Dietary Habits and Their Impact on Pediatric Obesity and Asthma: A Narrative Review with Emphasis on the Mediterranean Diet
Abstract
1. Introduction
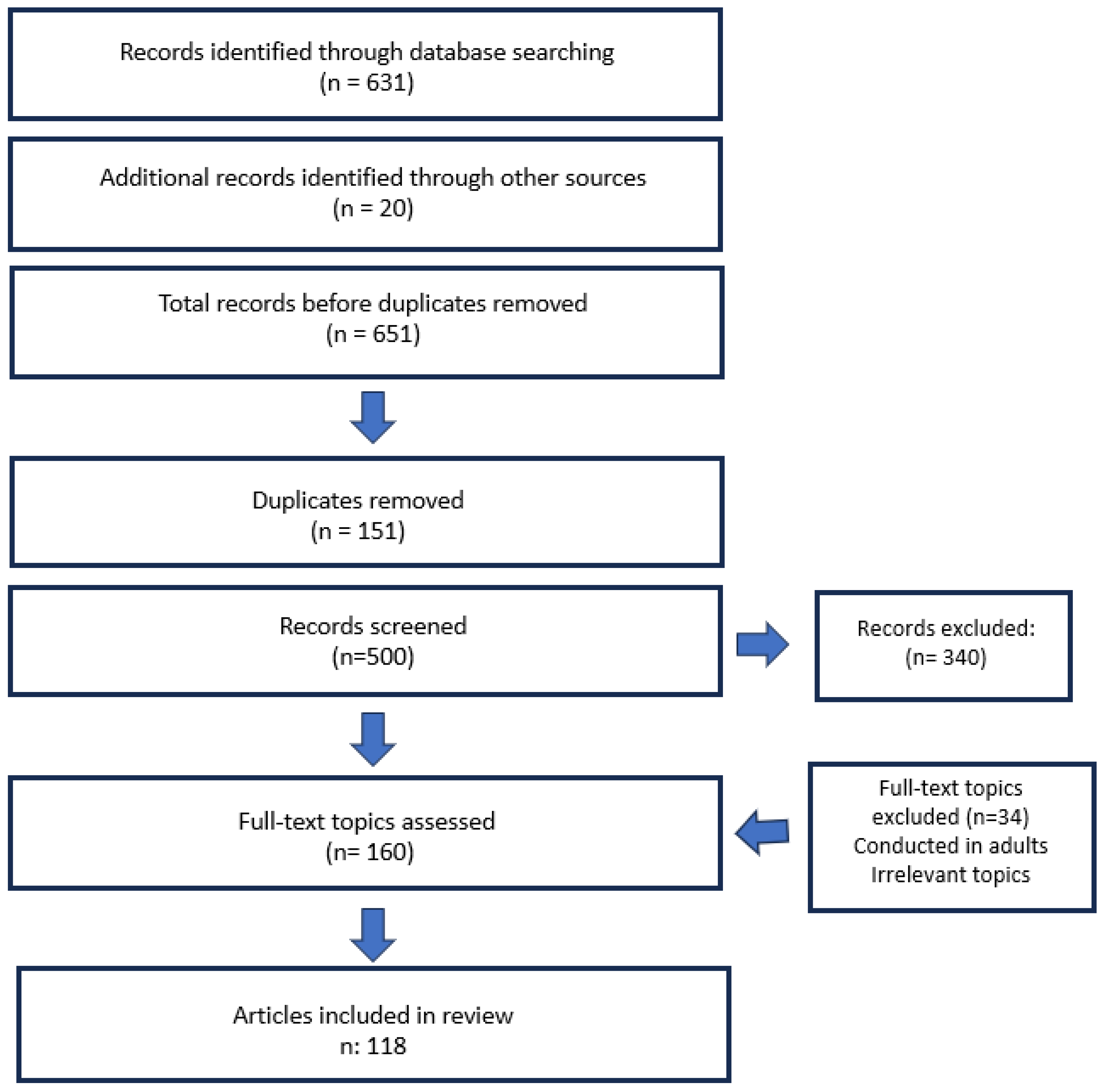
2. Methods
3. Discussion
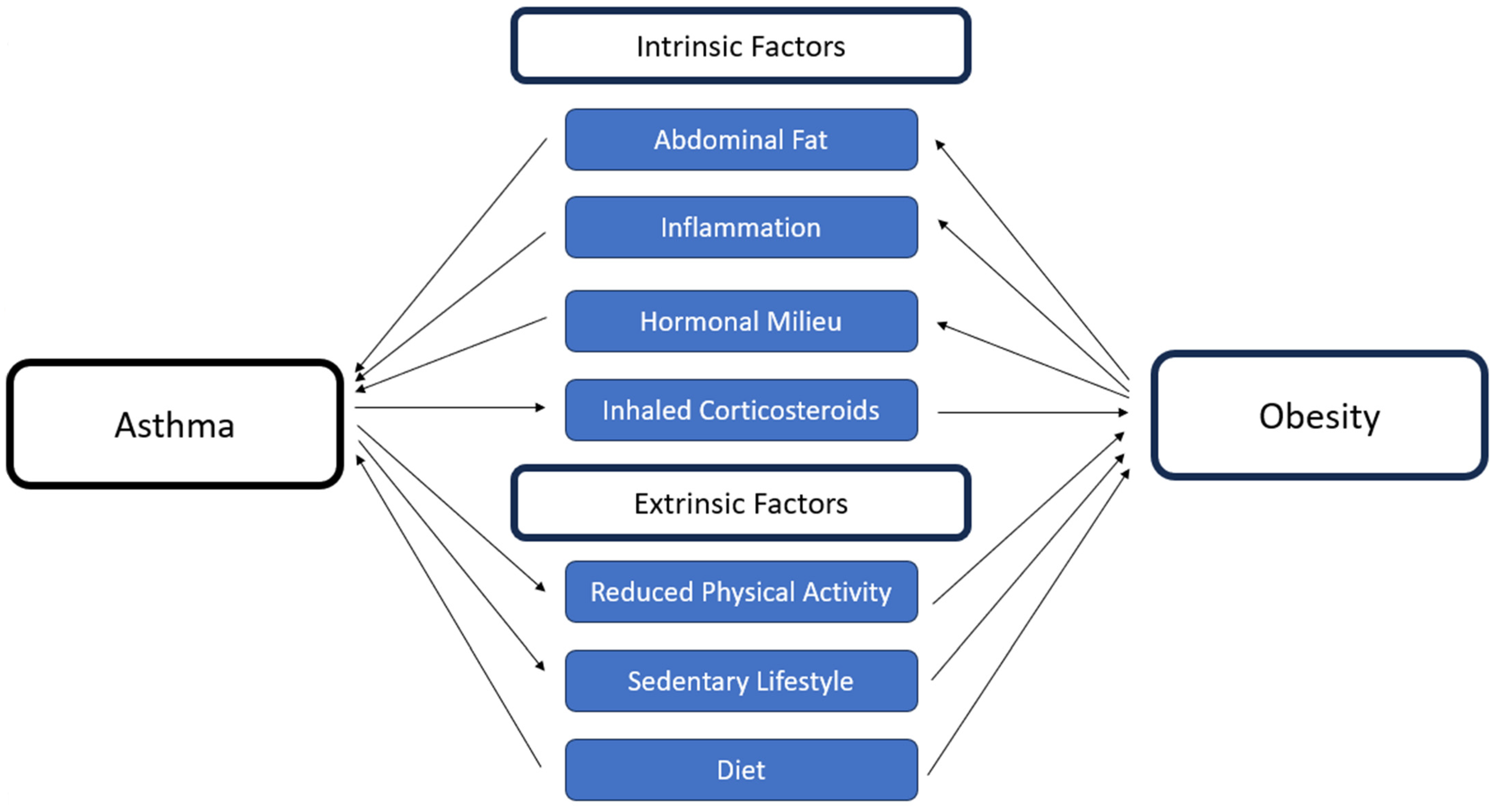
- Interconnection between obesity and asthma
3.1. Intrinsic Factors
3.1.1. Mechanical Factors
3.1.2. Hormonal Milieu—Inflammation
3.2. Extrinsic Factors
3.2.1. Sedentary Lifestyle and Activity
3.2.2. Westernized Inflammatory Diet
- Nutrition as a core intervention in the management of pediatric obesity and asthma and the role of the Mediterranean diet
- Effects of the Mediterranean diet on weight management and metabolic health
3.3. Mediterranean Diet: History and Components’ Benefits
3.4. Mediterranean Diet and Body Weight
3.5. Westernized Diet and Sedentary Lifestyle
- Antioxidant and anti-inflammatory effects of MD and asthma
3.6. Antioxidant-Rich Foods
3.7. Inflammatory Foods
3.8. Omega-3 Fatty Acids
3.9. Vitamin D
4. Limitations and Research Gaps
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The Epidemiological Burden of Obesity in Childhood: A Worldwide Epidemic Requiring Urgent Action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef]
- Uphoff, E.P.; Bird, P.K.; Antó, J.M.; Basterrechea, M.; von Berg, A.; Bergström, A.; Bousquet, J.; Chatzi, L.; Fantini, M.P.; Ferrero, A.; et al. Variations in the Prevalence of Childhood Asthma and Wheeze in MeDALL Cohorts in Europe. ERJ Open Res. 2017, 3, 00150–02016. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.A.; Robertson, C.; ISAAC Phase Three Study Group. Worldwide Trends in the Prevalence of Asthma Symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef]
- García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; Ellwood, P.; Marks, G.B.; Mortimer, K.; et al. The Burden of Asthma, Hay Fever and Eczema in Children in 25 Countries: GAN Phase I Study. Eur. Respir. J. 2022, 60, 2102866. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128.9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Parasuaraman, G.; Ayyasamy, L.; Aune, D.; Sen, A.; Nagarajan, R.; Rajkumar, P.; Velusamy, S.; Manickam, P.; Sivaprakasam, S. The Association between Body Mass Index, Abdominal Fatness, and Weight Change and the Risk of Adult Asthma: A Systematic Review and Meta-Analysis of Cohort Studies. Sci. Rep. 2023, 13, 9136. [Google Scholar] [CrossRef]
- Lang, J.E.; Bunnell, H.T.; Hossain, J.; Wysocki, T.; Lima, J.J.; Finkel, T.H.; Bacharier, L.; Dempsey, A.; Sarzynski, L.; Test, M.; et al. Being Overweight or Obese and the Development of Asthma. Pediatrics 2018, 142, e20182119. [Google Scholar] [CrossRef]
- Ahmadizar, F.; Vijverberg, S.J.H.; Arets, H.G.M.; de Boer, A.; Lang, J.E.; Kattan, M.; Palmer, C.N.A.; Mukhopadhyay, S.; Turner, S.; Maitland-van der Zee, A.H. Childhood Obesity in Relation to Poor Asthma Control and Exacerbation: A Meta-Analysis. Eur. Respir. J. 2016, 48, 1063–1073. [Google Scholar] [CrossRef]
