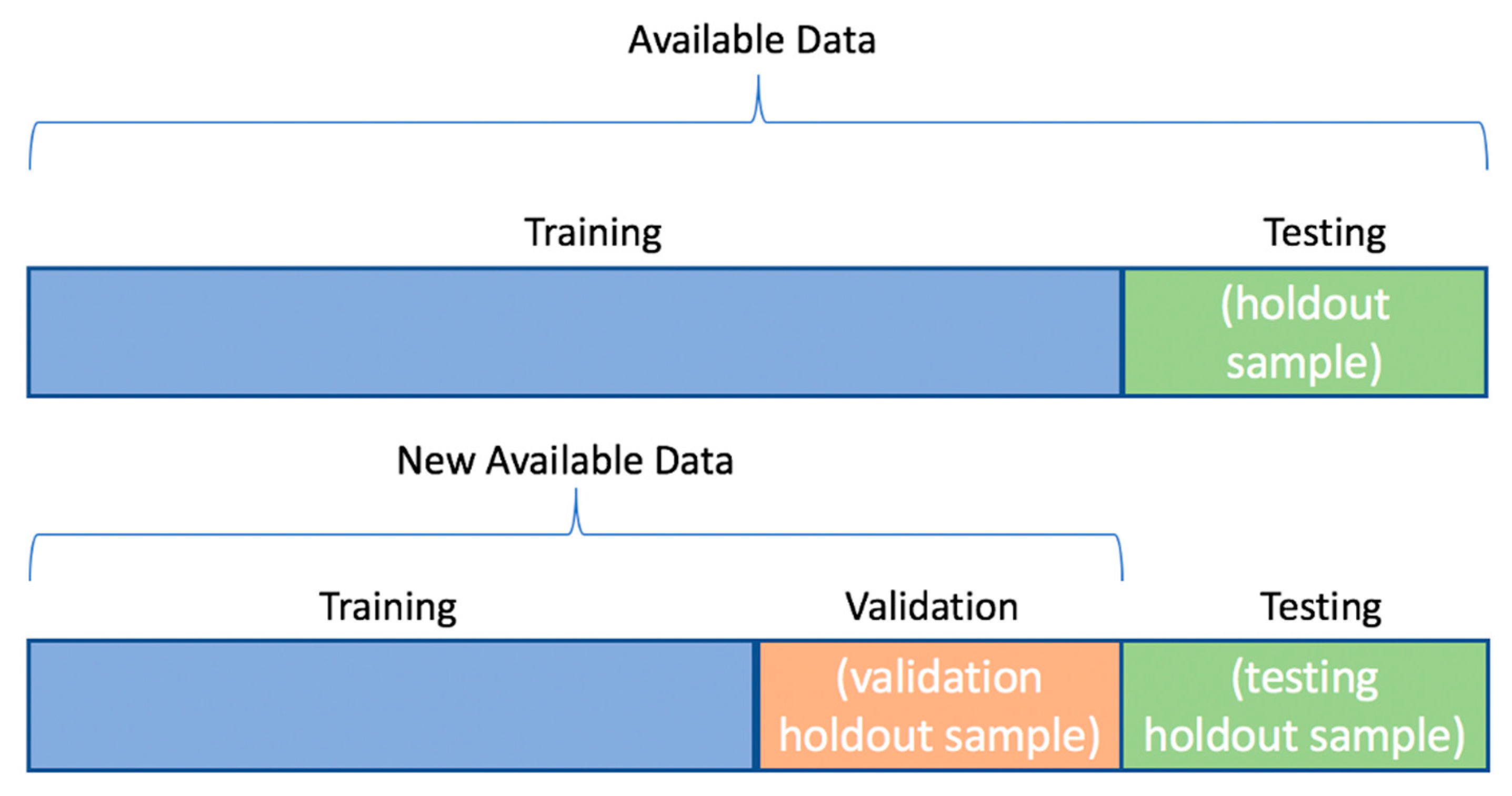
2. Materials and Methods
3. Results
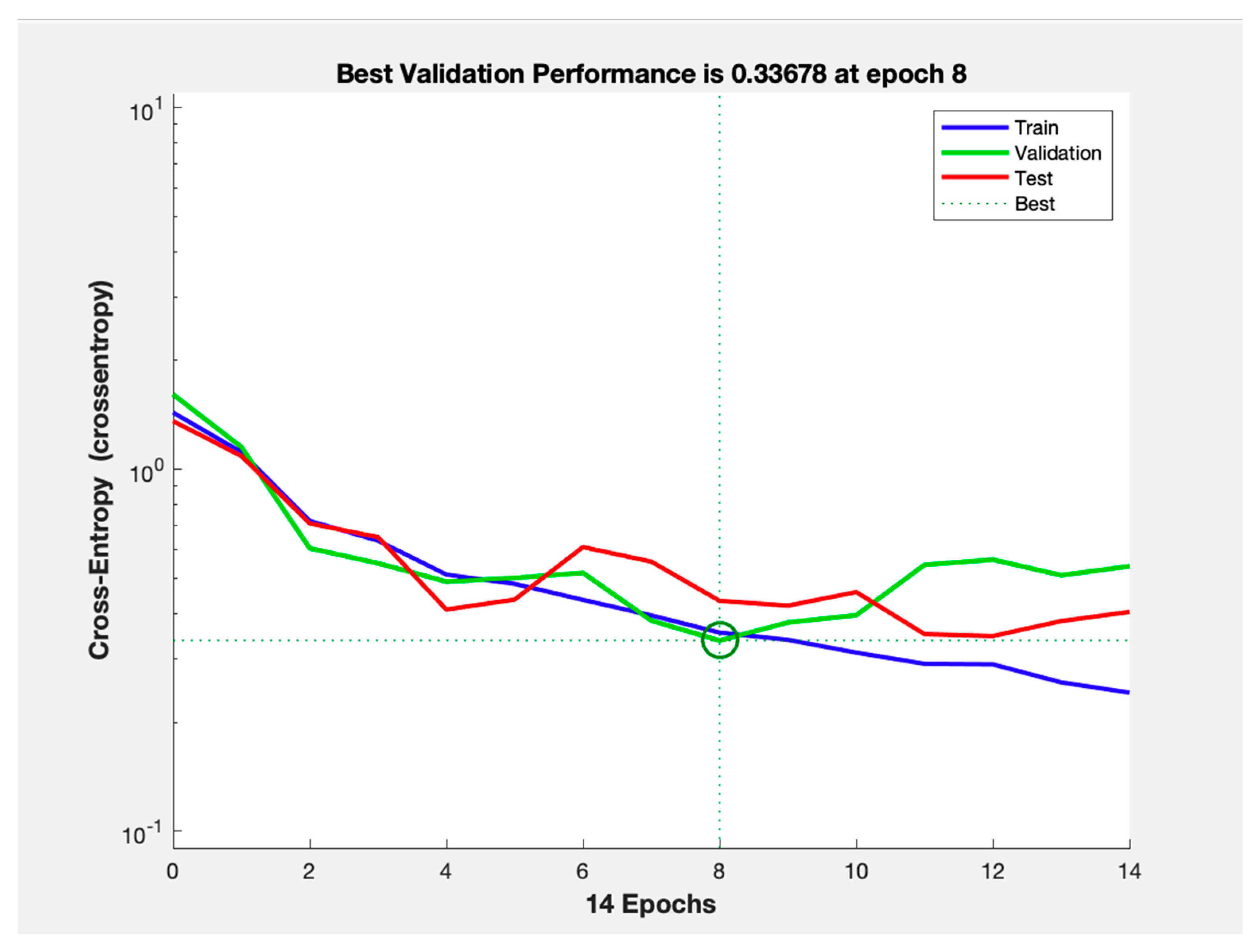
3.1. Cross-Entropy Loss and Training
3.2. Accuracy, Sensitivity, and Specificity by Dataset
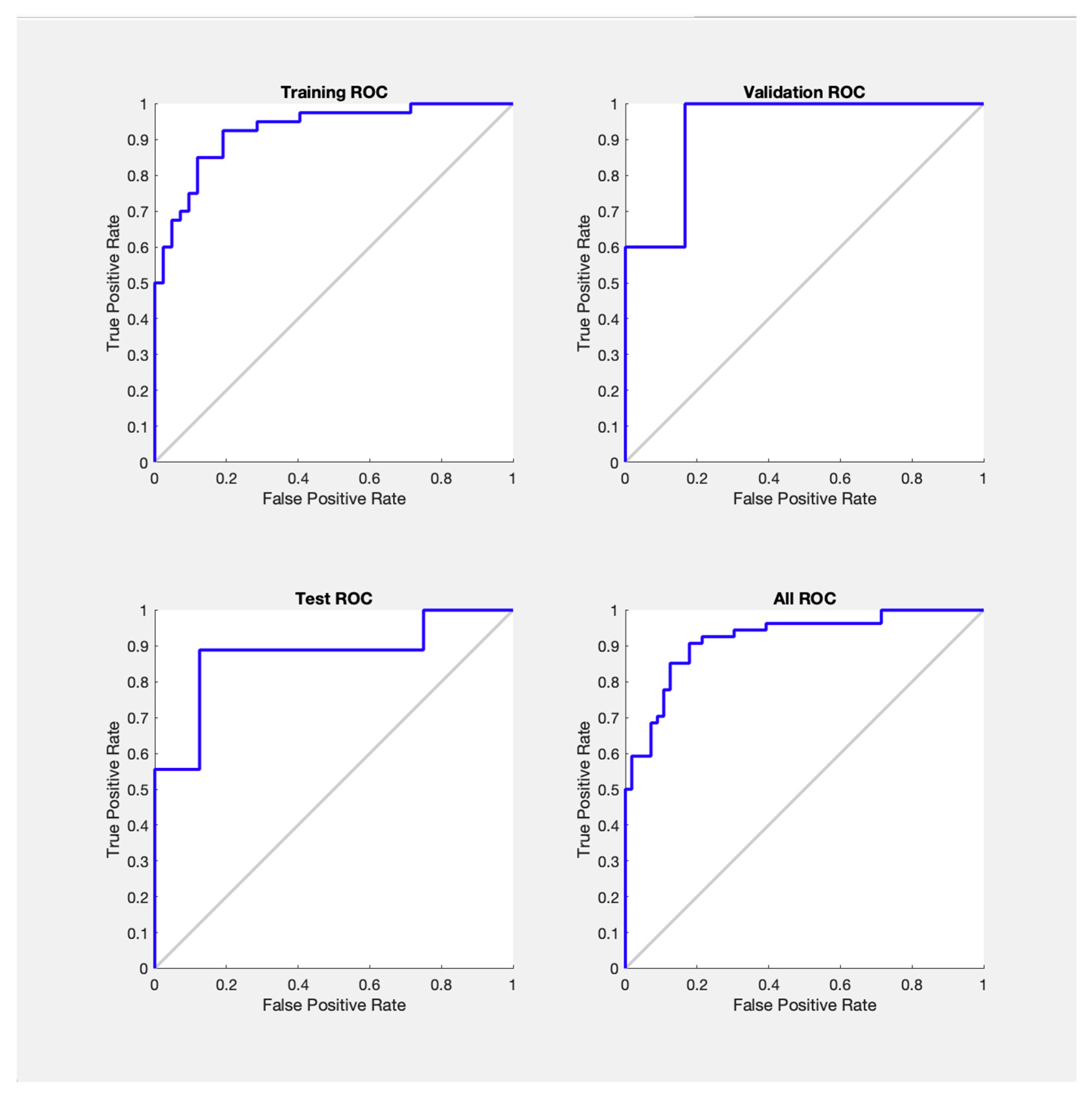
3.3. Receiver Operating Characteristic (ROC) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOL | Intraocular Lens |
| VA | Visual Acuity |
| ML | Machine Learning |
| AI | Artificial Intelligence |
| BCVA | Best Corrected Visual Acuity |
| ROC | Receiver Operating Characteristic Curve |
| AUC | Area Under the Curve |
References
- Katre, D.; Selukar, K. The Prevalence of Cataract in Children. Cureus 2022, 14, e30135. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Long, E.; Lin, H.; Liu, Y. Prevalence and Epidemiological Characteristics of Congenital Cataract: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 28564. [Google Scholar] [CrossRef]
- Al Shamrani, M.; Al Turkmani, S. Update of Intraocular Lens Implantation in Children. Saudi J. Ophthalmol. 2012, 26, 271–275. [Google Scholar] [CrossRef]
- Lenhart, P.D.; Lambert, S.R. Current Management of Infantile Cataracts. Surv. Ophthalmol. 2022, 67, 1476–1505. [Google Scholar] [CrossRef]
- Lambert, S.R.; Purohit, A.; Superak, H.M.; Lynn, M.J.; Beck, A.D. Long-Term Risk of Glaucoma After Congenital Cataract Surgery. Am. J. Ophthalmol. 2013, 156, 355–361.e2. [Google Scholar] [CrossRef]
