The Role of Pharmacotherapy in Social Cognition, Empathy, and Serum Oxytocin Levels in Children with Attention Deficit Hyperactivity Disorder: A Case–Control Study
Abstract
Highlights
- In children with attention-deficit/hyperactivity disorder (ADHD), regular pharmacological treatment with either methylphenidate (MPH) or atomoxetine (ATX) was associated with significant improvements in social skills, with ATX specifically linked to enhanced social cognition performance.
- Empathy (BEI scores) and serum oxytocin levels did not significantly differ across treatment and control groups.
- The findings suggest that pharmacotherapy may improve social cognition, though long-term effects on empathy or serum oxytocin levels remain unclear.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Psychometric Evaluation and Measurements
2.2.1. Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children–Present and Lifetime (K-SADS-PL)
2.2.2. Social Skills Rating Scale (SSRS)
2.2.3. Reading the Mind in the Eyes Test–Revised Child Version (RMET)
2.2.4. Bryant Empathy Index (BEI)
2.2.5. The Swanson, Nolan, and Pelham Questionnaire (SNAP-IV) Scale
2.2.6. Sample Collection
2.2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| ADHD-IA | Attention-Deficit/Hyperactivity Disorder, Inattentive Subtype |
| ADHD-CT | Attention-Deficit/Hyperactivity Disorder, Combined Subtype |
| ATX | Atomoxetine |
| BEI | Bryant Empathy Index |
| DA | Dopamine |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| K-SADS-5-PL | Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version |
| MPH | Methylphenidate |
| NE | Norepinephrine |
| PFC | Prefrontal cortex |
| RMET | Reading the Mind in the Eyes Test—Revised Child Version |
| SNAP-IV | The Swanson, Nolan, and Pelham Questionnaire |
| SRSS | Social Skills Rating Scale |
| ToM | Theory of Mind |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; DSM-5-TR; American Psychiatric Association Publishing: Washington, DC, USA, 2022; ISBN 978-0-89042-575-6. [Google Scholar]
- Barkley, R.A. (Ed.) Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 4th ed.; Guilford Press: New York, NY, USA; London, UK, 2015; ISBN 978-1-4625-3887-4. [Google Scholar]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative Efficacy and Tolerability of Medications for Attention-Deficit Hyperactivity Disorder in Children, Adolescents, and Adults: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Cortese, S. Pharmacologic Treatment of Attention Deficit–Hyperactivity Disorder. N. Engl. J. Med. 2020, 383, 1050–1056. [Google Scholar] [CrossRef]
- Oner, O.; Turkcapar, H.; Isli, F.; Karadag, H.; Akbulat, A.; Basci, A.B.; Aksoy, M.; Seckin, C.; Alkan, A. Attention Deficit Hyperactivity Disorder Treatment Practice in Turkey. Klin. Psikofarmakol. Bülteni-Bull. Clin. Psychopharmacol. 2016, 26, 265–272. [Google Scholar] [CrossRef]