- Deng, X.; Ma, J.; Yuan, Y.; Zhang, Z.; Niu, W. Association between Overweight or Obesity and the Risk for Childhood Asthma and Wheeze: An Updated Meta-Analysis. Pediatr. Obes. 2019, 14, e12532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K.; Gilliland, F.D. Effects of Childhood Asthma on the Development of Obesity among School-Aged Children. Am. J. Respir. Crit. Care Med. 2017, 195, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Alvarez-Puebla, M.J.; Esteban-Gorgojo, I.; Barranco, P.; Olaguibel, J.M. Obesity and Asthma: Key Clinical Questions. J. Investig. Allergol. Clin. Immunol. 2019, 29, 262–271. [Google Scholar] [CrossRef]
- Beuther, D.A.; Weiss, S.T.; Sutherland, E.R. Obesity and Asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 112–119. [Google Scholar] [CrossRef]
- Davidson, W.J.; Mackenzie-Rife, K.A.; Witmans, M.B.; Montgomery, M.D.; Ball, G.D.C.; Egbogah, S.; Faulkner, G.; McMorris, C.; Poulin, P.; Thabane, L.; et al. Obesity Negatively Impacts Lung Function in Children and Adolescents. Pediatr. Pulmonol. 2014, 49, 1003–1010. [Google Scholar] [CrossRef]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Ortega, V.E.; Coverstone, A.; et al. Plasma Interleukin-6 Concentrations, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.E.; Motawie, A.A.M.; Abd Al-Aziz, A.M.; Mostafa, N.A.A.; Hasan, N.S.; El-Baz, M.S. Role of Adiponectin, Resistin and Monocyte Chemo-Attractant Protein-1 in Overweight/Obese Asthma Phenotype in Children. BMC Pediatr. 2023, 23, 316. [Google Scholar] [CrossRef]
- Arshi, M.; Cardinal, J.; Hill, R.J.; Davies, P.S.W.; Wainwright, C. Asthma and Insulin Resistance in Children. Respirology 2010, 15, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawwa, B.A.; Al-Huniti, N.H.; DeMattia, L.; Gershan, W. Asthma and Insulin Resistance in Morbidly Obese Children and Adolescents. J. Asthma 2007, 44, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Cui, X.; Qualls, C.; Beckett, W.S.; Gross, M.D.; Steffes, M.W.; Smith, L.J.; Jacobs, D.R., Jr. Association between Asthma and Serum Adiponectin Concentration in Women. Thorax 2008, 63, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Shore, S.A. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J. Allergy 2013, 2013, 785835. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F.; García-Hermoso, A.; Sotos-Prieto, M.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Kales, S.N. Mediterranean Diet-Based Interventions to Improve Anthropometric and Obesity Indicators in Children and Adolescents: A Systematic Review with Meta-Analysis. Adv. Nutr. 2023, 14, 858–879. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulou, A.; Giannakopoulou, S.P.; Notara, V.; Antonogeorgos, G.; Rojas-Gil, A.P.; Kornilaki, E.N.; Konstantinou, E.; Lagiou, A.; Panagiotakos, D.B. The Association between Adherence to the Mediterranean Diet and Childhood Obesity; the Role of Family Structure. Nutr. Health 2021, 27, 39–47. [Google Scholar] [CrossRef]
