Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis
Abstract
Highlights
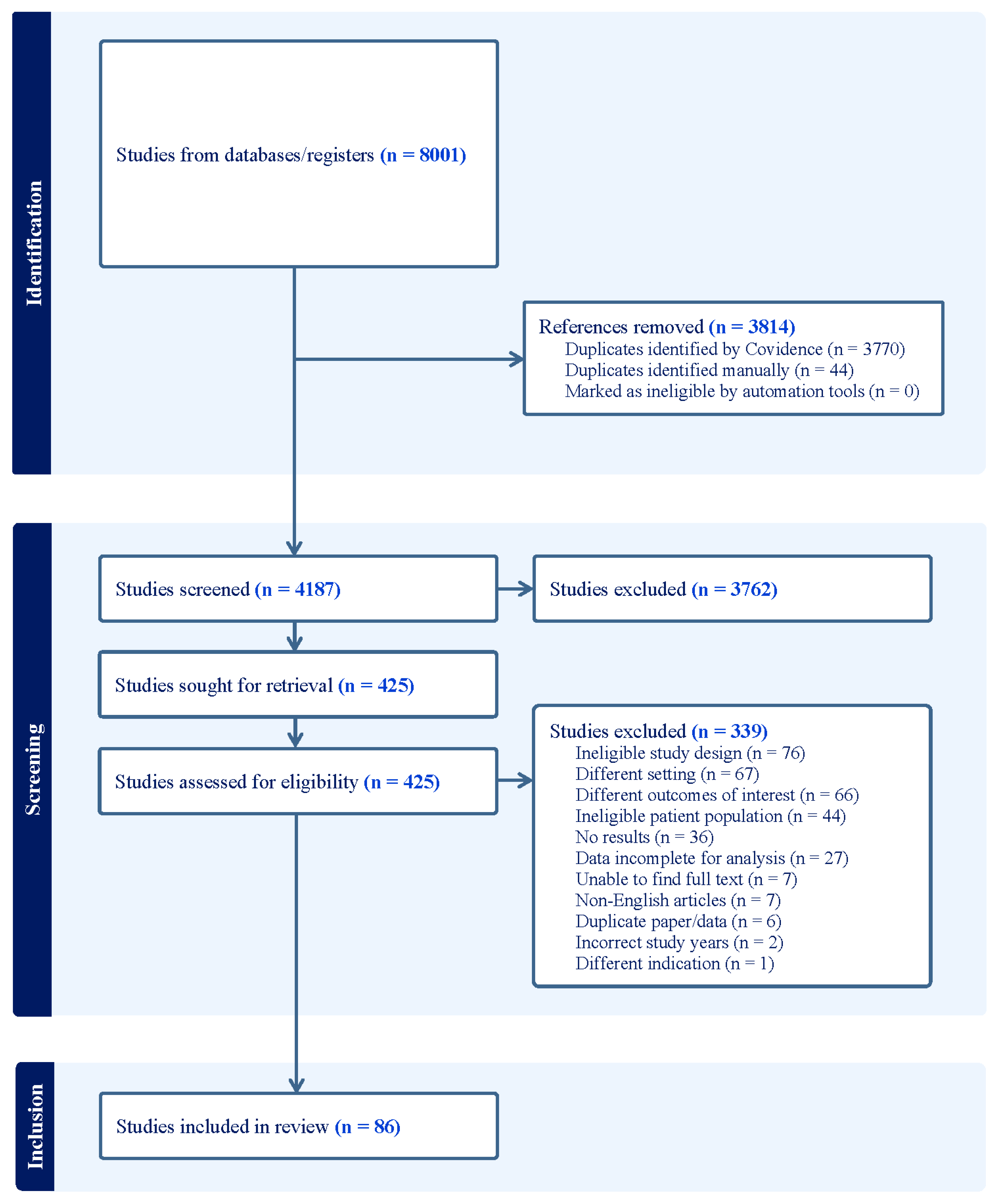
- Prescribing data for the treatment of acute otitis media (AOM) were abstracted from 83 studies in this meta-analysis.
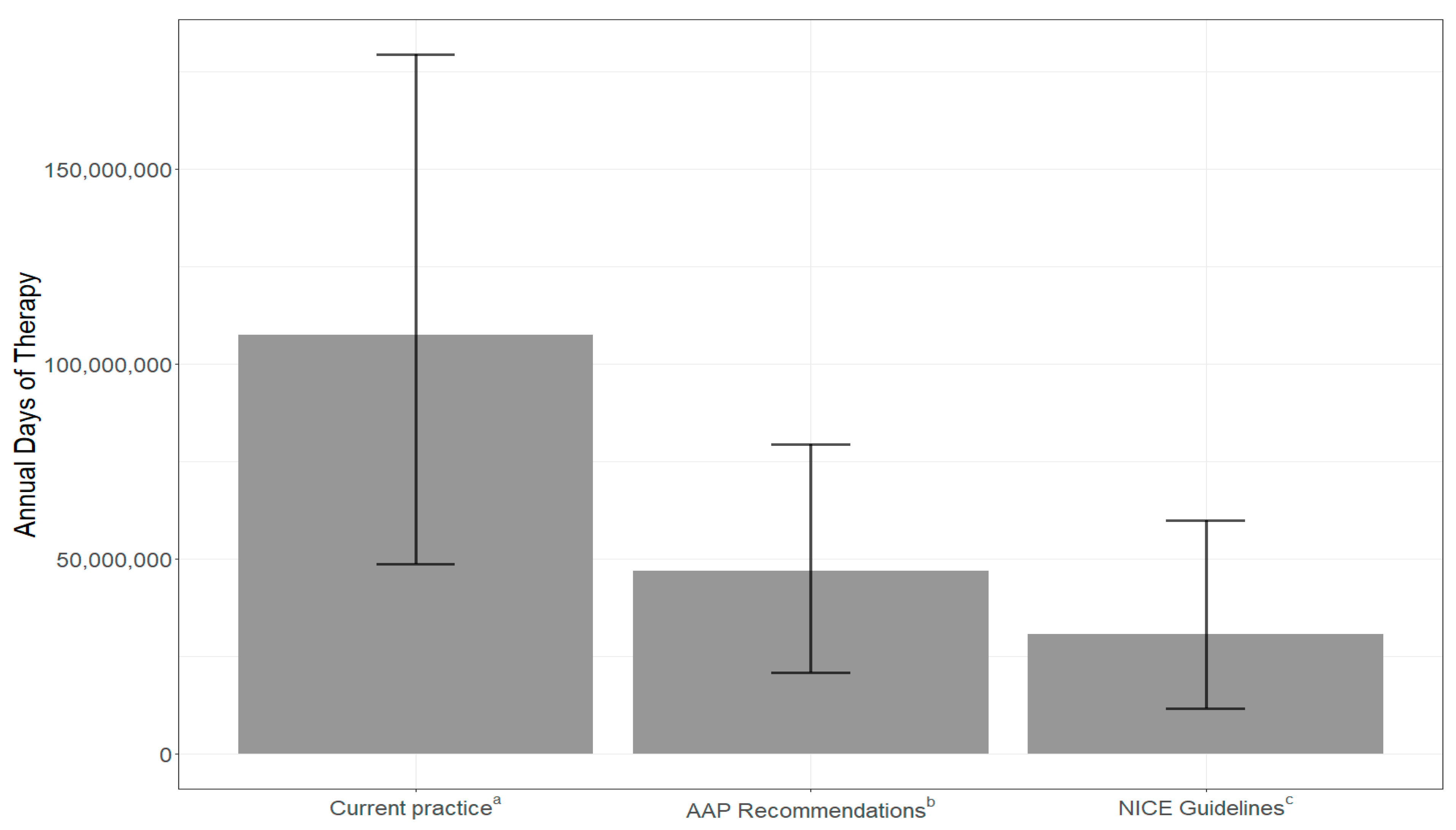
- If prescribers followed the American Academy of Pediatrics guidelines for prescribing, annual antibiotic days of therapy (DOT) could be reduced