- Nijmeijer, J.S.; Minderaa, R.B.; Buitelaar, J.K.; Mulligan, A.; Hartman, C.A.; Hoekstra, P.J. Attention-Deficit/Hyperactivity Disorder and Social Dysfunctioning. Clin. Psychol. Rev. 2008, 28, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.Y.; Normand, S. The Importance of Social Contextual Factors in Peer Relationships of Children with ADHD. Curr. Dev. Disord. Rep. 2015, 2, 30–37. [Google Scholar] [CrossRef]
- Zöggeler-Burkhardt, L.; Embacher, E.-M.; Smidt, W. Social Relationships, Interactions and Learning in Early Childhood—Theoretical Approaches, Empirical Findings and Challenges. Early Child Dev. Care 2023, 193, 1199–1203. [Google Scholar] [CrossRef]
- Humphreys, K.L.; Galán, C.A.; Tottenham, N.; Lee, S.S. Impaired Social Decision-Making Mediates the Association Between ADHD and Social Problems. J. Abnorm. Child Psychol. 2016, 44, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.M.; Lefler, E.K. ADHD Symptomology and Social Functioning in College Students. J. Atten. Disord. 2017, 21, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.M.; Gerdes, A.C. A Review of Peer Relationships and Friendships in Youth with ADHD. J. Atten. Disord. 2015, 19, 844–855. [Google Scholar] [CrossRef]
- Morris, S.; Sheen, J.; Ling, M.; Foley, D.; Sciberras, E. Interventions for Adolescents with ADHD to Improve Peer Social Functioning: A Systematic Review and Meta-Analysis. J. Atten. Disord. 2021, 25, 1479–1496. [Google Scholar] [CrossRef]
- Ros, R.; Graziano, P.A. Social Functioning in Children with or At Risk for Attention Deficit/Hyperactivity Disorder: A Meta-Analytic Review. J. Clin. Child Adolesc. Psychol. 2018, 47, 213–235. [Google Scholar] [CrossRef]
- Spender, K.; Chen, Y.-W.R.; Wilkes-Gillan, S.; Parsons, L.; Cantrill, A.; Simon, M.; Garcia, A.; Cordier, R. The Friendships of Children and Youth with Attention-Deficit Hyperactivity Disorder: A Systematic Review. PLoS ONE 2023, 18, e0289539. [Google Scholar] [CrossRef]
- Suri, D.; Teixeira, C.M.; Cagliostro, M.K.C.; Mahadevia, D.; Ansorge, M.S. Monoamine-Sensitive Developmental Periods Impacting Adult Emotional and Cognitive Behaviors. Neuropsychopharmacology 2015, 40, 88–112. [Google Scholar] [CrossRef]
- Sharp, J.L.; Smith, M.A. The Effects of Drugs on Behavior Maintained by Social Contact: Role of Monoamines in Social Reinforcement. Front. Behav. Neurosci. 2022, 15, 805139. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Zhang, X.; Gao, L. ASD and ADHD: Divergent Activating Patterns of Prefrontal Cortex in Executive Function Tasks? J. Psychiatr. Res. 2024, 172, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, F.; Kausel, L.; Albornoz, C.; Lavin, C.; Figueroa-Vargas, A.; Stecher, X.; Aragón-Caqueo, D.; Carrasco, X.; Aboitiz, F.; Billeke, P. Lateral Prefrontal Theta Oscillations Reflect Proactive Cognitive Control Impairment in Males with Attention Deficit Hyperactivity Disorder. Front. Syst. Neurosci. 2020, 14, 37. [Google Scholar] [CrossRef]