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does Adherence to the Mediterranean Diet Have a Protective Effect against Asthma and Allergies in Children? A Systematic Review. Nutrients 2022, 14, 1618. [Google Scholar] [CrossRef]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Scholtens, S.; Postma, D.S.; Kerkhof, M.; Gerritsen, J.; de Jongste, J.C.; Smit, H.A. Childhood Diet and Asthma and Atopy at 8 Years of Age: The PIAMA Birth Cohort Study. Eur. Respir. J. 2011, 37, 1060–1067. [Google Scholar] [CrossRef]
- Douros, K.; Thanopoulou, M.I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Tsabouri, S.; Nicolaidou, P.; Priftis, K.N. Adherence to the Mediterranean Diet and Inflammatory Markers in Children with Asthma. Allergol. Immunopathol. 2019, 47, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Papadopoulou, S.K.; Fasoulas, A.; Papaliagkas, V.; Alexatou, O.; Chatzidimitriou, M.; Tsaliki, M.; Petridis, D.; Tzimos, C.; Tsonidis, C.; et al. Diabesity and Dietary Interventions: Evaluating the Impact of Mediterranean Diet and Other Types of Diets on Obesity and Type 2 Diabetes Management. Nutrients 2024, 16, 34. [Google Scholar] [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Stuppia, L.; Puca, A.A.; Romeo, G.; Colonna, V.; et al. Modern Vision of the Mediterranean Diet. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E36–E46. [Google Scholar] [CrossRef]
- Shah Gupta, R.; Koteci, A.; Morgan, A.; George, P.M.; Quint, J.K. Incidence and Prevalence of Interstitial Lung Diseases Worldwide: A Systematic Literature Review. BMJ Open Respir. Res. 2023, 10, e001552. [Google Scholar] [CrossRef]
- Schatz, M.; Hsu, J.W.Y.; Zeiger, R.S.; Chen, W.; Dorenbaum, A.; Chipps, B.E.; Haselkorn, T.; Borish, L.; Weiss, S.T.; Peters, S.P.; et al. Phenotypes Determined by Cluster Analysis in Severe or Difficult-to-Treat Asthma. J. Allergy Clin. Immunol. 2014, 133, 1549–1556. [Google Scholar] [CrossRef]
- Ali, Z.; Ulrik, C.S. Obesity and Asthma: A Coincidence or a Causal Relationship? A Systematic Review. Respir. Med. 2013, 107, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Asthma and Obesity in Children. Biomedicines 2020, 8, 231. [Google Scholar] [CrossRef]
- Rastogi, D.; Bhalani, K.; Hall, C.B.; Isasi, C.R. Association of Pulmonary Function with Adiposity and Metabolic Abnormalities in Urban Minority Adolescents. Ann. Am. Thorac. Soc. 2014, 11, 744–752. [Google Scholar] [CrossRef]
- Vijayakanthi, N.; Greally, J.M.; Rastogi, D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics 2016, 137, e20150812. [Google Scholar] [CrossRef]
- Shore, S.A.; Johnston, R.A. Obesity and Asthma. Pharmacol. Ther. 2006, 110, 83–102. [Google Scholar] [CrossRef]