by 60.6 million days (56%), while following the National Institutes for Health and Care Excellence guidelines for prescribing could reduce DOT by 76.7 million days (71%).
- Adherence to national guidelines for AOM management could avert millions of antibiotic DOT annually.
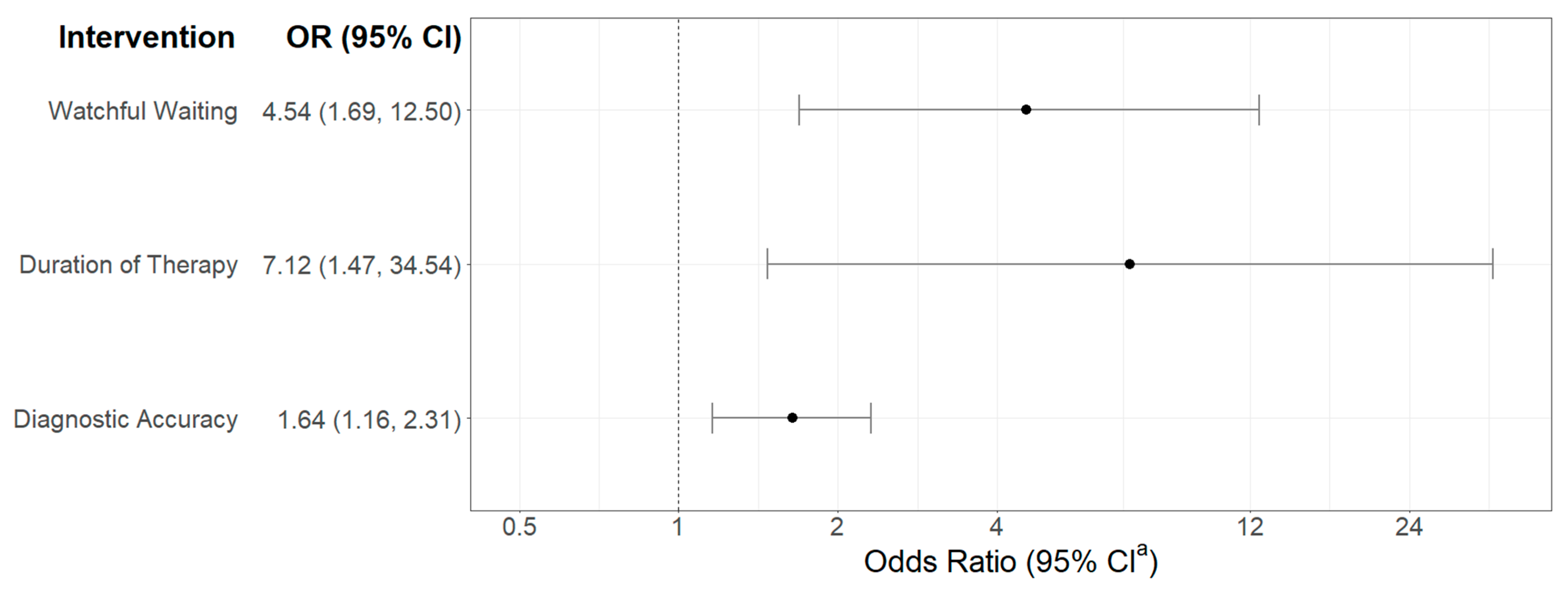
- Watchful waiting and short duration interventions have the greatest impact on antibiotic overprescribing.
