Screening for Familial Hypercholesterolemia in Childhood: An Overview of Current Practices Around the World
Abstract
1. Introduction
2. Materials and Methods
3. Familial Hypercholesterolemia Worldwide
3.1. FH Prevalence in Distinct Countries
3.2. The Prague Declaration
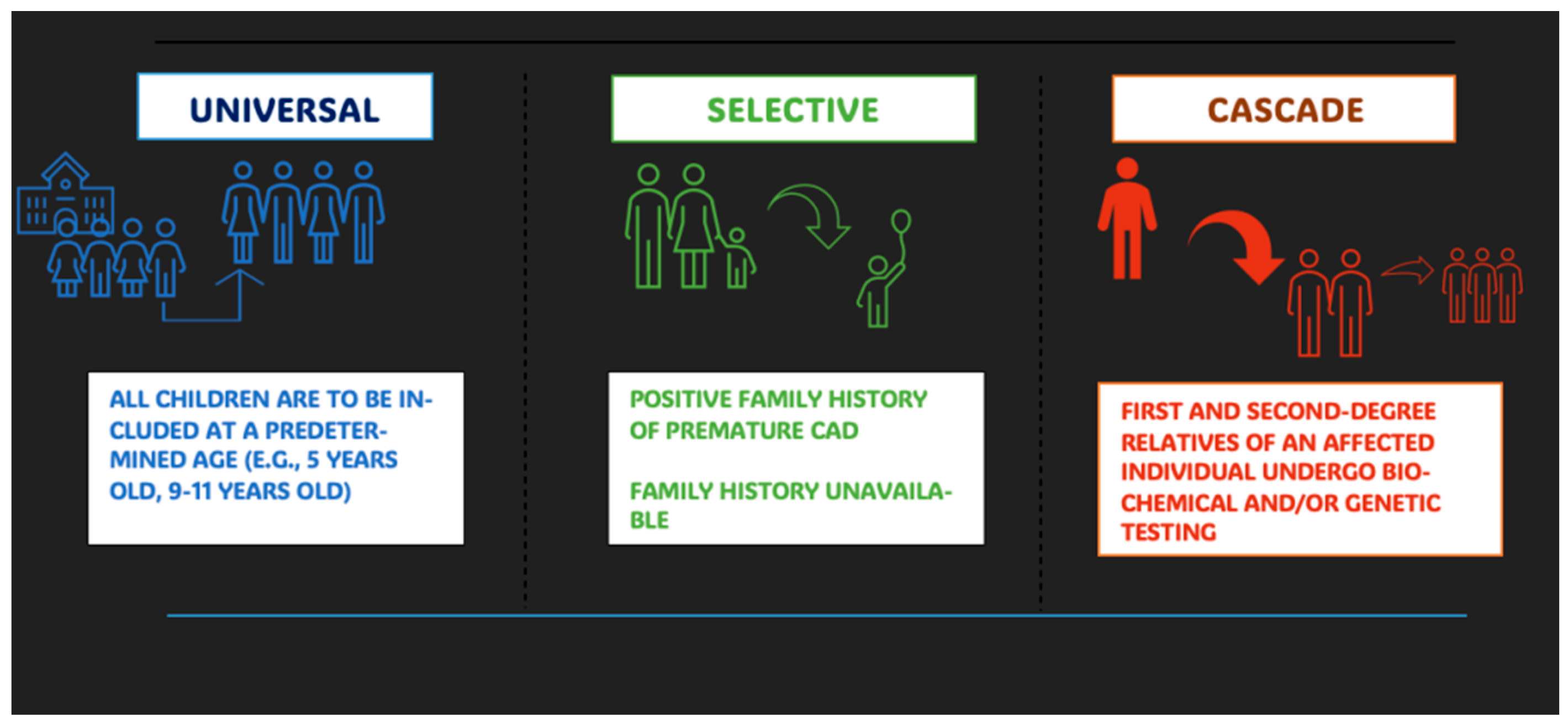
3.3. Screening Strategies
4. Familial Hypercholesterolemia Screening in Europe
5. Familial Hypercholesterolemia Screening in North America, Australia, and Japan
5.1. North America
5.2. Canada
5.3. Australia
5.4. Japan
6. Familial Hypercholesterolemia Screening in Low-Income Countries
6.1. Africa
6.2. Latin America
6.3. India
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ApoB | Apolipoprotein B |
| CVD | Cardiovascular Disease |
| EAS | European Atherosclerosis Society |
| FH | Familial Hypercholesterolemia |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HoFH | Homozygous Familial Hypercholesterolemia |
| HeFH | Heterozygous Familial Hypercholesterolemia |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LDL-R | Low-Density Lipoprotein Receptor |
| LDL-RAP1 | Low-Density Lipoprotein Receptor-Related Protein-Associated Protein 1 |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin type 9 |
References
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Glass, C.K.; Witztum, J.L.; Deutsch, R.; D’Armiento, F.P.; Palinski, W. Influence of maternal hypercholes-terolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999, 354, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kusters, D.M.; Wiegman, A.; Kastelein, J.J.; Hutten, B.A. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ. Res. 2014, 114, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.; Masana, L.; Comas-Cufí, M.; Vila, À.; Plana, N.; García-Gil, M.; Alves-Cabratosa, L.; Marrugat, J.; Roman, I.; Ramos, R. Familial hypercholesterolemia in a European Mediterranean popu-lation-Prevalence and clinical data from 2.5 million primary care patients. J. Clin. Lipidol. 2017, 11, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; Rodday, A.M.; Mendelson, M.; Wong, J.B.; Leslie, L.K.; Sheldrick, R.C. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016, 133, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, H.; Nohara, A.; Noguchi, T.; Kobayashi, J.; Kawashiri, M.-A.; Tada, H.; Nakanishi, C.; Mori, M.; Yamagishi, M.; Inazu, A.; et al. Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis 2011, 214, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ain, Q.; Sikonja, J.; Batool, H.; Hayat, M.Q.; Khan, M.I.; Groselj, U.; Sadiq, F. Prevalence of Familial Hypercholesterolemia in Pakistan: A Pooled Analysis of 1.5 Million Individuals and Comparison with Other Coun-tries of the Region. Glob. Heart 2025, 20, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhabib, K.F.; Al-Rasadi, K.; Almigbal, T.H.; Batais, M.A.; Al-Zakwani, I.; Al-Allaf, F.A.; Al-Waili, K.; Zadjali, F.; Alghamdi, M.; Alnouri, F.; et al. Familial Hypercholesterolemia in the Arabian Gulf Region: Clinical results of the Gulf FH Registry. PLoS ONE 2021, 16, e0251560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bamimore, M.A.; Zaid, A.; Banerjee, Y.; Al-Sarraf, A.; Abifadel, M.; Seidah, N.G.; Al-Waili, K.; Al-Rasadi, K.; Awan, Z. Familial hypercholesterolemia mutations in the Middle Eastern and North African region: A need for a national registry. J. Clin. Lipidol. 2015, 9, 187–194. [Google Scholar] [CrossRef]
- Santos, R.D.; Bourbon, M.; Alonso, R.; Cuevas, A.; Vasques-Cardenas, N.A.; Pereira, A.C.; Merchan, A.; Alves, A.C.; Medeiros, A.M.; Jannes, C.E.; et al. Clinical and molecular aspects of familial hypercholesterolemia in Ibero-American countries. J. Clin. Lipidol. 2017, 11, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.D.; Blom, D.J.; Raal, F.J.; The Lipid and Atherosclerosis Society of Southern Africa OBOTLA. Familial hypercholesterolaemia in South Africa: A reminder. S. Afr. Med. J. 2021, 111, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Smyth, N.; Ramsay, M.; Raal, F.J. Population specific genetic heterogeneity of familial hypercholesterolemia in South Africa. Curr. Opin. Lipidol. 2018, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Sullivan, D.R.; Hare, D.L.; Colquhoun, D.M.; Bates, T.R.; Ryan, J.D.; Bishop, W.; Burnett, J.R.; Bell, D.A.; Simons, L.A.; et al. Gaps in the Care of Familial Hypercholesterolaemia in Australia: First Report from the National Registry. Heart Lung Circ. 2021, 30, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Bedlington, N.; Abifadel, M.; Beger, B.; Bourbon, M.; Bueno, H.; Ceska, R.; Cillíková, K.; Cimická, Z.; Daccord, M.; de Beaufort, C.; et al. The time is now: Achieving FH paediatric screening across Europe—The Prague Declaration. GMS Health Innov. Technol. 2022, 16, Doc04. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ose, L. An update on familial hypercholesterolaemia. Ann. Med. 1999, 31, 13–18. [Google Scholar] [CrossRef]
- Pederiva, C.; Capra, M.E.; Biasucci, G.; Banderali, G.; Fabrizi, E.; Gazzotti, M.; Casula, M.; Catapano, A.L. Lipoprotein(a) and family history for cardiovascular disease in paediatric patients: A new frontier in cardiovascular risk stratification. Data from the LIPIGEN paediatric group. Atherosclerosis 2022, 349, 233–239. [Google Scholar] [CrossRef] [PubMed]
- European Atherosclerosis Society. Lipid Clinics Network. Available online: https://eas-society.org/ (accessed on 1 September 2025).