- Nagel, G.; Koenig, W.; Rapp, K.; Wabitsch, M.; Zoellner, I.; Weiland, S.K. Associations of Adipokines with Asthma, Rhinoconjunctivitis, and Eczema in German Schoolchildren. Pediatr. Allergy Immunol. 2009, 20, 81–88. [Google Scholar] [CrossRef]
- Baek, H.S.; Kim, Y.D.; Shin, J.H.; Kim, J.H.; Oh, J.W.; Lee, H.B. Serum Leptin and Adiponectin Levels Correlate with Exercise-Induced Bronchoconstriction in Children with Asthma. Ann. Allergy Asthma Immunol. 2011, 107, 14–21. [Google Scholar] [CrossRef]
- Pacifico, L.; Di Renzo, L.; Anania, C.; Osborn, J.F.; Ippoliti, F.; Schiavo, E.; Chiesa, C. Increased T-Helper Interferon-γ-Secreting Cells in Obese Children. Eur. J. Endocrinol. 2006, 154, 691–697. [Google Scholar] [CrossRef]
- Youssef, D.; Mohamed Elbehidy, R.; Mahamoud Shokry, D.; Mohamed Elbehidy, E. The Influence of Leptin on Th1/Th2 Balance in Obese Children with Asthma. J. Bras. Pneumol. 2013, 39, 562–568. [Google Scholar] [CrossRef]
- Rastogi, D.; Canfield, S.M.; Andrade, A.; Isasi, C.R.; Hall, C.B.; Rubinstein, A.; Arens, R.; Chung, K.F. Obesity-Associated Asthma in Children: A Distinct Entity. Chest 2012, 141, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Brunner, E.; et al. Inflammation, Metabolic Dysregulation, and Pulmonary Function among Obese Urban Adolescents with Asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, A.; Maitra, A.; Ness, A.R.; Mattocks, C.; Riddoch, C.; Reilly, J.J.; Paton, J.Y.; Henderson, A.J. Association of Duration of Television Viewing in Early Childhood with the Subsequent Development of Asthma. Thorax 2009, 64, 321–325. [Google Scholar] [CrossRef]
- Rasmussen, F.; Lambrechtsen, J.; Siersted, H.C.; Hansen, H.S.; Hansen, N.C. Low Physical Fitness in Childhood Is Associated with the Development of Asthma in Young Adulthood: The Odense Schoolchild Study. Eur. Respir. J. 2000, 16, 866–870. [Google Scholar] [CrossRef]
- Firrincieli, V.; Keller, A.; Ehrensberger, R.; Platts-Mills, J.; Shufflebarger, C.; Geldmaker, B.; Metayer, C.; Etzel, R.; Stigler, S.; Wagner, L.; et al. Decreased Physical Activity among Head Start Children with a History of Wheezing: Use of an Accelerometer to Measure Activity. Pediatr. Pulmonol. 2005, 40, 57–63. [Google Scholar] [CrossRef]
- Koinis-Mitchell, D.; Kopel, S.J.; Dunsiger, S.; McQuaid, E.L.; Miranda, L.G.; Mitchell, P.; Boergers, J.; Fritz, G.K. Asthma and Physical Activity in Urban Children. J. Pediatr. Psychol. 2021, 46, 970–981. [Google Scholar] [CrossRef]
- Glazebrook, C.; McPherson, A.C.; Macdonald, I.A.; Swift, J.A.; Ramsay, C.; Newbould, R.; Smyth, A. Asthma as a Barrier to Children’s Physical Activity: Implications for Body Mass Index and Mental Health. Pediatrics 2006, 118, 2443–2449. [Google Scholar] [CrossRef]
- Groth, S.W.; Rhee, H.; Kitzman, H. Relationships among obesity, physical activity and sedentary behavior in young adolescents with and without lifetime asthma. J. Asthma 2016, 53, 19–24. Available online: https://pubmed.ncbi.nlm.nih.gov/26288155/ (accessed on 24 July 2025).