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Data Collection and Bias Assessment
2.4. Data Items
2.5. Statistical Analysis
3. Results
3.1. Meta-Analysis
3.2. Simulation of Days of Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAP: | American Academy of Pediatrics |
| AOM: | Acute otitis media |
| CI: | Confidence interval |
| DOT: | Days of therapy |
| NICE: | National Institute for Health and Care Excellence |
References
- Finkelstein, J.A.; Metlay, J.P.; Davis, R.L.; Rifas-Shiman, S.L.; Dowell, S.F.; Platt, R. Antimicrobial use in defined populations of infants and young children. Arch. Pediatr. Adolesc. Med. 2000, 154, 395–400. [Google Scholar] [CrossRef]
- Lieberthal, A.S.; Carroll, A.E.; Chonmaitree, T.; Ganiats, T.G.; Hoberman, A.; Jackson, M.A.; Joffe, M.D.; Miller, D.T.; Rosenfeld, R.M.; Sevilla, X.D.; et al. The diagnosis and management of acute otitis media. Pediatrics 2013, 131, e964–e999. [Google Scholar] [CrossRef] [PubMed]
- Overview|Otitis Media (Acute): Antimicrobial Prescribing|Guidance|,N.I.C.E. 28 March 2018. Available online: https://www.nice.org.uk/guidance/ng91 (accessed on 20 November 2024).
- Antimicrobial Resistance. 20 November 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 November 2024).
- Khazanchi, R.; Brewster, R.; Butler, A.; O’Meara, D.; Bagchi, D.P.; Michelson, K. 1652. Impact of the 2022–2023 Amoxicillin Shortage on Antibiotic Prescribing for Acute Otitis Media: A Regression Discontinuity Study. Open Forum Infect. Dis. 2023, 10 (Suppl. 2), ofad500.1486. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Improving Outpatient Antibiotic Prescribing: A Toolkit for Healthcare Payers. 2021. Available online: https://www.cdc.gov/antibiotic-use/core-elements/pdfs/AU-Outpatient-Payer-Toolkit-508.pdf (accessed on 20 November 2024).
- Zay Ya, K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar] [CrossRef]
- Nedved, A.; Lee, B.R.; Hamner, M.; Wirtz, A.; Burns, A.; El Feghaly, R.E. Impact of an antibiotic stewardship program on antibiotic choice, dosing, and duration in pediatric urgent cares. Am. J. Infect. Control. 2023, 51, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Podmore, B.; Cuñado Moral, A.; Weiss, T.; Matthews, I.; Sarpong, E.; Méndez, I.; Qizilbash, N. Incidence of acute otitis media from 2003 to 2019 in children ≤17 years in England. BMC Public Health 2023, 23, 201. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 11 November 2023).
- Moons, K.G.M.; de Groot, J.A.H.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.G.; Dewez, J.E.; Nijman, R.G.; Yeung, S. Clinical practice guidelines for acute otitis media in children: A systematic review and appraisal of European national guidelines. BMJ Open 2020, 10, e035343. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45 Pt A, 139–145. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, N.E.; Antonelli, P.J.; Winterstein, A.G. Watchful Waiting for Acute Otitis Media. Pediatrics 2022, 150, e2021055613. [Google Scholar] [CrossRef] [PubMed]
- King, L.M.; Tsay, S.V.; Hicks, L.A.; Bizune, D.; Hersh, A.L.; Fleming-Dutra, K. Changes in outpatient antibiotic prescribing for acute respiratory illnesses, 2011 to 2018. Antimicrob. Steward. Heal. Epidemiol. ASHE 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Hu, T.; Done, N.; Petigara, T.; Petigara, T.; Mohanty, S.; Song, Y.; Liu, Q.; Lemus-Wirtz, E.; Signorovitch, J.; Sarpong, E.; et al. Incidence of acute otitis media in children in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998–2018. BMC Infect Dis. 2022, 22, 294. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Shapiro, D.J.; Hicks, L.A.; Gerber, J.S.; Hersh, A.L. Race, otitis media, and antibiotic selection. Pediatrics 2014, 134, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.S.; Maneno, M.K.; Daftary, M.N.; Wingate, L.; Ettienne, E.B. Factors Associated with Prescribing Broad-Spectrum Antibiotics for Children with Upper Respiratory Tract Infections in Ambulatory Care Settings. Clin. Med. Insights Pediatr. 2018, 12, 1179556518784300. [Google Scholar] [CrossRef]
- Kleinman, K.; Kim, J.; Solomon, B.; Canares, T. Evaluation of digital otoscopy in pediatric patients: A prospective randomized controlled clinical trial. Acad. Emerg. Med. 2021, 28 (Suppl. 1), S42. [Google Scholar] [CrossRef]
- Daggett, A.; Wyly, D.R.; Stewart, T.; Phillips, P.; Newell, C.; Lee, B.R.; Burns, A.; Sharma, N.; Shastri, N.; Rodean, J.; et al. Improving Emergency Department Use of Safety-Net Antibiotic Prescriptions for Acute Otitis Media. Pediatr. Emerg. Care 2022, 38, e1151–e1158. [Google Scholar] [CrossRef]
- Anderson, J.L.; Silva, L.O.J.; Bellolio, F.; VanMeter, D.E.; Mullan, A.F. Effect of the Ear Pain Conversation Aid in Parents of Children with Acute Otitis Media. Acad. Emerg. Med. 2023, 30, 183–184. [Google Scholar] [CrossRef]
- Chao, J.H.; Kunkov, S.; Reyes, L.B.; Lichten, S.; Crain, E.F. Comparison of two approaches to observation therapy for acute otitis media in the emergency department. Pediatrics 2008, 121, e1352–e1356. [Google Scholar] [CrossRef]