- Expert Panel of Blood Cholesterol Levels in Children and Adolescents. National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 1992, 89, 495–501. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Nutrition. American Academy of Pediatrics. Committee on Nutrition. Cholesterol in childhood. Pediatrics 1998, 101 Pt 1, 141–147. [Google Scholar] [PubMed]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef] [PubMed]
- Leren, T.P. Cascade genetic screening for familial hypercholesterolemia. Clin. Genet. 2004, 66, 483–487. [Google Scholar] [CrossRef]
- Casula, M.; Gazzotti, M.; Capra, M.E.; Olmastroni, E.; Galimberti, F.; Catapano, A.L.; Pederiva, C.; Anesi, A.; Arca, M.; Auricchio, R.; et al. Refinement of the diagnostic approach for the identification of children and adolescents affected by familial hypercholesterolemia: Evidence from the LIPIGEN study. Atherosclerosis 2023, 385, 117231. [Google Scholar] [CrossRef]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar] [CrossRef]
- Nherera, L.; Marks, D.; Minhas, R.; Thorogood, M.; Humphries, S.E. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart 2011, 97, 1175–1181. [Google Scholar] [CrossRef]
- Spencer, S.J.; Jones, L.K.; Guzauskas, G.F.; Hao, J.; Williams, M.S.; Peterson, J.F.; Veenstra, D.L. Cost-effectiveness of population-wide genomic screening for familial hypercholesterolemia in the United States. J. Clin. Lipidol. 2022, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Beeso, J.; Wong, N.; Ayling, R.; Eldridge, P.; Marshall, W.; Sherwood, R.; Peters, T. Screening for hypercholesterolaemia in 10,000 neonates in a multi-ethnic population. Eur. J. Pediatr. 1999, 158, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Held, P.K.; Lasarev, M.; Zhang, X.; Wiberley-Bradford, A.E.; Campbell, K.; Horner, V.; Shao, X.; Benoy, M.; Dodge, A.M.; Peterson, A.L. Familial Hypercholesterolemia Biomarker Distribution in Dried Blood Spots. J. Pediatr. 2023, 259, 113469. [Google Scholar] [CrossRef]
- Shao, X.; Steiner, R.; Peterson, A.L. Newborn screening for lipid disorders. Curr. Opin. Lipidol. 2024, 35, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tobik, K.; Orland, K.M.; Zhang, X.; Garcia, K.; Peterson, A.L. Parental Attitudes and Ideas Regarding Newborn Screening for Familial Hypercholesterolemia. Matern. Child Heal. J. 2023, 27, 978–983. [Google Scholar] [CrossRef]
- Umans-Eckenhausen, M.A.; Defesche, J.C.; Sijbrands, E.J.; Scheerder, R.L.; Kastelein, J.J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001, 357, 165–168. [Google Scholar] [CrossRef]
- Galema-Boers, J.M.; Versmissen, J.; Roeters van Lennep, H.W.; Dusault-Wijkstra, J.E.; Williams, M.; Roeters van Lennep, J.E. Cascade screening of familial hypercholesterolemia must go on. Atherosclerosis 2015, 242, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, L.C.; Defesche, J.C.; Wiegman, A. Successful Genetic Screening and Creating Awareness of Familial Hypercholesterolemia and Other Heritable Dyslipidemias in the Netherlands. Genes 2021, 12, 1168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leren, T.P.; Bogsrud, M.P. The importance of cascade genetic screening for diagnosing autosomal dominant hypercholesterolemia: Results from twenty years of a national screening program in Norway. J. Clin. Lipidol. 2021, 15, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, U.; Humphries, S.E.; Priestley-Barnham, L.; Green, P.; Wald, D.S.; Capps, N.; Anderson, M.; Dale, P.; Morris, A.A. Current management of children and young people with heterozygous familial hypercholesterolaemia—HEART UK statement of care. Atherosclerosis 2019, 290, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Groselj, U.; Kovac, J.; Sustar, U.; Mlinaric, M.; Fras, Z.; Podkrajsek, K.T.; Battelino, T. Universal screening for familial hypercholesterolaemia in children: The Slovenian model and literature review. Atherosclerosis 2018, 277, 383–391. [Google Scholar] [CrossRef]
- Diakou, M.; Miltiadous, G.; Xenophontos, S.L.; Manoli, P.; Cariolou, M.A.; Elisaf, M. Spectrum of LDLR gene mutations, including a novel mutation causing familial hypercholesterolaemia, in North-western Greece. Eur. J. Intern. Med. 2011, 22, e55–e59. [Google Scholar] [CrossRef] [PubMed]