- Freedman, S.F.; Lynn, M.J.; Beck, A.D.; Bothun, E.D.; Örge, F.H.; Lambert, S.R. Infant Aphakia Treatment Study Group Glaucoma-Related Adverse Events in the First 5 Years After Unilateral Cataract Removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015, 133, 907–914. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Shirzadeh, E.; Eslani, M.; Akbari, M. Optical Correction of Aphakia in Children. J. Ophthalmic Vis. Res. 2014, 9, 71–82. [Google Scholar]
- Stopyra, W.; Grzybowski, A. Intraocular Lens Power Calculation Formulas in Children—A Systematic Review. J. Clin. Med. 2024, 13, 4400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-H.; Zhao, Y.-E. Commentary Review: Challenges of Intraocular Lens Implantation for Congenital Cataract Infants. Int. J. Ophthalmol. 2021, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.R. Changes in Ocular Growth after Pediatric Cataract Surgery. Dev. Ophthalmol. 2016, 57, 29–39. [Google Scholar] [CrossRef]
- Panahibazaz, M.R.; Mohammadpour, S.; Samaeili, A. Overcoming Myopic Shift by the Initial Inductive Hypermetropia in Pediatric Cataract Surgery. Indian J. Ophthalmol. 2021, 69, 3515–3519. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ramteke, S.; Chattannavar, G.; Kekunnaya, R. Myopic Shift, Refractive, and Visual Outcomes after 5 Years of Infantile Cataract Surgery: Our Experience and Review of Literature. Taiwan J. Ophthalmol. 2024, 14, 236–241. [Google Scholar] [CrossRef]
- Milad, D.; Antaki, F.; Mikhail, D.; Farah, A.; El-Khoury, J.; Touma, S.; Durr, G.M.; Nayman, T.; Playout, C.; Keane, P.A.; et al. Code-Free Deep Learning Glaucoma Detection on Color Fundus Images. Ophthalmol. Sci. 2025, 5, 100721. [Google Scholar] [CrossRef]