- Wolf, R.M.; Langford, K.T.; Patterson, B.L. Improving Adherence to AAP Acute Otitis Media Guidelines in an Academic Pediatrics Practice through a Quality Improvement Project. Pediatr. Qual. Saf. 2022, 7, e553. [Google Scholar] [CrossRef] [PubMed]
- Dona, D.; Baraldi, M.; Brigadoi, G.; Lundin, R.S.; Perilongo, G.; Hamdy, R.F.; Zaoutis, T.M.; Da Dalt, L.; Giaquinto, C. The Impact of Clinical Pathways on Antibiotic Prescribing for Acute Otitis Media and Pharyngitis in the Emergency Department. Pediatr. Infect. J. 2018, 37, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mangione-Smith, R.; McGlynn, E.A.; Elliott, M.N.; Krogstad, P.; Brook, R.H. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics 1999, 103 Pt 1, 711–718. [Google Scholar] [CrossRef]
- Altiner, A.; Knauf, A.; Moebes, J.; Sielk, M.; Wilm, S. Acute cough: A qualitative analysis of how GPs manage the consultation when patients explicitly or implicitly expect antibiotic prescriptions. Fam. Pract. 2004, 21, 500–506. [Google Scholar] [CrossRef]
- Davis, R.L.; Wright, J.; Chalmers, F.; Levenson, L.; Brown, J.C.; Lozano, P.; Christakis, D.A. A cluster randomized clinical trial to improve prescribing patterns in ambulatory pediatrics. PLoS Clin. Trials 2007, 2, e25. [Google Scholar] [CrossRef]
- Dube, A.R.; Zhao, A.R.B.; Odozor, C.U.M.; Jordan, K.; Garuba, F.O.B.; Kennedy, A.C.; Niesen, A.M.; Kyrouac, R.C.P.-C.; Stortz, D.C.; Lodhi, H.M.; et al. Improving Prescribing for Otitis Media in a Pediatric Emergency Unit: A Quality Improvement Initiative. Pediatr. Qual. Saf. 2023, 8, e625. [Google Scholar] [CrossRef]
- Zahlanie, Y.; Mang, N.; Lin, K.; Hynan, L.S.; Prokesch, B.C. Improved antibiotic prescribing practices for respiratory infections through use of computerized order sets and educational sessions in pediatric clinics. Open Forum Infect. Dis. 2020, 7 (Suppl. 1), S684–S685. [Google Scholar] [CrossRef]
- Frost, H.M.; Becker, L.F.; Knepper, B.C.; Shihadeh, K.C.; Jenkins, T.C. Antibiotic Prescribing Patterns for Acute Otitis Media for Children 2 Years and Older. J. Pediatr. 2020, 220, 109–115.e1. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M.; Keith, A.; Sebastian, T.; Jenkins, T.C. Caregiver perspectives and preferences for acute otitis media management. Antimicrob. Steward. Heal. Epidemiol. 2021, 1, e69. [Google Scholar] [CrossRef]
- Rinehart, D.J.; Gilbert, A.; O’Leary, S.; Katz, S.E.; Frost, H.M. Reducing antibiotic duration for acute otitis media: Clinician, administrator, and parental insights to inform implementation of system-level interventions. Antimicrob. Steward. Heal. Epidemiol. ASHE 2025, 5, e3. [Google Scholar] [CrossRef]
- Spoială, E.-L.; Stârcea, I.M.; Ioniuc, I.K.; Cozma, R.S.; Rusu, D.C.; Bozomitu, L.; Lupu, V.V.; Haliţchi, C.O.I.; Roşu, V.E.; Roşu, S.T.; et al. Watchful Waiting in Pediatric Acute Otitis Media: A Real Practice Approach or an Intangible Desideratum? Medicina 2023, 59, 520. [Google Scholar] [CrossRef]
- Folino, F.; Caruso, M.; Bosi, P.; Aldè, M.; Torretta, S.; Marchisio, P. Acute otitis media diagnosis in childhood: Still a problem in 2023? Ital. J. Pediatr. 2024, 50, 19. [Google Scholar] [CrossRef]
- McGrath, L.J.; Frost, H.M.; Newland, J.G.; O’Neil, C.A.; Sahrmann, J.M.; Ma, Y.; Butler, A.M. Utilization of nonguideline concordant antibiotic treatment following acute otitis media in children in the United States. Pharmacoepidemiol. Drug Saf. 2023, 32, 256–265. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.M.; Brown, D.S.; Durkin, M.J.; Sahrmann, J.M.; Nickel, K.B.; O’nEil, C.A.; Olsen, M.A.; Hyun, D.Y.; Zetts, R.M.; Newland, J.G. Association of Inappropriate Outpatient Pediatric Antibiotic Prescriptions with Adverse Drug Events and Health Care Expenditures. JAMA Netw. Open 2022, 5, e2214153. [Google Scholar] [CrossRef] [PubMed]
- Abuali, M.; Zivot, A.; Guerguis, S.; Valladares, E.; Aleem, S.; Gonzalez-Salazar, F.; Rouchou, B.; Mottola, N.; Braitman, L.; Paoletti, A. Outpatient antibiotic prescribing patterns in pediatric academic and community practices. Am. J. Infect. Control. 2019, 47, 1151–1153. [Google Scholar] [CrossRef]
- Barrera, S.C.; Cancino, R.; Barreto, T. The impact of continuity of care on antibiotic prescribing in acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 2019, 126, 109616. [Google Scholar] [CrossRef]
- Boatright, C.; Holcomb, L.; Replogle, W. Treatment Patterns for Pediatric Acute Otitis Media: A Gap in Evidence-Based Theory and Clinical Practice. Pediatr. Nurs. 2015, 41, 271–276. [Google Scholar] [PubMed]
- Bondy, J.; Berman, S.; Glazner, J.; Lezotte, D. Direct expenditures related to otitis media diagnoses: Extrapolations from a pediatric medicaid cohort. Pediatrics 2000, 105, E72. [Google Scholar] [CrossRef]
- Bradley, M.; Bacharouch, A.; Hart-Johnson, T.; Burrows, H.L.; Blackwood, R.A. Adopting otitis media practice guidelines increases adherence within a large primary care network. J. Paediatr. Child. Health 2021, 57, 1054–1059. [Google Scholar] [CrossRef]