- Xing, B.; Li, Y.-C.; Gao, W.-J. Norepinephrine versus Dopamine and Their Interaction in Modulating Synaptic Function in the Prefrontal Cortex. Brain Res. 2016, 1641, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.R.; Arnsten, A.F.T. Neuronal Mechanisms Underlying Attention Deficit Hyperactivity Disorder: The Influence of Arousal on Prefrontal Cortical Function. Ann. N. Y. Acad. Sci. 2008, 1129, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.L.; Keightley, M.L. Studies of Altered Social Cognition in Neuropsychiatric Disorders Using Functional Neuroimaging. Can. J. Psychiatry 2002, 47, 327–336. [Google Scholar] [CrossRef]
- Baron-Cohen, S. Theory of Mind in Normal Development and Autism. Prisme 2001, 34, 74–183. [Google Scholar]
- Uekermann, J.; Kraemer, M.; Abdel-Hamid, M.; Schimmelmann, B.G.; Hebebrand, J.; Daum, I.; Wiltfang, J.; Kis, B. Social Cognition in Attention-Deficit Hyperactivity Disorder (ADHD). Neurosci. Biobehav. Rev. 2010, 34, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Dvash, J.; Shamay-Tsoory, S.G. Theory of Mind and Empathy as Multidimensional Constructs: Neurological Foundations. Top. Lang. Disord. 2014, 34, 282–295. [Google Scholar] [CrossRef]
- Maoz, H.; Gvirts, H.Z.; Sheffer, M.; Bloch, Y. Theory of Mind and Empathy in Children with ADHD. J. Atten. Disord. 2019, 23, 1331–1338. [Google Scholar] [CrossRef]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef]
- Ebert, A.; Brüne, M. Oxytocin and Social Cognition. In Behavioral Pharmacology of Neuropeptides: Oxytocin; Hurlemann, R., Grinevich, V., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2017; Volume 35, pp. 375–388. ISBN 978-3-319-63738-9. [Google Scholar]
- Erdozain, A.M.; Peñagarikano, O. Oxytocin as Treatment for Social Cognition, Not There Yet. Front. Psychiatry 2020, 10, 930. [Google Scholar] [CrossRef]
- Ross, H.E.; Young, L.J. Oxytocin and the Neural Mechanisms Regulating Social Cognition and Affiliative Behavior. Front. Neuroendocrinol. 2009, 30, 534–547. [Google Scholar] [CrossRef]
- Burenkova, O.V.; Dolgorukova, T.A.; An, I.; Kustova, T.A.; Podturkin, A.A.; Shurdova, E.M.; Talantseva, O.I.; Zhukova, M.A.; Grigorenko, E.L. Endogenous Oxytocin and Human Social Interactions: A Systematic Review and Meta-Analysis. Psychol. Bull. 2023, 149, 549–579. [Google Scholar] [CrossRef]
- Gallagher, S.; Varga, S. Social Cognition and Psychopathology: A Critical Overview. World Psychiatry 2015, 14, 5–14. [Google Scholar] [CrossRef]