- Mollaki, V.; Drogari, E. Genetic causes of monogenic familial hypercholesterolemia in the Greek population: Lessons, mistakes, and the way forward. J. Clin. Lipidol. 2016, 10, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, U.; Futema, M.; Bogsrud, M.P.; Holven, K.B.; Roeters van Lennep, J.; Wiegman, A.; Descamps, O.S.; Vrablik, M.; Freiberger, T.; Dieplinger, H.; et al. Comparison of the characteristics at diagnosis and treatment of children with heterozygous familial hy-percholesterolaemia (FH) from eight European countries. Atherosclerosis 2020, 292, 178–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreissl, A.; Walleczek, N.; Espina, P.R.; Hallwirth, U.; Greber-Platzer, S. Selective screening for familial hypercholesterolemia in Austrian children—First year results. BMC Pediatr. 2019, 19, 208. [Google Scholar] [CrossRef]
- Sanin, V.; Schunkert, H. Nie zu früh: Screening auf familiäre Hypercholesterinämie. MMW-Fortschritte Med. 2025, 167, 56–59. [Google Scholar] [CrossRef]
- Sanin, V.; Schmieder, R.; Ates, S.; Schlieben, L.D.; Wiehler, J.; Sun, R.; Decker, M.; Sander, M.; Holdenrieder, S.; Kohlmayer, F.; et al. Population-based screening in children for early diagnosis and treatment of familial hypercholesterolemia: Design of the VRONI study. Eur. J. Public Health 2022, 32, 422–428. [Google Scholar] [CrossRef]
- Averna, M.; Cefalù, A.B.; Casula, M.; Noto, D.; Arca, M.; Bertolini, S.; Calandra, S.; Catapano, A.L.; Tarugi, P.; Pellegatta, F.; et al. Familial hypercholesterolemia: The Italian Atherosclerosis Society Network (LIPIGEN). Atheroscler. Suppl. 2017, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, M.; Casula, M.; Olmastroni, E.; Averna, M.; Arca, M.; Catapano, A.L. How registers could enhance knowledge and characterization of genetic dyslipidaemias: The experience of the LIPIGEN in Italy and of other networks for familial hypercholesterolemia. Atheroscler. Suppl. 2020, 42, e35–e40. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, C.; Gazzotti, M.; Arca, M.; Averna, M.; Banderali, G.; Biasucci, G.; Brambilla, M.; Buonuomo, P.S.; Calabrò, P.; Cipollone, F.; et al. Clinical Approach in the Management of Paediatric Patients with Familial Hypercholesterolemia: A National Survey Conducted by the LIPIGEN Paediatric Group. Nutrients 2023, 15, 3468. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, C.; Galimberti, F.; Casula, M.; Banderali, G.; Beccuti, G.; Bianconi, V.; Biasucci, G.; Biolo, M.; Bucci, M.; Buonuomo, P.S.; et al. Diagnosis and Screening Strategies for Detection of Familial Hypercholesterolaemia in Children and Adolescents in Italy: A Survey from the LIPIGEN Paediatric Group. Children 2025, 12, 288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibarretxe, D.; Rodríguez-Borjabad, C.; Feliu, A.; Bilbao, J.Á.; Masana, L.; Plana, N. Detecting familial hypercholester-olemia earlier in life by actively searching for affected children:The DECOPIN project. Atherosclerosis 2018, 278, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, P.; de Isla, L.P.; Watts, G.F.; Alonso, R.; Norman, R.; Muñiz, O.; Fuentes, F.; Mata, N.; López-Miranda, J.; González-Juanatey, J.R.; et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J. Clin. Lipidol. 2017, 11, 260–271. [Google Scholar] [CrossRef]
- Zamora, A.; Paluzie, G.; García-Vilches, J.; Alonso Gisbert, O.; Méndez Martínez, A.I.; Plana, N.; Rodríguez-Borjabad, C.; Ibarretxe, D.; Martín-Urda, A.; Masana, L. Massive data screening is a second opportunity to improve the management of patients with familial hypercholesterolemia phenotype. Clin. Investig. Arterioscler. 2021, 33, 138–147, (In English/Spanish). [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Bestwick, J.P.; Morris, J.K.; Whyte, K.; Jenkins, L.; Wald, N.J. Child-Parent Familial Hypercholesterolemia Screening in Primary Care. N. Engl. J. Med. 2016, 375, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Neely, D. The UK National Screening Committee’s position on child-parent screening for familial hypercholesterolaemia. J. Med. Screen. 2021, 28, 217–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKay, A.J.; Hogan, H.; Humphries, S.E.; Marks, D.; Ray, K.K.; Miners, A. Universal screening at age 1-2 years as an adjunct to cascade testing for familial hypercholesterolaemia in the UK: A cost-utility analysis. Atherosclerosis 2018, 275, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, U.; Cooper, J.; E Humphries, S. The UK Paediatric Familial Hypercholesterolaemia Register: Preliminary data. Arch. Dis. Child. 