- Brinker, D.L., Jr.; MacGeorge, E.L.; Hackman, N. Diagnostic Accuracy, Prescription Behavior, and Watchful Waiting Efficacy for Pediatric Acute Otitis Media. Clin. Pediatr. 2019, 58, 60–65. [Google Scholar] [CrossRef]
- Chiappini, E.; Motisi, M.A.; Becherucci, P.; Pierattelli, M.; Galli, L.; Marchisio, P. Italian primary care paediatricians’ adherence to the 2019 National Guideline for the management of acute otitis media in children: A cross-sectional study. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110282. [Google Scholar] [CrossRef] [PubMed]
- Coco, A.S.; Horst, M.A.; Gambler, A.S. Trends in broad-spectrum antibiotic prescribing for children with acute otitis media in the United States, 1998–2004. BMC Pediatr. 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Coco, A.; Vernacchio, L.; Horst, M.; Anderson, A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics 2010, 125, 214–220. [Google Scholar] [CrossRef]
- Cox, E.D.; Saluja, S. Criteria-based diagnosis and antibiotic overuse for upper respiratory infections. Ambul. Pediatr. 2008, 8, 250–254. [Google Scholar] [CrossRef]
- Crowson, M.G.; Bates, D.W.; Suresh, K.; Cohen, M.S.; Hartnick, C.J. “Human vs. Machine” Validation of a Deep Learning Algorithm for Pediatric Middle Ear Infection Diagnosis. Otolaryngol. Head. Neck Surg. 2023, 169, 41–46. [Google Scholar] [CrossRef]
- Cushen, R.; Francis, N.A. Antibiotic use and serious complications following acute otitis media and acute sinusitis: A retrospective cohort study. Br. J. Gen. Pract. 2020, 70, e255–e263. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, R.; van Balen, F.A.M.; Hoes, A.W.; Verheij, T.J.M.; de Melker, R.A. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. Br. Med. J. 2000, 320, 350–354. [Google Scholar] [CrossRef]
- Di, S.; McCarthy, T.J.; Liberman, D.B. Cost-Effectiveness of Watchful Waiting in Acute Otitis Media. Pediatrics 2017, 139, 1–9. [Google Scholar] [CrossRef]
- Esposito, S.; Lizioli, A.; Lastrico, A.; Begliatti, E.; Rognoni, A.; Tagliabue, C.; Cesati, L.; Carreri, V.; Principi, N. Impact on respiratory tract infections of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months of age. Respir. Res. 2007, 8, 12. [Google Scholar] [CrossRef]
- Fiks, A.G.; Zhang, P.; Localio, A.R.; Khan, S.; Grundmeier, R.W.; Karavite, D.J.; Bailey, C.; Alessandrini, E.A.; Forrest, C.B. Adoption of electronic medical record-based decision support for otitis media in children. Health Serv. Res. 2015, 50, 489–513. [Google Scholar] [CrossRef]
- Fischer, T.; Singer, A.J.; Chale, S. Observation option for acute otitis media in the emergency department. Pediatr. Emerg. Care 2009, 25, 575–578. [Google Scholar] [CrossRef]
- Frost, H.M.; Bizune, D.; Gerber, J.S.; Hersh, A.L.; Hicks, L.A.; Tsay, S.V. Amoxicillin Versus Other Antibiotic Agents for the Treatment of Acute Otitis Media in Children. J. Pediatr. 2022, 251, 98–104.e5. [Google Scholar] [CrossRef]
- Frost, H.M.; Lou, Y.; Keith, A.; Byars, A.; Jenkins, T.C. Increasing Guideline-Concordant Durations of Antibiotic Therapy for Acute Otitis Media. J. Pediatr. 2022, 240, 221–227.e9. [Google Scholar] [CrossRef]
- Frost, H.M.; Keith, A.; Fletcher, D.R.; Sebastian, T.; Dominguez, S.R.; Kurtz, M.; Parker, S.K.; Wilson, M.L.; Jenkins, T.C. Clinical Outcomes Associated with Amoxicillin Treatment for Acute Otitis Media in Children. J. Pediatric Infect. Dis. Soc. 2024, 13, 203–210. [Google Scholar] [CrossRef]
- Gaboury, I.; Coyle, K.; Coyle, D.; Le Saux, N. Treatment cost effectiveness in acute otitis media: A watch-and-wait approach versus amoxicillin. Paediatr. Child. Health 2010, 15, e14–e18. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, J.; Jeffe, D.B.; Shackelford, P. Diagnosis and treatment of acute otitis media: An assessment. Pediatrics 2003, 112 Pt 1, 143–149. [Google Scholar] [CrossRef] [PubMed]
- García Ventura, M.; García Vera, C.; Ruiz-Canela Cáceres, J. Therapeutic approach to acute otitis media in primary care in an urban area. Delayed antibiotic prescription evaluation. An. Pediatr. 2022, 96, 422–430. [Google Scholar] [CrossRef]
- Groth, A.; Enoksson, F.; Hermansson, A.; Hultcrantz, M.; Stalfors, J.; Stenfeldt, K. Acute mastoiditis in children in Sweden 1993–2007—No increase after new guidelines. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Gurnaney, H.; Spor, D.; Johnson, D.G.; Propp, R. Diagnostic accuracy and the observation option in acute otitis media: The Capital Region Otitis Project. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1315–1325. [Google Scholar] [CrossRef]
- Hoberman, A.; Paradise, J.L.; Rockette, H.E.; Shaikh, N.; Wald, E.R.; Kearney, D.H.; Colborn, D.K.; Kurs-Lasky, M.; Bhatnagar, S.; Haralam, M.A.; et al. Treatment of acute otitis media in children under 2 years of age. N. Engl. J. Med. 2011, 364, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hoberman, A.; Paradise, J.L.; Rockette, H.E.; Kearney, D.H.; Bhatnagar, S.; Shope, T.R.; Martin, J.M.; Kurs-Lasky, M.; Copelli, S.J.; Colborn, D.K.; et al. Shortened Antimicrobial Treatment for Acute Otitis Media in Young Children. N. Engl. J. Med. 2016, 375, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Hullegie, S.; Schilder, A.G.M.; Marchisio, P.; de Sévaux, J.L.H.; van der Velden, A.W.; van de Pol, A.C.; Boeijen, J.A.; Platteel, T.N.; Torretta, S.; Damoiseaux, R.A.M.J.; et al. A Strong Decline in the Incidence of Childhood Otitis Media During the COVID-19 Pandemic in the Netherlands. Front. Cell Infect. Microbiol. 2021, 11, 768377. [Google Scholar] [CrossRef]