- Haza, B.; Gosling, C.J.; Ciminaghi, F.; Conty, L.; Pinabiaux, C. Research Review: Social Cognition and Everyday Social Skills in Children and Adolescents with Attention-deficit/Hyperactivity Disorder: A Meta-analysis of Case–Control Studies. Child Psychol. Psychiatry 2024, 65, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V. Reading Mind from the Eyes in Individuals with Attention Deficit-Hyperactivity Disorder (ADHD): A Meta-Analysis. Expert Rev. Neurother. 2022, 22, 889–896. [Google Scholar] [CrossRef]
- Bora, E.; Pantelis, C. Meta-Analysis of Social Cognition in Attention-Deficit/Hyperactivity Disorder (ADHD): Comparison with Healthy Controls and Autistic Spectrum Disorder. Psychol. Med. 2016, 46, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Casula, A.; Belluardo, G.; Antenucci, C.; Bianca, F.; Corallo, F.; Ferraioli, F.; Gargano, D.; Giuffrè, S.; Giunta, A.L.C.; La Torre, A.; et al. The Role of Empathy in ADHD Children: Neuropsychological Assessment and Possible Rehabilitation Suggestions—A Narrative Review. Medicina 2025, 61, 505. [Google Scholar] [CrossRef]
- Dessoki, H.H.; Amin, O.R.; Soltan, M.R.; Abbas, M.M.; Dawoud, M.E. Social Cognitive Deficits in Male Children with Attention Deficit Hyperactivity Disorder in Relation to Salivary Oxytocin Level. Middle East Curr. Psychiatry 2020, 27, 15. [Google Scholar] [CrossRef]
- Keech, B.; Crowe, S.; Hocking, D.R. Intranasal Oxytocin, Social Cognition and Neurodevelopmental Disorders: A Meta-Analysis. Psychoneuroendocrinology 2018, 87, 9–19. [Google Scholar] [CrossRef]
- Sasaki, T.; Hashimoto, K.; Oda, Y.; Ishima, T.; Kurata, T.; Takahashi, J.; Kamata, Y.; Kimura, H.; Niitsu, T.; Komatsu, H.; et al. Decreased Levels of Serum Oxytocin in Pediatric Patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res. 2015, 228, 746–751. [Google Scholar] [CrossRef]
- Wernicke, J.; Zhang, Y.; Felten, A.; Du, J.; Yao, S.; Kou, J.; Chen, Y.; Kendrick, K.M.; Becker, B.; Reuter, M.; et al. Blood Oxytocin Levels Are Not Associated with ADHD Tendencies and Emotionality in Healthy Adults. Neurosci. Lett. 2020, 738, 135312. [Google Scholar] [CrossRef]
- Boyle, D.; Levi-Shachar, O.; Gvirts, H.Z.; Zagoory-Sharon, O.; Feldman, R.; Bloch, Y.; Nitzan, U.; Maoz, H. Lack of Association between Severity of ADHD Symptoms and Salivary Oxytocin Levels. Psychoneuroendocrinology 2021, 131, 105293. [Google Scholar] [CrossRef] [PubMed]
- Alkalay, S.; Dan, O. Effect of Short-Term Methylphenidate on Social Impairment in Children with Attention Deficit/Hyperactivity Disorder: Systematic Review. Child. Adolesc. Psychiatry Ment. Health 2022, 16, 93. [Google Scholar] [CrossRef]