2017, 102, 255–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Global perspective of familial hypercholesterolaemia: A cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021, 398, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Groselj, U.; Wiegman, A.; Gidding, S.S. Screening in children for familial hypercholesterolaemia: Start now. Eur. Heart J. 2022, 43, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Stuhldreher, W.L.; Orchard, T.J.; Donahue, R.P.; Kuller, L.H.; Gloninger, M.F.; Drash, A.L. Cholesterol screening in childhood: Sixteen-year Beaver County Lipid Study experience. J. Pediatr. 1991, 119, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Henrikson, N.B.; Dunn, J.; Morrison, C.C.; Nguyen, M.; Blasi, P.R.; Anderson, M.L.; Whitlock, E.P. Lipid Screening in Childhood and Adolescence for Detection of Familial Hypercholesterolemia: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 316, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pletcher, M.J.; Vittinghoff, E.; Clemons, A.M.; Jacobs, D.R.; Allen, N.B.; Alonso, A.; Bellows, B.K.; Oelsner, E.C.; Al Hazzouri, A.Z.; et al. Association Between Cumulative Low-Density Lipoprotein Cholesterol Exposure During Young Adulthood and Middle Age and Risk of Cardiovascular Events. JAMA Cardiol. 2021, 6, 1406–1413. [Google Scholar] [CrossRef]
- Shah, N.P.; Ahmed, H.M.; Wilson Tang, W.H. Familial hypercholesterolemia: Detect, treat, and ask about family. Cleve. Clin. J. Med. 2020, 87, 109–120, Erratum in Cleve. Clin. J. Med. 2020, 87, 311. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e603–e634. [Google Scholar] [CrossRef]
- Drastal, M.S.; de Ferranti, S.; Gooding, H. Recent updates on the screening, diagnosis, and management of lipids disorders in children and adolescents. Curr. Opin. Pediatr. 2025, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Ruel, I.; Aljenedil, S.; Rivière, J.B.; Baass, A.; Tu, J.V.; Mancini, G.B.J.; Raggi, P.; Gupta, M.; Couture, P.; et al. Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018. Can. J. Cardiol. 2018, 34, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Bigras, J.-L.; Cummings, E.A.; Harris, K.C.; Hegele, R.A.; Henderson, M.; Morrison, K.M.; St-Pierre, J.; Wong, P.D.; McCrindle, B.W. The detection, evaluation, and management of dyslipidemia in children and adolescents: A Canadian Cardiovascular Society/Canadian Pediatric Cardiology Association clinical practice update. Can. J. Cardiol. 2022, 38, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Rodday, A.M.; Mackie, A.S.; Gill, P.; McLaughlin, T.; Harris, K.C.; Wong, P.; McCrindle, B.W.; Birken, C.S.; de Ferranti, S.D. Pediatric lipid screening and treatment in Canada: Practices, attitudes, and barriers. Can. J. Cardiol. 2020, 36, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Zahavich, L.; Rush, J.; Hewson, S.; Chitayat, D.; McCrindle, B.W.; Chahal, N. Exploring Barriers and Facilitators to Indirect Cascade Screening for Familial Hypercholesteraemia in a Paediatric/Parent Population. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 211–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, A.C.; Hooper, A.J.; Norman, R.; Nguyen, L.T.; Burnett, J.R.; Bell, D.A.; Brett, T.; Garton-Smith, J.; Pang, J.; Nowak, K.J.; et al. Pilot study of universal screening of children and child-parent cascade testing for familial hypercholesterolaemia in Australia. J. Paediatr. Child Health 2022, 58, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Martin, A.C.; Mori, T.A.; Beilin, L.J.; Watts, G.F. Prevalence of Familial Hypercholesterolemia in Adolescents: Potential Value of Universal Screening? J. Pediatr. 2016, 170, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.A.; Pang, J.; Burrows, S.; Bates, T.R.; van Bockxmeer, F.M.; Hooper, A.J.; O’Leary, P.; Burnett, J.R.; Watts, G.F. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally co ordinated clinical service: An Australian experience. Atherosclerosis 2015, 239, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Minamizuka, T.; Tada, H.; Yokote, K. Familial hypercholesterolemia with special focus on Japan. Clin. Chim. Acta 2024, 556, 117847. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shiba, M.; Ohta, T.; Ohtake, A.; Ogura, M.; Dobashi, K.; Nohara, A.; Yamashita, S.; Yokote, K.; Joint Working Group by Japan Pediatric Society and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial Hypercholesterolemia. Guidance for Pediatric Familial Hypercholesterolemia 2017. J. Atheroscler. Thromb. 2018, 25, 539–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsunaga, K.; Mizobuchi, A.; Ying Fu, H.; Ishikawa, S.; Tada, H.; Kawashiri, M.A.; Yokota, I.; Sasaki, T.; Ito, S.; Kunikata, J.; et al. Universal Screening for Familial Hypercholesterolemia in Children in Kagawa, Japan. J. Atheroscler. Thromb. 