- Islam, S.; Mannix, M.K.; Breuer, R.K.; Hassinger, A.B. Guideline Adherence and Antibiotic Utilization by Community Pediatricians, Private Urgent Care Centers, and a Pediatric Emergency Department. Clin. Pediatr. 2020, 59, 21–30. [Google Scholar] [CrossRef]
- Jokinen, S.; Ruohola, A.; Tähtinen, P.A. Parental experiences and opinions regarding the management of acute otitis media in Finland-a comparative questionnaire between 2006 and 2019. Fam. Pract. 2024, 41, 321–325. [Google Scholar] [CrossRef]
- Kalu, S.U.; Ataya, R.S.; McCormick, D.P.; Patel, J.A.; Revai, K.; Chonmaitree, T. Clinical spectrum of acute otitis media complicating upper respiratory tract viral infection. Pediatr. Infect. Dis. J. 2011, 30, 95–99. [Google Scholar] [CrossRef]
- Katz, S.E.; Jenkins, T.C.; Stein, A.B.; Thomas, G.; Koenig, N.; Starnes, G.L.; Newland, J.G.; Banerjee, R.; Frost, H.M. Durations of Antibiotic Treatment for Acute Otitis Media and Variability in Prescribed Durations Across Two Large Academic Health Systems. J. Pediatric Infect. Dis. Soc. 2024, 13, 455–465. [Google Scholar] [CrossRef]
- Kautz-Freimuth, S.; Redaèlli, M.; Samel, C.; Civello, D.; Altin, S.V.; Stock, S. Parental views on acute otitis media (AOM) and its therapy in children--results of an exploratory survey in German childcare facilities. BMC Pediatr. 2015, 15, 199. [Google Scholar] [CrossRef]
- King, L.M.; Hersh, A.L.; Hicks, L.A.; Fleming-Dutra, K.E. Duration of Outpatient Antibiotic Therapy for Common Outpatient Infections, 2017. Clin. Infect. Dis. 2021, 72, E663–E666. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, A.; Shaikh, N.; Hoberman, A.; Kovačević, J. Automated diagnosis of otitis media: Vocabulary and grammar. Int. J. Biomed. Imaging 2013, 2013, 327515. [Google Scholar] [CrossRef]
- Le Saux, N.; Gaboury, I.; Baird, M.; Klassen, T.P.; MacCormick, J.; Blanchard, C.; Pitters, C.; Sampson, M.; Moher, D. A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age. Cmaj 2005, 172, 335–341. [Google Scholar] [CrossRef]
- Linsk, R.; Cooke, J. Diagnosis and management of acute otitis media in Michigan. Clin. Pediatr. 2004, 43, 159–169. [Google Scholar] [CrossRef]
- Little, P.; Gould, C.; Williamson, I.; Moore, M.; Warner, G.; Dunleavey, J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001, 322, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Ronfani, L.; Nibali, S.C.; Tamburlini, G. Delayed prescription may reduce the use of antibiotics for acute otitis media: A prospective observational study in primary care. Arch. Pediatr. Adolesc. Med. 2005, 159, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Marom, T.; Tan, A.; Wilkinson, G.S.; Pierson, K.S.; Freeman, J.L.; Chonmaitree, T. Trends in Otitis Media-Related Health Care Use in the United States, 2001–2011. JAMA Pediatr. 2014, 168, 68–75. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.P.; Chonmaitree, T.; Pittman, C.; Saeed, K.; Friedman, N.R.; Uchida, T.; Baldwin, C.D. Nonsevere acute otitis media: A clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics 2005, 115, 1455–1465. [Google Scholar] [CrossRef]
- McGrath, L.J.; Becker-Dreps, S.; Pate, V.; Brookhart, M.A. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000–2011. PLoS ONE 2013, 8, e81210. [Google Scholar] [CrossRef]
- McKinsey, J.; Lee, B.R.; Wyly, D.; Austin, H.; Dosdos, D.; Murdock, E.; Patel, A.; El Feghaly, R.E.; Nedved, A. Increasing Safety Net Antibiotic Prescriptions for Acute Otitis Media in Urgent Care Clinics. J. Pediatr. Clin. Pract. 2024, 14, 200122. [Google Scholar] [CrossRef]
- Merenstein, D.; Diener-West, M.; Krist, A.; Pinneger, M.; Cooper, L.A. An assessment of the shared-decision model in parents of children with acute otitis media. Pediatrics 2005, 116, 1267–1275. [Google Scholar] [CrossRef]
- Nedved, A.; Bizune, D.; Fung, M.; Liu, C.M.; Tsay, S.; Hamdy, R.F.M.; Montalbano, A. Communication Strategies to Improve Antibiotic Prescribing in Pediatric Urgent Care Centers. Pediatr. Emerg. Care 2023, 40, 265–269. [Google Scholar] [CrossRef]
- Neumark, T.; Mölstad, S.; Rosén, C.; Persson, L.-G.; Törngren, A.; Brudin, L.; Eliasson, I. Evaluation of phenoxymethylpenicillin treatment of acute otitis media in children aged 2–16. Scand. J. Prim. Health Care 2007, 25, 166–171. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Marques, J.S.; Costa, I.S.; Reis, S.; Antunes, J.; Baptista, C. Acute otitis media in children, diagnosis and management. Arch. Dis. Child. 2021, 106 (Suppl. 2), A29. [Google Scholar]
- Olsen, J.K.; Lykkegaard, J.; Hansen, M.P.; Waldorff, F.B.; Lous, J.; Andersen, M.K. Prescription of antibiotics to children with acute otitis media in Danish general practice. BMC Fam. Pract. 2020, 21, 177. [Google Scholar] [CrossRef]