- Contreras, Z.A.; Chen, Z.; Roumeliotaki, T.; Annesi-Maesano, I.; Baïz, N.; von Berg, A.; Bauer, C.P.; Berdel, D.; Berhane, K.; Chatzi, L.; et al. Does Early Onset Asthma Increase Childhood Obesity Risk? A Pooled Analysis of 16 European Cohorts. Eur. Respir. J. 2018, 52, 1800504. [Google Scholar] [CrossRef]
- Shan, L.S.; Zhou, Q.L.; Shang, Y.X. Bidirectional Association between Asthma and Obesity during Childhood and Adolescence: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 576858. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.; Ogston, S.; Mukhopadhyay, S. Annual Increase in Body Mass Index in Children with Asthma on Higher Doses of Inhaled Steroids. J. Pediatr. 2005, 147, 549–553. [Google Scholar] [CrossRef]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Mihatsch, W.; Vandenplas, Y.; van Goudoever, J.B.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Patel, S.; Custovic, A.; Smith, J.A.; Simpson, A.; Kerry, G.; Murray, C.S. Cross-Sectional Association of Dietary Patterns with Asthma and Atopic Sensitization in Childhood: A Cohort Study. Pediatr. Allergy Immunol. 2014, 25, 565–571. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A High-Fat Challenge Increases Airway Inflammation and Impairs Bronchodilator Recovery in Asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange Juice Neutralizes the Proinflammatory Effect of a High-Fat, High-Carbohydrate Meal and Prevents Endotoxin Increase and Toll-Like Receptor Expression. Am. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef] [PubMed]
- NICE. Physical Activity and Diet: Overweight and Obesity Management. NICE Guidance. Available online: https://www.nice.org.uk/guidance (accessed on 24 July 2025).
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Daniels, S.R.; de Ferranti, S.D.; Golden, N.H.; Kelly, A.S.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Olivieri, F.; Valerio, G.; Verduci, E.; Licenziati, M.R.; Calcaterra, V.; Pietrobelli, A.; Miraglia Del Giudice, E.; Brufani, C.; Grugni, G.; et al. The Treatment of Obesity in Children and Adolescents: Consensus Position Statement of the Italian Societies. Ital. J. Pediatr. 2023, 49, 69. [Google Scholar] [CrossRef]
- Abu-Qiyas, S.; Radwan, H.; Cheikh Ismail, L.; Alameddine, M.; Muayyad, M.; Naja, F. Knowledge, Attitudes, and Use of the Mediterranean Diet in Practice among Dietitians in the UAE. Sci. Rep. 2025, 15, 2301. [Google Scholar] [CrossRef]
- Venter, C.; Greenhawt, M.; Meyer, R.W.; Agostoni, C.; Reese, I.; du Toit, G.; Fleischer, D.M.; Maslin, K.; Nwaru, B.I.; Roduit, C.; et al. EAACI Position Paper on Diet Diversity in Pregnancy, Infancy and Childhood. Allergy 2020, 75, 497–523. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Itsiopoulos, C.; Susanto, N.H.; Erbas, B. Does Adherence to the Mediterranean Dietary Pattern Reduce Asthma Symptoms in Children? A Systematic Review. Public Health Nutr. 2017, 20, 2722–2734. [Google Scholar] [CrossRef]
- Stoodley, I.; Williams, L.; Thompson, C.; Scott, H.; Wood, L. Evidence for Lifestyle Interventions in Asthma. Breathe 2019, 15, e50–e61. [Google Scholar] [CrossRef]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Santangelo, C.; Serafini, M.; Storniolo, C.E.; Visioli, F. Nutrigenomics of Extra-Virgin Olive Oil: A Review. Biofactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet: Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Guibas, G.V.; Papadopoulos, N.G. Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy. Nutrients 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Mediterranean Diet, Dietary Polyphenols and Low Grade Inflammation: Results from the MOLI-SANI Study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.W.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Chen, J.; Carmody, R.N.; Gaskins, H.R.; Swanson, K.S.; et al. Gut Microbiome Variation Modulates the Effects of Dietary Fiber on Host Metabolism. Microbiome 2021, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Qualls, C.; Schuyler, M.; Thyagarajan, B.; Steffes, M.W.; Smith, L.J.; Jacobs, D.R., Jr. Low Serum Adiponectin Predicts Future Risk for Asthma in Women. Am. J. Respir. Crit. Care Med. 2012, 186, 41–47. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 Fatty Acid-Derived Mediators That Control Inflammation and Tissue Homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef]