- Chu, Y.; Hu, S.; Li, Z.; Yang, X.; Liu, H.; Yi, X.; Qi, X. Image Analysis-Based Machine Learning for the Diagnosis of Retinopathy of Prematurity: A Meta-Analysis and Systematic Review. Ophthalmol. Retin. 2024, 8, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, D.; Milad, D.; Antaki, F.; Hammamji, K.; Qian, C.X.; Rezende, F.A.; Duval, R. The Role of Artificial Intelligence in Epiretinal Membrane Care: A Scoping Review. Ophthalmol. Sci. 2025, 5, 100689. [Google Scholar] [CrossRef]
- Alexeeff, S.E.; Uong, S.; Liu, L.; Shorstein, N.H.; Carolan, J.; Amsden, L.B.; Herrinton, L.J. Development and Validation of Machine Learning Models: Electronic Health Record Data to Predict Visual Acuity After Cataract Surgery. Perm. J. 2020, 25, 1. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tabuchi, H.; Takase, K.; Masumoto, H. Use of a Machine Learning Method in Predicting Refraction after Cataract Surgery. J. Clin. Med. 2021, 10, 1103. [Google Scholar] [CrossRef]
- Brown, J.M.; Campbell, J.P.; Beers, A.; Chang, K.; Ostmo, S.; Chan, R.V.P.; Dy, J.; Erdogmus, D.; Ioannidis, S.; Kalpathy-Cramer, J.; et al. Automated Diagnosis of Plus Disease in Retinopathy of Prematurity Using Deep Convolutional Neural Networks. JAMA Ophthalmol. 2018, 136, 803–810. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Zhao, X.; Tang, J.; Yu, Z.; Wu, Z.; Tian, R.; Chen, Y.; Chen, M.; Ntentakis, D.P.; et al. Automated Detection of Nine Infantile Fundus Diseases and Conditions in Retinal Images Using a Deep Learning System. EPMA J. 2024, 15, 39–51. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; He, M.; Wu, T.; Hao, Z.; Zheng, C.; Ma, J.; Zhou, J. Development of a Prognostic Model for Predicting Long-Term Visual Acuity after Cataract Surgery in Children with Bilateral Congenital Cataracts: A Single Centre Retrospective, Observational Study. BMC Ophthalmol. 2024, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Chen, J.; Wu, X.; Liu, Z.; Wang, L.; Jiang, J.; Li, W.; Zhu, Y.; Chen, C.; Lin, Z.; et al. Artificial Intelligence Manages Congenital Cataract with Individualized Prediction and Telehealth Computing. npj Digit. Med. 2020, 3, 112. [Google Scholar] [CrossRef]
- Rong, X.; Ji, Y.; Fang, Y.; Jiang, Y.; Lu, Y. Data from: Long-Term Visual Outcomes of Secondary Intraocular Lens Implantation in Children with Congenital Cataracts. PLoS ONE 2015, 10, e0134864. [Google Scholar] [CrossRef]
- Møller, M.F. A Scaled Conjugate Gradient Algorithm for Fast Supervised Learning. Neural Netw. 1993, 6, 525–533. [Google Scholar] [CrossRef]
- Wilson, M.E.; Trivedi, R.H. Pediatric Cataract Surgery: Operative and Postoperative Issues. In Pediatric Ophthalmology: Current Thought and A Practical Guide; Wilson, M.E., Trivedi, R.H., Saunders, R.A., Eds.; Springer: Berlin, Heidelberg, 2009; pp. 325–343. ISBN 978-3-540-68632-3. [Google Scholar]
- Wójcik-Niklewska, B.; Nocoń-Bratek, M.; Szala, K. Intraocular Lens Power Calculation in Pediatric Cataract Surgery: A Narrative Review. Medicine 2025, 104, e42072. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.R.; Drack, A.V. Infantile Cataracts. Surv. Ophthalmol. 1996, 40, 427–458. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, I.C.; Wilson, M.E.; Trivedi, R.H.; Biswas, S.; Ashworth, J.L.; Green, E.; Self, J.; Voltz, K.; Archambault, C.; McClatchey, T.S.; et al. Update on Pediatric Cataract Surgery. Asia-Pac. J. Ophthalmol. 2025, 14, 100229. [Google Scholar] [CrossRef]
- Wood, K.S.; Tadros, D.; Trivedi, R.H.; Wilson, M.E. Secondary Intraocular Lens Implantation Following Infantile Cataract Surgery: Intraoperative Indications, Postoperative Outcomes. Eye 2016, 30, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, D.K.; Drews-Botsch, C.D.; Nizam, A.; Bothun, E.D.; Wilson, L.B.; Wilson, M.E.; Lambert, S.R.; for the Infant Aphakia Treatment Study. Outcomes of Secondary Intraocular Lens Implantation in the Infant Aphakia Treatment Study. J. Cataract. Refract. Surg. 2021, 47, 172. [Google Scholar] [CrossRef]
- Vision|Determining Medical Fitness to Operate Motor Vehicles. Available online: https://driversguide.ca/sections/vision (accessed on 14 August 2024).
- Steinkuller, P.G. Legal Vision Requirements for Drivers in the United States. AMA J. Ethics 2010, 12, 938–940. [Google Scholar] [CrossRef]
- Aljied, R.; Aubin, M.-J.; Buhrmann, R.; Sabeti, S.; Freeman, E.E. Prevalence and Determinants of Visual Impairment in Canada: Cross-Sectional Data from the Canadian Longitudinal Study on Aging. Can. J. Ophthalmol. 2018, 53, 291–297. [Google Scholar] [CrossRef]
- Government of Canada; Statistics Canada. The Daily—Canadian Health Measures Survey, 2018–2019. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/201214/dq201214d-eng.htm (accessed on 14 August 2024).