2022, 29, 839–849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okamura, T.; Tsukamoto, K.; Arai, H.; Fujioka, Y.; Ishigaki, Y.; Koba, S.; Ohmura, H.; Shoji, T.; Yokote, K.; Yoshida, H.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. J. Atheroscler. Thromb. 2024, 31, 641–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, H.Y.; Matsunaga, K.; Inoue, T.; Tani, R.; Funatsuki, K.; Iwase, T.; Kondo, S.; Nishioka, K.; Ito, S.; Sasaki, T.; et al. Improved Efficiency of the Clinical Diagnostic Criteria for Familial Hypercholesterolemia in Children: A Com-parison of the Japan Atherosclerosis Society Guidelines of 2017 and 2022. J. Atheroscler. Thromb. 2024, 31, 1048–1057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsunaga, K.; Harada-Shiba, M.; Yamashita, S.; Tada, H.; Uda, A.; Mori, K.; Yoshimura, M.; Inoue, S.; Kamae, I.; Yoko-yama, S.; et al. A Cost-Effectiveness Analysis for the Combination of Universal Screening at 9–10 Years Old and Reverse Cascade Screening of Relatives for Familial Hypercholesterolemia in Japan. J. Atheroscler. Thromb. 2025, 32, 962–981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raal, F.J.; Bahassi, E.M.; Stevens, B.; Turner, T.A.; Stein, E.A. Cascade Screening for Familial Hypercholesterolemia in South Africa: The Wits FIND-FH Program. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.R.S.; Jannes, C.E.; Oliveira, T.G.M.; Gómez, L.M.G.; Krieger, J.E.; Santos, R.D.; Pereira, A.C. Predictors of Family Enrollment in a Genetic Cascade Screening Program for Familial Hypercholesterolemia. Arq. Bras. Cardiol. 2018, 111, 578–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Setia, N.; Saxena, R.; Sawhney, J.P.S.; Verma, I.C. Familial Hypercholesterolemia: Cascade Screening in Children and Relatives of the Affected. Indian J. Pediatr. 2018, 85, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, J.P.S.; Gupta, R. Indian dyslipidaemia guidelines: Need of the hour. Indian Heart J. 2024, 76 (Suppl. S1), S2–S5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, J.; David Marais, A.; Blom, D.J.; Brice, B.C.; Silva, P.R.; Jannes, C.E.; Pereira, A.C.; Hooper, A.J.; Ray, K.K.; Santos, R.D.; et al. Heterozygous familial hypercholesterolaemia in specialist centres in South Africa, Australia and Brazil: Importance of early detection and lifestyle advice. Atherosclerosis 2018, 277, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Almahmeed, W.; Al-Rasadi, K.; Azuri, J.; Daclin, V.; Kayikcioglu, M.; Mercier, F.; Ruiz, A.J.; Santos, R.D.; ICLPS Study Group. Low-density lipoprotein cholesterol goal achievement in patients with familial hypercholesterolemia in countries outside Western Europe: The International ChoLesterol management Practice Study. J. Clin. Lipidol. 2019, 13, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.D.; Kotze, M.J.; Raal, F.J.; Khine, A.A.; Talmud, P.J.; Humphries, S.E. Familial hypercholesterolaemia workshop for leveraging point-of-care testing and personalised medicine in association with the Lipid and Atherosclerosis Society of Southern Africa. Cardiovasc. J. Afr. 2019, 30, 297–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsayed, N.; Almahmeed, W.; Alnouri, F.; Al-Waili, K.; Sabbour, H.; Sulaiman, K.; Zubaid, M.; Ray, K.K.; Al-Rasadi, K. Consensus clinical recommendations for the management of plasma lipid disorders in the Middle East: 2021 update. Atherosclerosis 2022, 343, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Brink, P.A.; Steyn, L.T.; Coetzee, G.A.; Van der Westhuyzen, D.R. Familial hypercholesterolemia in South African Afrikaners. PvuII and StuI DNA polymorphisms in the LDL-receptor gene consistent with a predominating founder gene effect. Hum. Genet. 1987, 77, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Ranjith, N.; Joshi, P.; Naidoo, P.; van Tonder, A.; Musa, M.G.; Joshi, S.; Leisegang, R.; Trokis, J.S.; Makan, H.; et al. The therapeutic management of South African dyslipidaemic patients at very high cardiovascular risk (CARDIO TRACK): A cross-sectional study. Cardiovasc. J. Afr. 2020, 31, 245–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhababi, D.; Zayed, H. Spectrum of mutations of familial hypercholesterolemia in the 22 Arab countries. Atherosclerosis 2018, 279, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashwal, A.; Alsagheir, A.; Al Dubayee, M.; Al-Khnifsawi, M.; Al-Sarraf, A.; Awan, Z.; Ben-Omran, T.; Al-Yaarubi, S.; Almutair, A.; Habeb, A.; et al. Modern approaches to the management of homozygous familial hypercholesterolemia in the Middle East and North Africa. J. Clin. Lipidol. 2024, 18, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Zubirán, R.; Martagón, A.J.; Vázquez Cárdenas, A.; Segura Kato, Y.; Tusié Luna, M.T.; Aguilar Salinas, C.A. The panorama of familial hypercholesterolemia in Latin America: A systematic review. J. Lipid. Res. 2016, 57, 2115–2129. [Google Scholar] [CrossRef]