- Notario-Barandiaran, L.; Valera-Gran, D.; Gonzalez-Palacios, S.; Garcia-de-la-Hera, M.; Fernández-Barrés, S.; Pereda-Pereda, E.; Fernandez-Somoano, A.; Guxens, M.; Iniguez, C.; Romaguera, D.; et al. High Adherence to a Mediterranean Diet at Age 4 Reduces Overweight, Obesity and Abdominal Obesity Incidence in Children at the Age of 8. Int. J. Obes. 2020, 44, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Papadopoulou, S.K.; Alexatou, O.; Voulgaridou, G.; Mentzelou, M.; Biskanaki, F.; Psara, E.; Tsourouflis, G.; Lefantzis, N.; Dimoliani, S.; et al. Childhood Mediterranean Diet Adherence Is Associated with Lower Prevalence of Childhood Obesity, Specific Sociodemographic, and Lifestyle Factors: A Cross-Sectional Study in Pre-School Children. Epidemiologia 2023, 5, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Afonso, C.; Rodrigues, S.; Oliveira, A. Healthy and Sustainable Dietary Patterns in Children and Adolescents: A Systematic Review. Adv. Nutr. 2022, 13, 1144–1185. [Google Scholar] [CrossRef]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in Children and Adolescents: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Fitó, M.; Morales-Suárez-Varela, M.; Moya, A.; Gómez, S.F.; Schröder, H. Mediterranean Diet and Adiposity in Children and Adolescents: A Systematic Review. Obes. Rev. 2022, 23 (Suppl. 1), e13381. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Mirmiran, P.; Hooshmand, F.; Najafi, R.; Azizi, F. DASH Dietary Pattern and Reduced Incidence of Metabolic Syndrome in Children and Adolescents. J. Pediatr. 2016, 174, 178–184.e1. [Google Scholar] [CrossRef]
- Lioret, S.; McNaughton, S.A.; Cameron, A.J.; Crawford, D.; Campbell, K.J.; Cleland, V.J.; Ball, K. Three-Year Change in Diet Quality and Associated Changes in BMI among Schoolchildren Living in Socioeconomically Disadvantaged Neighbourhoods. Br. J. Nutr. 2014, 112, 260–268. [Google Scholar] [CrossRef]
- Martin-Calvo, N.; Chavarro, J.E.; Falbe, J.; Hu, F.B.; Field, A.E. Adherence to the Mediterranean Dietary Pattern and BMI Change among U.S. Adolescents. Int. J. Obes. 2016, 40, 1103–1108. [Google Scholar] [CrossRef]
- McCourt, H.J.; Draffin, C.R.; Woodside, J.V.; Cardwell, C.R.; Young, I.S.; Hunter, S.J.; Murray, L.J.; Boreham, C.A.; Gallagher, A.M.; Neville, C.E.; et al. Dietary Patterns and Cardiovascular Risk Factors in Adolescents and Young Adults: Northern Ireland Young Hearts Project. Br. J. Nutr. 2014, 112, 1685–1698. [Google Scholar] [CrossRef]
- Monjardino, T.; Lucas, R.; Ramos, E.; Barros, H. Associations between a Priori-Defined Dietary Patterns and Longitudinal Changes in Bone Mineral Density in Adolescents. Public Health Nutr. 2014, 17, 195–205. [Google Scholar] [CrossRef]
- Ojeda-Rodríguez, A.; Zazpe, I.; Morell-Azanza, L.; Chueca, M.J.; Azcona-Sanjulián, M.C.; Marti, A. Improved Diet Quality and Nutrient Adequacy after a Lifestyle Intervention in Children with Abdominal Obesity. Nutrients 2018, 10, 1500. [Google Scholar] [CrossRef]
- Ranucci, C.; Pippi, R.; Buratta, L.; Aiello, C.; Gianfredi, V.; Piana, N.; Reginato, E.; Romano, L.; Lorenzoni, V.; Pippi, R.; et al. Effects of an Intensive Lifestyle Intervention in Overweight/Obese Children and Adolescents. Biomed. Res. Int. 2017, 2017, 8573725. [Google Scholar] [CrossRef]
- Tognon, G.; Hebestreit, A.; Lanfer, A.; Moreno, L.A.; Pala, V.; Siani, A.; Veidebaum, T.; Tornaritis, M.; Molnár, D.; De Henauw, S.; et al. Mediterranean Diet, Overweight and Body Composition in Children from Eight European Countries: IDEFICS Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 205–213. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2021, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-Processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Luger, M.; Lafontan, M.; Bes-Rastrollo, M.; Winzer, E.; Yumuk, V.; Farpour-Lambert, N. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review from 2013 to 2015 and a Comparison with Previous Studies. Obes. Facts 2018, 10, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Schwarzfischer, P.; Gruszfeld, D.; Socha, P.; Luque, V.; Closa-Monasterolo, R.; Rousseaux, D.; Moretti, M.; Mariani, B.; Verduci, E.; Koletzko, B.; et al. Longitudinal Analysis of Physical Activity, Sedentary Behaviour and Anthropometry Ages 6 to 11 Years. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 126. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Psarra, G.; Georgoulis, M.; Tambalis, K.; Panagiotakos, D.B.; Sidossis, L.S.; EYZN Study Group. High and Moderate Adherence to Mediterranean Lifestyle Is Inversely Associated with Overweight, General and Abdominal Obesity in Children and Adolescents: The MediLIFE-Index. Nutr. Res. 2020, 73, 38–47. [Google Scholar] [CrossRef]