- Levi-Shachar, O.; Gvirts, H.Z.; Goldwin, Y.; Bloch, Y.; Shamay-Tsoory, S.; Zagoory-Sharon, O.; Feldman, R.; Maoz, H. The Effect of Methylphenidate on Social Cognition and Oxytocin in Children with Attention Deficit Hyperactivity Disorder. Neuropsychopharmacology 2020, 45, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Demirci, E.; Ozmen, S.; Kilic, E.; Oztop, D.B. The Relationship between Aggression, Empathy Skills and Serum Oxytocin Levels in Male Children and Adolescents with Attention Deficit and Hyperactivity Disorder. Behav. Pharmacol. 2016, 27, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.H.; Kim, Y.-H. Clinical Practice Guidelines for Attention-Deficit/Hyperactivity Disorder: Recent Updates. Clin. Exp. Pediatr. 2024, 67, 26–34. [Google Scholar] [CrossRef]
- Kaufman, J.; Townsend, L.D.; Kobak, K. The Computerized Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS): Development and Administration Guidelines. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, S357. [Google Scholar] [CrossRef]
- Unal, F.; Oktem, F.; Cetin Cuhadaroglu, F.; Cengel Kultur, S.E.; Akdemir, D.; Foto Ozdemir, D.; Cak, H.T.; Unal, D.; Tiras, K.; Aslan, C.; et al. Reliability and Validity of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version, DSM-5 November 2016-Turkish Adaptation (K-SADS-PL-DSM-5-T). Turk. J. Psychiatry 2019, 30, 42–50. [Google Scholar] [CrossRef]
- Akçamete, G.A.H. Sosyal Becerileri Değerlendirme Ölçeği’nin (7–12 Yaş) Geçerlik ve Güvenirlik Çalışması; Abant İzzet Baysal Üniversitesi Eğitim Fakültesi Dergisi: Gölköy, Turkey, 2016; pp. 61–77. [Google Scholar]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-Functioning Autism. J. Child Psychol. Psychiatry 2001, 42, 241–251. [Google Scholar] [CrossRef]
- Girli, A. Psychometric Properties of the Turkish Child and Adult Form of “Reading the Mind in the Eyes Test”. Psych 2014, 5, 1321–1337. [Google Scholar] [CrossRef]
- Bryant, B.K. An Index of Empathy for Children and Adolescents. Child Dev. 1982, 53, 413. [Google Scholar] [CrossRef]
- Yüksel, A. Empati Eğitim Programının İlköğretim Öğrencilerinin Empatik Becerilerine Etkisi. Ph.D. Thesis, Ankara Üniversitesi Sosyal Bilimler Enstitüsü, Ankara, Turkey, 2003. [Google Scholar]
- Bussing, R.; Fernandez, M.; Harwood, M.; Wei, H.; Garvan, C.W.; Eyberg, S.M.; Swanson, J.M. Parent and Teacher SNAP-IV Ratings of Attention Deficit Hyperactivity Disorder Symptoms: Psychometric Properties and Normative Ratings From a School District Sample. Assessment 2008, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.M.; Schuck, S.; Porter, M.M.; Carlson, C.; Hartman, C.A.; Sergeant, J.A.; Clevenger, W.; Wasdell, M.; McCleary, R.; Lakes, K.; et al. Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int. J. Educ. Psychol. Assess. 2012, 10, 51–70. [Google Scholar] [PubMed]