- Maberley, D.A.L.; Hollands, H.; Chuo, J.; Tam, G.; Konkal, J.; Roesch, M.; Veselinovic, A.; Witzigmann, M.; Bassett, K. The Prevalence of Low Vision and Blindness in Canada. Eye 2006, 20, 341–346. [Google Scholar] [CrossRef]
- Shah, P.; Schwartz, S.G.; Gartner, S.; Scott, I.U.; Flynn, H.W. Low Vision Services: A Practical Guide for the Clinician. Ther. Adv. Ophthalmol. 2018, 10, 2515841418776264. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.; Brown, G.C.; Brown, M.M.; Stein, J.D.; Sharma, S. Vision-Related Quality-of-Life Estimates in Adolescent Youths. Can. J. Ophthalmol. 2021, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Vision and Quality-of-Life. Trans. Am. Ophthalmol. Soc. 1999, 97, 473–511. [Google Scholar]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver Operating Characteristic Curve Analysis in Diagnostic Accuracy Studies: A Guide to Interpreting the Area under the Curve Value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, N.-E.; Haugen, O.H. Bag-in-the-Lens Intraocular Lens in Paediatric Cataract Surgery: Intraoperative and Postoperative Outcomes. Acta Ophthalmol. 2022, 100, e135–e141. [Google Scholar] [CrossRef]
- Sukhija, J.; Kaur, S.; Korla, S.; Kumari, K. Surgical Challenges of Posterior Optic Capture in Pediatric Cataract Surgery. Indian J. Ophthalmol. 2024, 72, 51. [Google Scholar] [CrossRef] [PubMed]
- Devebacak, A.; Biler, E.D.; Degirmenci, C.; Uretmen, O. Optic Capture Without Anterior Vitrectomy in Pediatric Cataract Surgery. Am. J. Ophthalmol. 2023, 247, 88–95. [Google Scholar] [CrossRef]
- Oshika, T.; Endo, T.; Kurosaka, D.; Matsuki, N.; Miyagi, M.; Mori, T.; Nagamoto, T.; Negishi, K.; Nishina, S.; Nomura, K.; et al. Long-Term Surgical Outcomes of Pediatric Cataract—Multivariate Analysis of Prognostic Factors. Sci. Rep. 2023, 13, 21645. [Google Scholar] [CrossRef]
- Yen, K.G.; Repka, M.X.; Sutherland, D.R.; Haider, K.M.; Hatt, S.R.; Kraker, R.T.; Galvin, J.A.; Li, Z.; Cotter, S.A.; Holmes, J.M.; et al. Complications Occurring Through 5 Years Following Primary Intraocular Lens Implantation for Pediatric Cataract. JAMA Ophthalmol. 2023, 141, 705–714. [Google Scholar] [CrossRef]
- Infant Aphakia Treatment Study Group; Lambert, S.R.; Buckley, E.G.; Drews-Botsch, C.; DuBois, L.; Hartmann, E.; Lynn, M.J.; Plager, D.A.; Wilson, M.E. The Infant Aphakia Treatment Study: Design and Clinical Measures at Enrollment. Arch. Ophthalmol. 2010, 128, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.; Balasubramanian, P.; Heralgi, M.M. Posterior Iris Fixated Intraocular Lens for Pediatric Traumatic Cataract. Middle East. Afr. J. Ophthalmol. 2016, 23, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, A.R.; Trivedi, R.H. Role of Optic Capture in Congenital Cataract and Intraocular Lens Surgery in Children. J. Cataract. Refract. Surg. 2000, 26, 824–831. [Google Scholar] [CrossRef] [PubMed]
| Performance Metrics | Training | Validation | Test | Total |
|---|---|---|---|---|
| Specificity | 85.7% | 83.3% | 87.5% | 85.7% |
| Sensitivity | 85.0% | 80.0% | 88.9% | 85.2% |
| Accuracy | 85.4% | 81.8% | 88.2% | 85.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farah, A.; Remtulla, R.; Koenekoop, R.K. Neural Network-Based Prediction of Post-Operative Visual Outcomes Following Secondary Pediatric Intraocular Lens Implantation. Children 2025, 12, 1413. https://doi.org/10.3390/children12101413
Farah A, Remtulla R, Koenekoop RK. Neural Network-Based Prediction of Post-Operative Visual Outcomes Following Secondary Pediatric Intraocular Lens Implantation. Children. 2025; 12(10):1413. https://doi.org/10.3390/children12101413
Chicago/Turabian StyleFarah, Andrew, Raheem Remtulla, and Robert K. Koenekoop. 2025. "Neural Network-Based Prediction of Post-Operative Visual Outcomes Following Secondary Pediatric Intraocular Lens Implantation" Children 12, no. 10: 1413. https://doi.org/10.3390/children12101413
APA StyleFarah, A., Remtulla, R., & Koenekoop, R. K. (2025). Neural Network-Based Prediction of Post-Operative Visual Outcomes Following Secondary Pediatric Intraocular Lens Implantation. Children, 12(10), 1413. https://doi.org/10.3390/children12101413