- Seaton, A.; Godden, D.J.; Brown, K. Increase in Asthma: A More Toxic Environment or a More Susceptible Population? Thorax 1994, 49, 171–174. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Johnston, S.L.; Freezer, N.J.; Ritter, W.; O’Toole, S.; Howarth, P.H. Prostaglandin D2-Induced Bronchoconstriction: Role of Thromboxane Prostanoid Receptor. Eur. Respir. J. 1995, 8, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Chang, S.S.; Sun, K.; Talegawkar, S.; Ferrucci, L.; Fried, L.P. Serum Carotenoids and Pulmonary Function in Older Community-Dwelling Women. J. Nutr. Health Aging 2012, 16, 291–296. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; ISAAC Phase III Study Group. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef]
- Romieu, I.; Trenga, C. Diet and Obstructive Lung Diseases. Epidemiol. Rev. 2001, 23, 268–287. [Google Scholar] [CrossRef]
- Shams, M.H.; Jafari, R.; Eskandari, N.; Masjedi, M.; Kheirandish, F.; Ganjalikhani Hakemi, M.; Ahmadi, M.; Kalantari, H.; Rezaei, N. Anti-Allergic Effects of Vitamin E in Allergic Diseases: An Updated Review. Int. Immunopharmacol. 2021, 90, 107196. [Google Scholar] [CrossRef]
- Cook-Mills, J.M. Isoforms of Vitamin E Differentially Regulate PKCα and Inflammation: A Review. J. Clin. Cell. Immunol. 2013, 4, 1000137. [Google Scholar] [CrossRef]
- Ghaffari, J.; Hossaini, R.F.; Khalilian, A.; Nahanmoghadam, N.; Salehifar, E.; Rafatpanah, H. Vitamin E Supplementation, Lung Functions and Clinical Manifestations in Children with Moderate Asthma: A Randomized Double-Blind Placebo-Controlled Trial. Iran. J. Allergy Asthma Immunol. 2014, 13, 348–355. Available online: http://ijaai.tums.ac.ir (accessed on 24 July 2025).
- Allan, K.M.; Prabhu, N.; Craig, L.C.A.; McNeill, G.; Kirby, B.; McLay, J.; Helms, P.J.; Ayres, J.G.; Seaton, A.; Turner, S.W. Maternal Vitamin D and E Intakes during Pregnancy and Childhood Asthma. Eur. Respir. J. 2015, 45, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Abalkhail, B.; Seaton, A. Diet and Childhood Asthma in a Society in Transition: A Study in Urban and Rural Saudi Arabia. Thorax 2000, 55, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.V.E.; Nash, A.A.; Powers, H.J.; Primhak, R.A. Antioxidant Status in Asthma. Pediatr. Pulmonol. 1994, 18, 34–38. [Google Scholar] [CrossRef]
- Zajac, D.; Wojciechowski, P. The Role of Vitamins in the Pathogenesis of Asthma. Int. J. Mol. Sci. 2023, 24, 8574. [Google Scholar] [CrossRef]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; Dell’Orco, V.; Ciccone, G.; Bisanti, L.; SIDRIA Collaborative Group. Consumption of Fresh Fruit Rich in Vitamin C and Wheezing Symptoms in Children. Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef]
- Kaur, B.; Rowe, B.H.; Arnold, E. Vitamin C supplementation for asthma. Cochrane Database Syst. Rev. 2009, 2009, CD000993. [Google Scholar] [CrossRef]
- Cohen, H.A.; Neuman, I.; Nahum, H.; Nahum, M. Blocking Effect of Vitamin C in Exercise-Induced Asthma. Arch. Pediatr. Adolesc. Med. 1997, 151, 367–370. [Google Scholar] [CrossRef]
- Nagel, G.; Linseisen, J. Dietary Intake of Fatty Acids, Antioxidants and Selected Food Groups and Asthma in Adults. Eur. J. Clin. Nutr. 2005, 59, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Wang, J.; Zhang, X.; Zhang, L.; Zhang, H.P.; Wang, L.; Yang, C.; Wang, Y. Fast Food Consumption, Dietary Factors and Asthma Prevalence in Adolescents. Respirology 2018, 23, 901–913. [Google Scholar] [CrossRef]
- Al-Zalabani, A.H.; Elahi, I.N.; Katib, A.; Alamri, A.G.; Halawani, A.; Alsindi, N.M.; Alharbi, K.K.; Abduljabbar, A.Z.; Al-Dakheel, F.M.; Alzahrani, A.S.; et al. Association between Soft Drinks Consumption and Asthma: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e029046. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Folsgaard, N.V.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze/Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2540. [Google Scholar] [CrossRef]