- Tabak, B.A.; Leng, G.; Szeto, A.; Parker, K.J.; Verbalis, J.G.; Ziegler, T.E.; Lee, M.R.; Neumann, I.D.; Mendez, A.J. Advances in Human Oxytocin Measurement: Challenges and Proposed Solutions. Mol. Psychiatry 2023, 28, 127–140. [Google Scholar] [CrossRef]
- Horvat-Gordon, M.; Granger, D.A.; Schwartz, E.B.; Nelson, V.J.; Kivlighan, K.T. Oxytocin Is Not a Valid Biomarker When Measured in Saliva by Immunoassay. Physiol. Behav. 2005, 84, 445–448. [Google Scholar] [CrossRef]
- Bowen, R.A.R.; Remaley, A.T. Interferences from Blood Collection Tube Components on Clinical Chemistry Assays. Biochem. Med. 2014, 24, 31–44. [Google Scholar] [CrossRef]
- Arango-Tobón, O.E.; Guevara Solórzano, A.; Orejarena Serrano, S.J.; Olivera-La Rosa, A. Social Cognition and Prosocial Behavior in Children with Attention Deficit Hyperactivity Disorder: A Systematic Review. Healthcare 2023, 11, 1366. [Google Scholar] [CrossRef]
- Humphreys, K.L.; Katz, S.J.; Lee, S.S.; Hammen, C.; Brennan, P.A.; Najman, J.M. The Association of ADHD and Depression: Mediation by Peer Problems and Parent–Child Difficulties in Two Complementary Samples. J. Abnorm. Psychol. 2013, 122, 854–867. [Google Scholar] [CrossRef]
- Shang, C.-Y.; Shih, H.-H.; Pan, Y.-L.; Lin, H.-Y.; Gau, S.S.-F. Comparative Efficacy of Methylphenidate and Atomoxetine on Social Adjustment in Youths with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2020, 30, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Pitzianti, M.B.; Spiridigliozzi, S.; Bartolucci, E.; Esposito, S.; Pasini, A. New Insights on the Effects of Methylphenidate in Attention Deficit Hyperactivity Disorder. Front. Psychiatry 2020, 11, 531092. [Google Scholar] [CrossRef] [PubMed]
- Aduen, P.A.; Day, T.N.; Kofler, M.J.; Harmon, S.L.; Wells, E.L.; Sarver, D.E. Social Problems in ADHD: Is It a Skills Acquisition or Performance Problem? J. Psychopathol. Behav. Assess. 2018, 40, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, C.J.; Faries, D.; Vaughan, B.; Perwien, A.; Busner, J.; Saylor, K.; Kaplan, S.; Buermeyer, C.; Swindle, R. Emotional Expression During Attention-Deficit/Hyperactivity Disorders Treatment: Initial Assessment of Treatment Effects. J. Child Adolesc. Psychopharmacol. 2007, 17, 51–62. [Google Scholar] [CrossRef]
- Wehmeier, P.M.; Schacht, A.; Lehmann, M.; Dittmann, R.W.; Silva, S.G.; March, J.S. Emotional Well-Being in Children and Adolescents Treated with Atomoxetine for Attention-Deficit/Hyperactivity Disorder: Findings from a Patient, Parent and Physician Perspective Using Items from the Pediatric Adverse Event Rating Scale (PAERS). Child. Adolesc. Psychiatry Ment. Health 2008, 2, 11. [Google Scholar] [CrossRef]