- Palma, S.; Rosafio, C.; Del Giovane, C.; Patianna, V.D.; Lucaccioni, L.; Genovese, E.; Bertolani, P.; Iughetti, L. The impact of the Italian guidelines on antibiotic prescription practices for acute otitis media in a paediatric emergency setting. Ital. J. Pediatr. 2015, 41, 37. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Diagnostic accuracy, tympanocentesis training performance, and antibiotic selection by pediatric residents in management of otitis media. Pediatrics 2002, 110, 1064–1070. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Poole, M.D. Assessing diagnostic accuracy and tympanocentesis skills in the management of otitis media. Arch. Pediatr. Adolesc. Med. 2001, 155, 1137–1142. [Google Scholar] [CrossRef]
- Rosenfeld, R.M. Diagnostic certainty for acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 2002, 64, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.; Pitaro, J.; Hackett, A.; Kozer, E.; Gavriel, H.; Muallem-Kalmovich, L.; Eviatar, E.; Marom, T. Appropriate and Inappropriate Treatment of Acute Otitis Media in the Pediatric Emergency Department. Pediatr. Infect. Dis. J. 2018, 37, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Ryborg, C.T.; Søndergaard, J.; Lous, J.; Munck, A.; Larsen, P.V.; Hansen, M.P.; Thomsen, J.L. Factors associated with antibiotic prescribing in children with otitis media. ISRN Family Med. 2013, 2013, 587452. [Google Scholar] [CrossRef]
- Shah-Becker, S.; Carr, M.M. Current management and referral patterns of pediatricians for acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 2018, 113, 19–21. [Google Scholar] [CrossRef]
- Shaikh, N.; Hoberman, A.; Kaleida, P.H.; Rockette, H.E.; Kurs-Lasky, M.; Hoover, H.; Pichichero, M.E.; Roddey, O.F.; Harrison, C.; Hadley, J.A.; et al. Otoscopic signs of otitis media. Pediatr. Infect. Dis. J. 2011, 30, 822–826. [Google Scholar] [CrossRef]
- Shireman, T.I.; Kelsey, K.A. Prescribing patterns and retreatment rates in patients with otitis media. Clin. Drug Investig. 2002, 22, 303–311. [Google Scholar] [CrossRef]
- Siegel, R.M.; Bien, J.; Lichtenstein, P.; Davis, J.; Khoury, J.C.; Knight, J.E.; Kiely, M.; Bernier, J. A safety-net antibiotic prescription for otitis media: The effects of a PBRN study on patients and practitioners. Clin. Pediatr. 2006, 45, 518–524. [Google Scholar] [CrossRef]
- Siegel, R.M.; Kiely, M.; Bien, J.P.; Joseph, E.C.; Davis, J.B.; Mendel, S.G.; Pestian, J.P.; DeWitt, T.G. Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics 2003, 112 Pt 1, 527–531. [Google Scholar] [CrossRef]
- Småbrekke, L.; Berild, D.; Giaever, A.; Myrbakk, T.; Fuskevåg, A.; Ericson, J.U.; Flaegstad, T.; Olsvik, Ø.; Ringertz, S.H. Educational intervention for parents and healthcare providers leads to reduced antibiotic use in acute otitis media. Scand. J. Infect. Dis. 2002, 34, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Spiro, D.M.; King, W.D.; Arnold, D.H.; Johnston, C.; Baldwin, S. A randomized clinical trial to assess the effects of tympanometry on the diagnosis and treatment of acute otitis media. Pediatrics 2004, 114, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Spiro, D.M.; Tay, K.Y.; Arnold, D.H.; Dziura, J.D.; Baker, M.D.; Shapiro, E.D. Wait-and-see prescription for the treatment of acute otitis media: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2006, 296, 1235–1241. [Google Scholar] [CrossRef]
- Stevanovic, T.; Komazec, Z.; Lemajic-Komazec, S.; Jovic, R. Acute otitis media: To follow-up or treat? Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Tähtinen, P.A.; Laine, M.K.; Ruuskanen, O.; Ruohola, A. Delayed versus immediate antimicrobial treatment for acute otitis media. Pediatr. Infect. Dis. J. 2012, 31, 1227–1232. [Google Scholar] [CrossRef]
- Tähtinen, P.A.; Laine, M.K.; Huovinen, P.; Jalava, J.; Ruuskanen, O.; Ruohola, A. A Placebo-Controlled Trial of Antimicrobial Treatment for Acute Otitis Media. N. Engl. J. Med. 2011, 364, 116–126. [Google Scholar] [CrossRef]
- Talathi, S.; Gupta, N.; Sethuram, S.; Khanna, S.; Sitnitskaya, Y. Otitis Media in Fully Vaccinated Preschool Children in the Pneumococcal Conjugate Vaccine Era. Glob. Pediatr. Health 2017, 4, 2333794x17749668. [Google Scholar] [CrossRef]
- Thompson, P.L.; Gilbert, R.E.; Long, P.F.; Saxena, S.; Sharland, M.; Wong, I.C. Has UK guidance affected general practitioner antibiotic prescribing for otitis media in children? J. Public Health 2008, 30, 479–486. [Google Scholar] [CrossRef]
- Tong, S.; Amand, C.; Kieffer, A.; Kyaw, M.H. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008–2014. BMC Health Serv. Res. 2018, 18, 318. [Google Scholar] [CrossRef]
- Uhl, B.D.; Boutzoukas, A.; Gallup, N.; Patrick, M.; Stultz, J.; Porter, C.R.; Watson, J.R. Increasing Adherence to Acute Otitis Media Treatment Duration Guidelines using a Quality Improvement Approach. Pediatr. Qual. Saf. 2021, 6, e501. [Google Scholar] [CrossRef] [PubMed]