- Birch, E.E.; Khoury, J.C.; Berseth, C.L.; Castañeda, Y.S.; Couch, J.M.; Bean, J.; Tamer, R.; Harris, C.L.; Mitmesser, S.H.; Scalabrin, D.M.F. The Impact of Early Nutrition on Incidence of Allergic Manifestations and Common Respiratory Illnesses in Children. J. Pediatr. 2010, 156, 902–906.e1. [Google Scholar] [CrossRef] [PubMed]
- Hodge, L.; Salome, C.M.; Peat, J.K.; Haby, M.M.; Xuan, W.; Woolcock, A.J. Consumption of Oily Fish and Childhood Asthma Risk. Med. J. Aust. 1996, 164, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Antova, T.; Pattenden, S.; Nikiforov, B.; Leonardi, G.S.; Boeva, B.; Fletcher, T.; Rudnai, P.; Slachtova, H.; Tabak, C.; Zlotkowska, R.; et al. Nutrition and Respiratory Health in Children in Six Central and Eastern European Countries. Thorax 2003, 58, 231–236. [Google Scholar] [CrossRef]
- Escamilla-Nuñez, M.C.; Barraza-Villarreal, A.; Hernández-Cadena, L.; Navarro-Olivos, E.; Sly, P.D.; Romieu, I. Omega-3 Fatty Acid Supplementation during Pregnancy and Respiratory Symptoms in Children. Chest 2014, 146, 373–382. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Sharmin, S.; Rezwan, R.; Rahman, R.; Neeletol, S. A Comprehensive Look into the Association of Vitamin D Levels and Vitamin D Receptor Gene Polymorphism with Obesity in Children. Biomed. Pharmacother. 2022, 153, 113285. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, M.M.; Itsiopoulos, C.; Katsardis, C.; Tsoukalas, D.; Erbas, B. Does BMI Modify the Association between Vitamin D and Pulmonary Function in Children of the Mild Asthma Phenotype? Int. J. Environ. Res. Public Health 2022, 19, 16768. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.Q.; Yin, J.; Yao, J.; Shen, J.; Sheng, G.J.; Li, K.; Lv, H.F.; Fang, X.; Wu, W.F.; et al. Meta-Analysis of Vitamin D and Lung Function in Asthma Patients. Respir. Res. 2019, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D Supplementation in Children May Prevent Asthma Exacerbation Triggered by Acute Respiratory Infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza A in Schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
| Author | Participants | Date | Ages (Years Old) | Duration of Follow-Up | Assessment Methods | Result |
|---|---|---|---|---|---|---|
| Asghari et al., Iran [77] | 424 | 2016 | 6–18 | 3.6 years | DASH | No significant decreasing trend (p = 0.192) |
| Lioret et al., Australia [78] | 216 | 2014 | 5–12 | 3 years | DQI | Score inversely related to BMI in overweight children at Baseline; (p = 0.078) |
| Martin-Calvo et al., United States [79] | 10,918 | 2016 | 8–15 | 2–3 years | KIDMED | 2-point increment in the index is associated −0.04 kg/m2; p = 0.001 |
| McCourt et al., Ireland [80] | 487 | 2014 | 12–15 | 10 years | MDS | No association |
| Monjardino et al., Portugal [81] | 1716 | 2012 | 13 | 4 years | DASH, OHS | No significant association |
| Notario et al., United States [72] | 1527 | 2020 | 4 | 4 years | rMED | Score inversely associated (p = 0.02) |
| Ojeda-Rodríguez et al., Pamplona [82] | 107 | 2018 | 10 | 2 years | KIDMED | Inversely associated |
| Ranucci et al., Perugia [83] | 74 | 2017 | 5–17 | 3 months | KIDMED | Inversely associated in children group; p < 0.01 |
| Tognon et al., 8 European countries [84] | 16.220 | 2014 | 2–9 | 2 years | fMDS | High fMDS scores were inversely associated with overweight, obesity (p = 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deligeorgopoulou, M.; Tsabouri, S.; Siomou, E.; Vlahos, A.P.; Serbis, A. Dietary Habits and Their Impact on Pediatric Obesity and Asthma: A Narrative Review with Emphasis on the Mediterranean Diet. Children 2025, 12, 1354. https://doi.org/10.3390/children12101354
Deligeorgopoulou M, Tsabouri S, Siomou E, Vlahos AP, Serbis A. Dietary Habits and Their Impact on Pediatric Obesity and Asthma: A Narrative Review with Emphasis on the Mediterranean Diet. Children. 2025; 12(10):1354. https://doi.org/10.3390/children12101354
Chicago/Turabian StyleDeligeorgopoulou, Marianna, Sophia Tsabouri, Ekaterini Siomou, Antonios P. Vlahos, and Anastasios Serbis. 2025. "Dietary Habits and Their Impact on Pediatric Obesity and Asthma: A Narrative Review with Emphasis on the Mediterranean Diet" Children 12, no. 10: 1354. https://doi.org/10.3390/children12101354
APA StyleDeligeorgopoulou, M., Tsabouri, S., Siomou, E., Vlahos, A. P., & Serbis, A. (2025). Dietary Habits and Their Impact on Pediatric Obesity and Asthma: A Narrative Review with Emphasis on the Mediterranean Diet. Children, 12(10), 1354. https://doi.org/10.3390/children12101354