- Golubchik, P.; Weizman, A. The Possible Effect of Methylphenidate Treatment on Empathy in Children Diagnosed with Attention-Deficit/Hyperactivity Disorder, Both With and Without Comorbid Oppositional Defiant Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 429–432. [Google Scholar] [CrossRef]
- Fantozzi, P.; Sesso, G.; Muratori, P.; Milone, A.; Masi, G. Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants. Brain Sci. 2021, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, F.; Yilmaz, I.; Yulaf, Y.; Gokce, S.; Sabuncuoglu, O. Empathy and Facial Expression Recognition in Children with and Without Attention-Deficit/Hyperactivity Disorder: Effects of Stimulant Medication on Empathic Skills in Children with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 433–439. [Google Scholar] [CrossRef]
- Belal, M.; Moussa, S.; Omnia, R.A.; Fakher, W. Salivary Oxytocin Levels, Empathy, and Executive Functions in Egyptian Children with ADHD: A Case–Control Study. Middle East Curr. Psychiatry 2025, 32, 47. [Google Scholar] [CrossRef]
| Non-Medicated Group (n = 50), Mean ± SD | MPH (n = 52), Mean ± SD | ATX (n = 50) Mean ± SD | F * | Partial Eta Squared | p | Post Hoc (Games–Howell) | |
|---|---|---|---|---|---|---|---|
| Basic Social Skills | 38.70 ± 7.33 | 54.67 ± 7.01 | 57.82 ± 4.36 | 126.17 | 0.635 | <0.001 | ATX > MPH > Control |
| Basic Communication Skills | 11.98 ± 3.02 | 15.31 ± 3.32 | 17.98 ± 1.58 | 80.55 | 0.444 | <0.001 | ATX > MPH > Control |
| Advanced Communication Skills | 12.94 ± 2.82 | 19.02 ± 4.54 | 20.48 ± 3.50 | 79.50 | 0.440 | <0.001 | ATX = MPH > Control |
| Initiating Relationships | 21.06 ± 3.78 | 22.31 ± 3.00 | 23.18 ± 2.40 | 5.70 | 0.073 | 0.005 | ATX > Control, MPH = ATX, MPH = Control |
| Maintaining Relationships | 22.98 ± 3.79 | 26.02 ± 4.37 | 27.34 ± 3.06 | 20.10 | 0.190 | <0.001 | ATX = MPH > Control |
| Working in Groups | 22.68 ± 7.08 | 28.83 ± 5.97 | 31.38 ± 4.62 | 26.29 | 0.273 | <0.001 | ATX > MPH > Control |
| Emotional Skills | 19.44 ± 6.38 | 23.50 ± 5.36 | 25.84 ± 3.32 | 20.31 | 0.208 | <0.001 | ATX > MPH > Control |
| Self-Control Skills | 17.70 ± 5.53 | 22.96 ± 5.26 | 24.26 ± 4.39 | 22.44 | 0.240 | <0.001 | ATX = MPH > Control |
| Coping with Aggressive Behaviors | 13.70 ± 3.56 | 15.48 ± 3.35 | 15.92 ± 2.15 | 7.13 | 0.089 | 0.001 | ATX = MPH > Control |
| Accepting Consequences | 7.60 ± 2.89 | 10.98 ± 3.22 | 11.38 ± 2.51 | 26.95 | 0.258 | <0.001 | ATX = MPH > Control |
| Giving Instructions | 12.76 ± 3.18 | 17.23 ± 2.26 | 17.74 ± 1.83 | 47.58 | 0.451 | <0.001 | ATX = MPH > Control |
| Cognitive Skills | 17.58 ± 4.73 | 23.40 ± 5.36 | 24.04 ± 3.85 | 30.36 | 0.279 | <0.001 | ATX = MPH > Control |
| Control (n = 50), Mean ± SD | MPH (n = 52), Mean ± SD | ATX (n = 50) Mean ± SD | F * | Partial Eta Squared | p | Post Hoc (Games–Howell) | |