- Silva, P.R.S.; Jannes, C.E.; Oliveira, T.G.M.; Miname, M.H.; Rocha, V.Z.; Chacra, A.P.; Gurgel, M.H.C.; Montenegro, R.M.; Ro-drigues Sobrinho, C.R.M.; Bello Moreira, A.S.; et al. Evaluation of clinical and laboratory parameters used in the identification of index cases for genetic screening of familial hypercholesterolemia in Brazil. Atherosclerosis 2017, 263, 257–262. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Silvino, J.P.; Jannes, C.E.; Tada, M.T.; Lima, I.R.; Silva, I.F.O.; Pereira, A.C.; Gomes, K.B. Cascade screening and genetic diagnosis of familial hypercholesterolemia in clusters of the Southeastern region from Brazil. Mol. Biol. Rep. 2020, 47, 9279–9288. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.R.S.; Jannes, C.E.; Oliveira, T.G.M.; Krieger, J.E.; Santos, R.D.; Pereira, A.C. Pharmacological treatment with lipid-lowering agents after molecular identification of familial hypercholesterolemia: Results from the Hipercol Brasil cohort. J. Clin. Lipidol. 2022, 16, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Alonso, R.; Diaz-Diaz, J.L.; Medeiros, A.M.; Jannes, C.E.; Merchan, A.; Vasques-Cardenas, N.A.; Cuevas, A.; Chacra, A.P.; Krieger, J.E.; et al. Phenotypical, Clinical, and Molecular Aspects of Adults and Children With Homozygous Familial Hypercholesterolemia in Iberoamerica. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2508–2515. [Google Scholar] [CrossRef]
- Mehta, R.; Martagon, A.J.; Ramirez, G.A.G.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Elias-Lopez, D.; Gonzalez-Retana, G.; Rodríguez-Encinas, B.; Ceballos-Macías, J.J.; Romero-Zazueta, A.; et al. Familial hypercholesterolemia in Mexico: Initial insights from the national registry. J. Clin. Lipidol. 2021, 15, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Pavía-López, A.A.; Alcocer-Gamba, M.A.; Ruiz-Gastelum, E.D.; Mayorga-Butrón, J.L.; Mehta, R.; Díaz-Aragón, F.A.; Aldrete-Velasco, J.A.; López-Juárez, N.; Cruz-Bautista, I.; Chávez-Mendoza, A.; et al. Guía de práctica clínica mexicana para el diagnóstico y tratamiento de las dislipidemias y enfermedad cardiovascular aterosclerótica. Arch. Cardiol. Mex. 2022, 9, 1–62. (In English) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawhney, J.; Prasad, S.R.; Sharma, M.; Madan, K.; Mohanty, A.; Passey, R.; Mehta, A.; Kandpal, B.; Makhija, A.; Jain, R.; et al. Prevalence of familial hypercholesterolemia in premature coro-nary artery disease patients admitted to a tertiary care hospital in North India. Indian Heart J. 2019, 71, 118–122. [Google Scholar] [CrossRef]
- Setia, N.; Movva, S.; Balakrishnan, P.; Biji, I.K.; Sawhney, J.P.S.; Puri, R.; Arora, A.; Puri, R.D.; Saxena, R.; Mishra, S.; et al. Genetic analysis of familial hypercholesterolemia in Asian Indians: A single-center study. J. Clin. Lipidol. 2020, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.L.; Shah, S.A.V.; Ashavaid, T.F. Shortcomings on genetic testing of Familial hypercholesterolemia (FH) in India: Can we collaborate to establish Indian FH registry? Indian Heart J. 2022, 74, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Atherosclerosis Society Familial Hypercholesterolaemia Studies Collaboration. Familial hypercholesterolaemia in children and adolescents from 48 countries: A cross-sectional study. Lancet 2024, 403, 55–66. [Google Scholar] [CrossRef]
- Luirink, I.K.; Wiegman, A.; Kusters, D.M.; Hof, M.H.; Groothoff, J.W.; de Groot, E.; Kastelein, J.J.P.; Hutten, B.A. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N. Engl. J. Med. 2019, 381, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
| Country | Main Screening Method | Consensus/Guideline Document | Bibliographic References |
|---|---|---|---|
| |||
| Netherlands (1994) | National cascade screening since 1994: index case via clinical/lab criteria + genetic testing; family tracing 1st/2nd degree | Dutch Ministry of Health FH Screening Program Guidelines (1994–2014) | Zuurbier et al., 2021 [34] |
| Slovenia (1995) | Universal pediatric screening at age 5 (total cholesterol); genetic confirmation; cascade family screening | National Pediatric Preventive Program—FH Screening Protocol | Groselj et al., 2018 [37]; |