- Vernacchio, L.; Vezina, R.M.; Mitchell, A.A. Knowledge and practices relating to the 2004 acute otitis media clinical practice guideline: A survey of practicing physicians. Pediatr. Infect Dis. J. 2006, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Vernacchio, L.; Vezina, R.M.; Mitchell, A.A. Management of acute otitis media by primary care physicians: Trends since the release of the 2004 American Academy of Pediatrics/American Academy of Family Physicians clinical practice guideline. Pediatrics 2007, 120, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wyly, D.R.; DeSchepper, A.; Nedved, A.; Lee, B.R.; El Feghaly, R.E. Appropriateness of Diagnosis and Management for Otitis Media with Effusion in Pediatric Urgent Care Clinics. Pediatr. Emerg. Care 2023, 39, 390–392. [Google Scholar] [CrossRef]
| Presentation | Child Age | AAP a Guidelines | NICE b Guidelines |
|---|---|---|---|
| Unilateral, Non-Severe AOM | 6–23 Months | 10 Days of Antibiotics; Consider Watchful Waiting | Watchful Waiting; If Prescribing, Consider 5–7 Days of Antibiotics |
| Unilateral, Severe AOM | 6–23 Months | 10 Days of Antibiotics | 5–7 Days of Antibiotics; |
| Bilateral, Severe and Non-Severe AOM | 6–23 Months | 10 Days of Antibiotics | 5–7 Days of Antibiotics; Consider Watchful Waiting if Non-Severe |
| Unilateral and Bilateral, Non-Severe AOM | 24+ Months | Consider Watchful Waiting; 7 Days of Antibiotics (children ages 2–5), 5–7 Days of Antibiotics (children ages 6+) | Watchful Waiting; If Prescribing, Consider 5–7 Days of Antibiotics |
| Unilateral and Bilateral, Severe AOM | 24+ Months | 10 Days of Antibiotics | 5–7 days of Antibiotics |
| AOM Outcome | Number of Studies | Heterogeneity | Pooled Estimate; % (95% CI a) | |
|---|---|---|---|---|
| I-Squared (%) | p-Value | |||
| Diagnostic Accuracy | 10 | 100% | <0.01 | 56.59 (42.76, 70.43) |
| Duration: 5 Days | 3 | 99% | <0.01 | 2.29 (0.00, 5.10) |
| Duration: 7 Days | 3 | 98% | <0.01 | 10.64 (1.69, 19.59 |
| Duration: 10 Days | 3 | 100% | <0.01 | 84.11 (74.98, 93.24) |
| Severe AOM b | 18 | 100% | <0.01 | 43.00 (31.18, 54.81) |
| Bilateral AOM b | 10 | 99% | <0.01 | 33.94 (23.42, 44.46) |
| AAP c Range: <2 years | 18 | 100% | <0.01 | 35.14 (25.67, 44.61) |
| AAP c Range: 2–5 years | 14 | 100% | <0.01 | 37.00 (26.25, 47.76) |
| AAP c Range: >5 years | 13 | 100% | <0.01 | 25.20 (20.40, 30.00) |
| Use of Watchful Waiting | 39 | 100% | <0.01 | 25.71 (18.44, 32.99) |
| Days of Therapy | Minimum Days of Therapy | Maximum Days of Therapy | ||
|---|---|---|---|---|
| Current Practice | 106,567,457 | 48,205,560 | 177,989,760 | |
| Correct Diagnosis | 60,306,524 | 27,279,526 | 100,724,405 | |
| Days of Therapy Saved | 46,260,933 | 20,926,034 | 77,265,355 | |
| % Decrease | 43.4% | 43.4% | 43.4% | |
| Correct Days of Therapy: AAP a | 98,019,085 | 43,592,120 | 166,471,296 | |
| Days of Therapy Saved | 8,547,652 | 4,613,440 | 11,518,464 | |
| % Decrease | 8.0% | 9.6% | 6.5% | |
| Use of Watchful Waiting: AAP a | 86,032,749 | 38,361,836 | 145,741,373 | |
| Days of Therapy Saved | 20,534,708 | 9,843,724 | 32,248,387 | |
| % Decrease | 19.3% | 20.4% | 18.1% | |
| Correct Days of Therapy: NICE b | 67,138,774 | 25,308,400 | 130,824,960 | |
| Days of Therapy Saved | 40,277,056 | 23,280,920 | 48,581,760 | |
| % Decrease | 37.5% | 47.9% | 27.1% | |
| Use of Watchful Waiting: NICE b | 57,299,788 | 21,599,530 | 111,652,955 | |
| Days of Therapy Saved | 49,267,669 | 26,606,030 | 66,336,805 | |
| % Decrease | 46.2% | 55.2% | 37.3% | |
| Intervention | Average DOT (Minimum–Maximum) | Reduction in DOT a | % Reduction |
|---|---|---|---|
| Current Practice—No Intervention | 106,567,457 (48,205,560–177,989,760) | Reference | Reference |
| Watchful Waiting: AAP b | 102,104,877 (46,066,333–170,959,844) | 4,462,580 | 4.2% |
| Watchful Waiting: NICE c | 95,860,661 (42,423,567–163,573,518) | 10,706,796 | 10.0% |
| Duration Therapy: AAP b | 103,896,628 (46,764,029–174,390,661) | 2,670,829 | 2.5% |
| Duration Therap: NICE c | 93,982,350 (40,931,124–162,809,737) | 12,585,107 | 11.8% |
| Diagnostic Accuracy | 102,183,717 (46,222,585–170,668,004) | 4,383,740 | 4.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, T.L.; Stein, A.B.; El Feghaly, R.E.; Nedved, A.C.; Katz, S.E.; Keith, A.; Laferriere, H.E.; Jenkins, T.C.; Frost, H.M. Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis. Children 2025, 12, 1408. https://doi.org/10.3390/children12101408
Morin TL, Stein AB, El Feghaly RE, Nedved AC, Katz SE, Keith A, Laferriere HE, Jenkins TC, Frost HM. Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis. Children. 2025; 12(10):1408. https://doi.org/10.3390/children12101408
Chicago/Turabian StyleMorin, Theresa L., Amy B. Stein, Rana E. El Feghaly, Amanda C. Nedved, Sophie E. Katz, Amy Keith, Heather E. Laferriere, Timothy C. Jenkins, and Holly M. Frost. 2025. "Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis" Children 12, no. 10: 1408. https://doi.org/10.3390/children12101408
APA StyleMorin, T. L., Stein, A. B., El Feghaly, R. E., Nedved, A. C., Katz, S. E., Keith, A., Laferriere, H. E., Jenkins, T. C., & Frost, H. M. (2025). Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis. Children, 12(10), 1408. https://doi.org/10.3390/children12101408