|---|---|---|---|---|---|---|---|
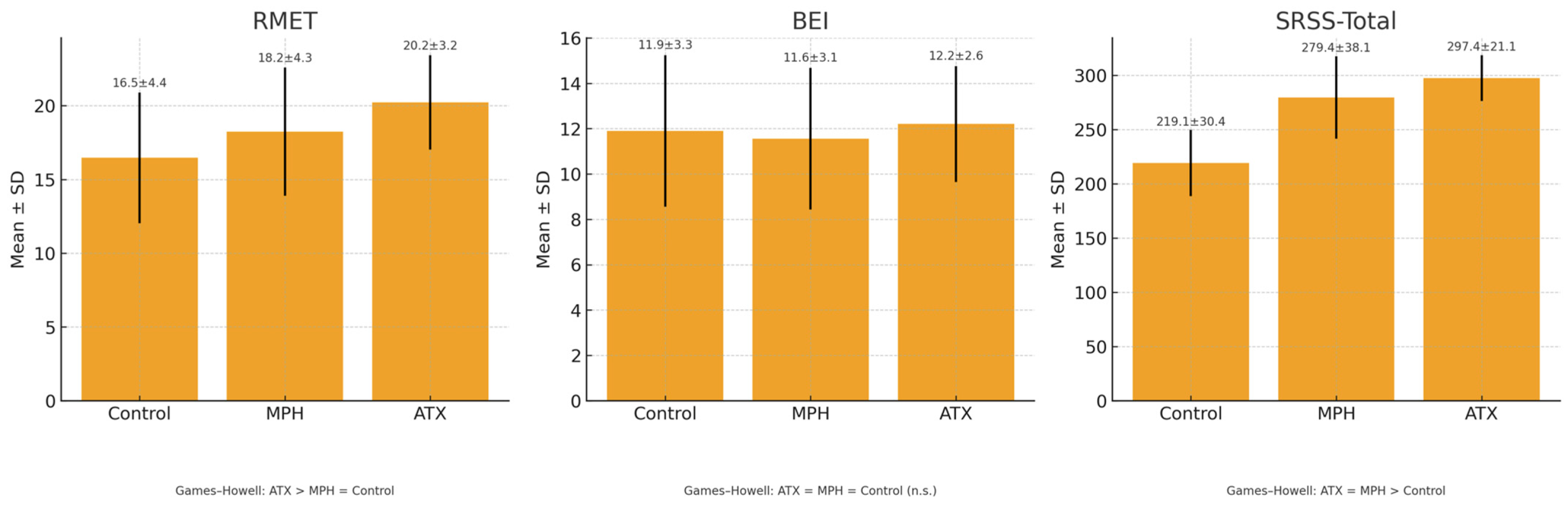
| RMET | 16.46 ± 4.43 | 18.23 ± 4.35 | 20.22 ± 3.20 | 12.24 | 0.127 | <0.001 | ATX > MPH = Control |
| BEI | 11.90 ± 3.34 | 11.56 ± 3.12 | 12.20 ± 2.56 | 0.65 | 0.008 | 0.525 | ATX = MPH = Control |
| SRSS-Total | 219.12 ± 30.40 | 279.44 ± 38.07 | 297.36 ± 21.13 | 111.72 | 0.544 | <0.001 | ATX = MPH > Control |
| Control (n = 50), Med (IQR) | MPH (n = 52), Med (IQR) | ATX (n = 50), Med (IQR) | H | Epsilon Squared | p | |
|---|---|---|---|---|---|---|
| Oxytocin | 50.65 (39.27–68.91) | 57.55 (43.23–87.04) | 46.64 (41.36–75.75) | 2.94 | 0.01 | 0.230 |
| Variables a | RMET | SRSS-Total Score | BEI | Oxytocin |
|---|---|---|---|---|
| SNAP-IV | −0.174 * | −0.527 ** | −0.005 | −0.056 |
| RMET | 0.593 ** | 0.002 | −0.049 | |
| SRSS-Total Score | 0.094 | 0.042 | ||
| BEI | −0.011 |
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | β | t | p | 95% CI | β | t | p | 95% CI |
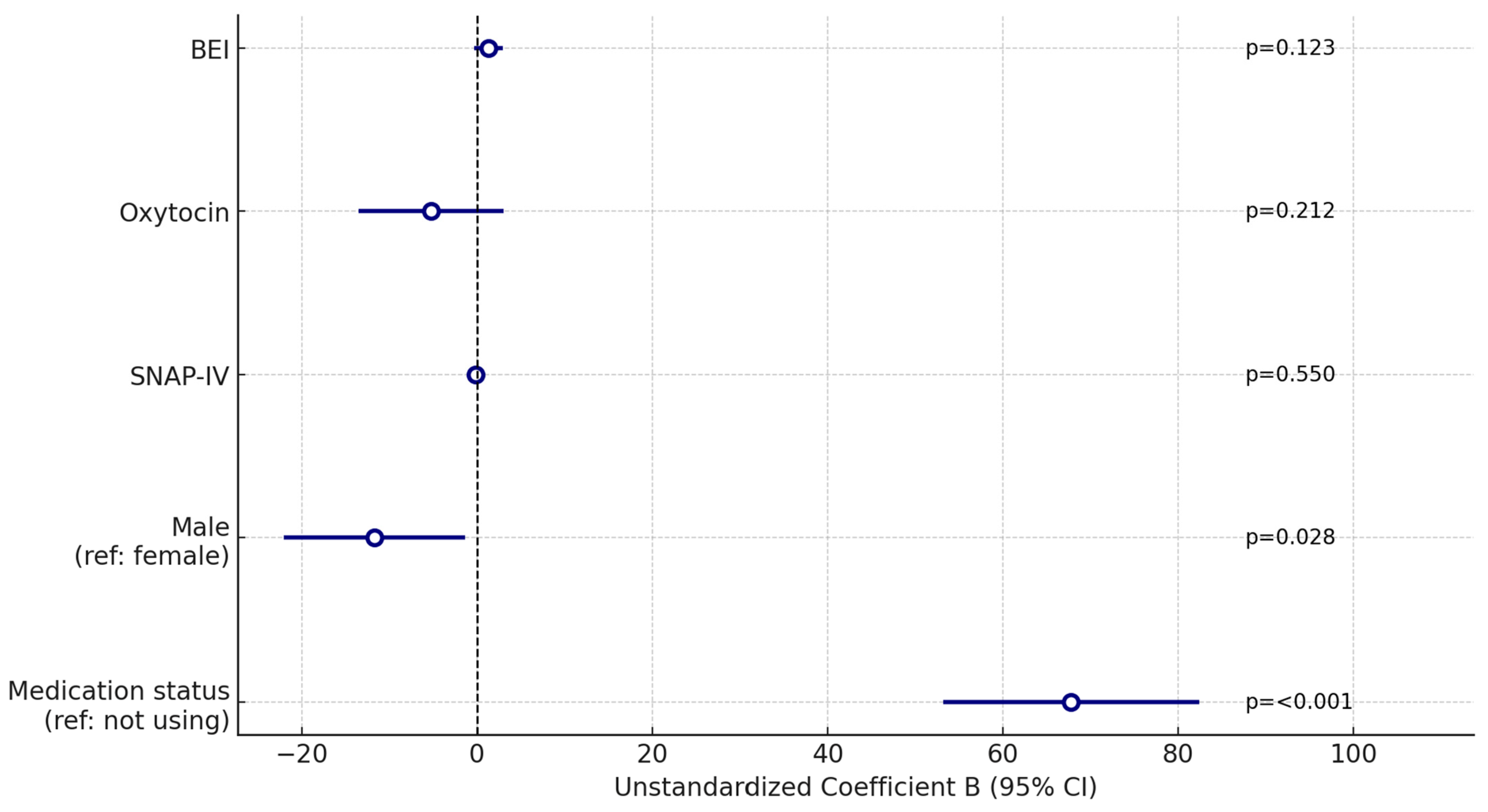
| Medication status (reference group: not using medication) | 0.720 | 12.691 | <0.001 | 58.346–79.865 | 0.706 | 7.386 | <0.001 | 53.175–82.372 |
| Male (reference: female) | −0.081 | −1.001 | 0.318 | −22.502–7.370 | −0.126 | −2.227 | 0.028 | −22.095–−1.316 |
| SNAP-IV | −0.527 | −7.592 | <0.001 | −2.554–−1.499 | −0.046 | −0.599 | 0.550 | −0.757–0.405 |
| Oxytocin | <0.001 | −0.003 | 0.998 | −12.132–12.093 | −0.070 | −1.253 | 0.212 | −13.516–3.026 |
| BEI | 0.102 | 1.261 | 0.209 | −0.872–3.949 | 0.087 | 1.553 | 0.123 | −0.358–2.980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekmez, H.O.; Suzer Gamli, I.; Karakus, O.B. The Role of Pharmacotherapy in Social Cognition, Empathy, and Serum Oxytocin Levels in Children with Attention Deficit Hyperactivity Disorder: A Case–Control Study. Children 2025, 12, 1367. https://doi.org/10.3390/children12101367
Pekmez HO, Suzer Gamli I, Karakus OB. The Role of Pharmacotherapy in Social Cognition, Empathy, and Serum Oxytocin Levels in Children with Attention Deficit Hyperactivity Disorder: A Case–Control Study. Children. 2025; 12(10):1367. https://doi.org/10.3390/children12101367
Chicago/Turabian StylePekmez, Hasibe Ozlem, Ipek Suzer Gamli, and Oguz Bilal Karakus. 2025. "The Role of Pharmacotherapy in Social Cognition, Empathy, and Serum Oxytocin Levels in Children with Attention Deficit Hyperactivity Disorder: A Case–Control Study" Children 12, no. 10: 1367. https://doi.org/10.3390/children12101367
APA StylePekmez, H. O., Suzer Gamli, I., & Karakus, O. B. (2025). The Role of Pharmacotherapy in Social Cognition, Empathy, and Serum Oxytocin Levels in Children with Attention Deficit Hyperactivity Disorder: A Case–Control Study. Children, 12(10), 1367. https://doi.org/10.3390/children12101367