| Norway (late 1990s) | Cascade screening integrated with genetic registries and electronic health records; >50% of expected cases identified by 2020 | Norwegian Directorate of Health FH Recommendations | Bogsrud et al., 2010 [35] |
| Sweden (early 2000s) | Cascade screening using clinical-genetic algorithms; strong primary-specialist care integration | HEART UK statement adapted for Sweden | Ramaswami et al., 2020 [36] |
| Greece (1993–2018, pilot) | Universal pediatric lipid screening integrated with vaccination visits | Greek Pediatric Lipid Screening Project Protocol | Diakou et al., 2011; Mollaki & Drogari, 2016 [38,39] |
| Spain (2004) | National SAFEHEART registry, cascade clinical-genetic screening; cost-effectiveness proven | SAFEHEART National Registry Protocol | Lázaro et al., 2017 [49] |
| Italy (2010s) | LIPIGEN network: specialist centers, registries, genetic testing; cascade screening | Italian Society of Atherosclerosis—LIPIGEN network | Averna et al., 2017; [44] |
| Austria (2017) | Selective screening (FH Kids Austria) in school entry visits (5–7 y); questionnaire + lipid testing | FH Kids Austria Screening Protocol | Kreissl et al., 2019; [41] |
| Germany (2021) | Universal pediatric screening (VRONI) age 5–14 during preventive visits; LDL-C capillary + genetic testing; reverse cascade | VRONI Pediatric FH Screening Protocol | Sanin et al., 2022 [42,43] |
| |||
| USA (AAP rec. 2011) | Targeted screening + cascade; AAP recommends universal at 9–11 and 17–21 y; USPSTF no recommendation | American Academy of Pediatrics & USPSTF Positions | Shah et al., 2020; Lozano et al., 2016 [58,60] |
| United Kingdom (2016) | Child–parent screening: universal cholesterol testing in toddlers (1–2 y) during vaccination; if positive, genetic testing and parental cascade | UK Child–parent Screening Program (pilot, NHS-supported) | Wald et al., NEJM 2016 [51] |
| Australia (2015–2016 pilot) | Proposed universal pediatric screening at 1–2 years with vaccination; cascade clinical-genetic screening; WA pilot | Australian FH Screening Consensus Statement | Martin et al., 2022; Bell et al., 2015 [67,69] |
| Japan (Kagawa pilot 2012; JAS 2022) | JAS 2022: updated criteria, recommends family screening; Kagawa pilot universal screening at 9–10 y | Japan Atherosclerosis Society (JAS) 2022 Guidelines | JAS 2022; Matsunaga 2025 [73,74,75] |
| Canada (2014; update 2018, 2022) | CCS 2018 and 2022: cascade screening for 1st-degree relatives; universal pediatric screening recommended but low adherence | Canadian Cardiovascular Society FH Guidelines | Brunham et al., 2018; Khoury et al., 2022 [63,64] |
| South Africa (1990s) | Targeted screening in founder populations + cascade (Wits FIND-FH) based on clinical criteria | Wits FIND-FH Program Protocol | Raal et al., 2020 [76] |
| Brazil (2008–2015) | Cascade screening + centralized genetic testing; high prevalence in children | HipercolBrasil National Screening Guidelines | Silva et al., 2018 [77] |
| India (2018 pilot; 2024 guidelines call) | Cascade screening in children and relatives (pilot studies); no national registry yet, but recent national guidelines stress urgent need for structured FH screening | Indian Dyslipidemia Guidelines (Indian Heart Journal, 2024) | Setia et al., 2018; Sawhney & Gupta, 2024 [78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, M.E.; Sodero, R.; Travaglia, E.; Banderali, G.; Biasucci, G.; Pederiva, C. Screening for Familial Hypercholesterolemia in Childhood: An Overview of Current Practices Around the World. Children 2025, 12, 1364. https://doi.org/10.3390/children12101364
Capra ME, Sodero R, Travaglia E, Banderali G, Biasucci G, Pederiva C. Screening for Familial Hypercholesterolemia in Childhood: An Overview of Current Practices Around the World. Children. 2025; 12(10):1364. https://doi.org/10.3390/children12101364
Chicago/Turabian StyleCapra, Maria Elena, Roberta Sodero, Elisa Travaglia, Giuseppe Banderali, Giacomo Biasucci, and Cristina Pederiva. 2025. "Screening for Familial Hypercholesterolemia in Childhood: An Overview of Current Practices Around the World" Children 12, no. 10: 1364. https://doi.org/10.3390/children12101364
APA StyleCapra, M. E., Sodero, R., Travaglia, E., Banderali, G., Biasucci, G., & Pederiva, C. (2025). Screening for Familial Hypercholesterolemia in Childhood: An Overview of Current Practices Around the World. Children, 12(10), 1364. https://doi.org/10.3390/children12101364






