Effects of Intramuscular Vasopressin on Pharmacokinetics and Pharmacodynamics in Healthy Neonatal Piglets: A Dose–Response Study
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Randomization
2.3. Blinding
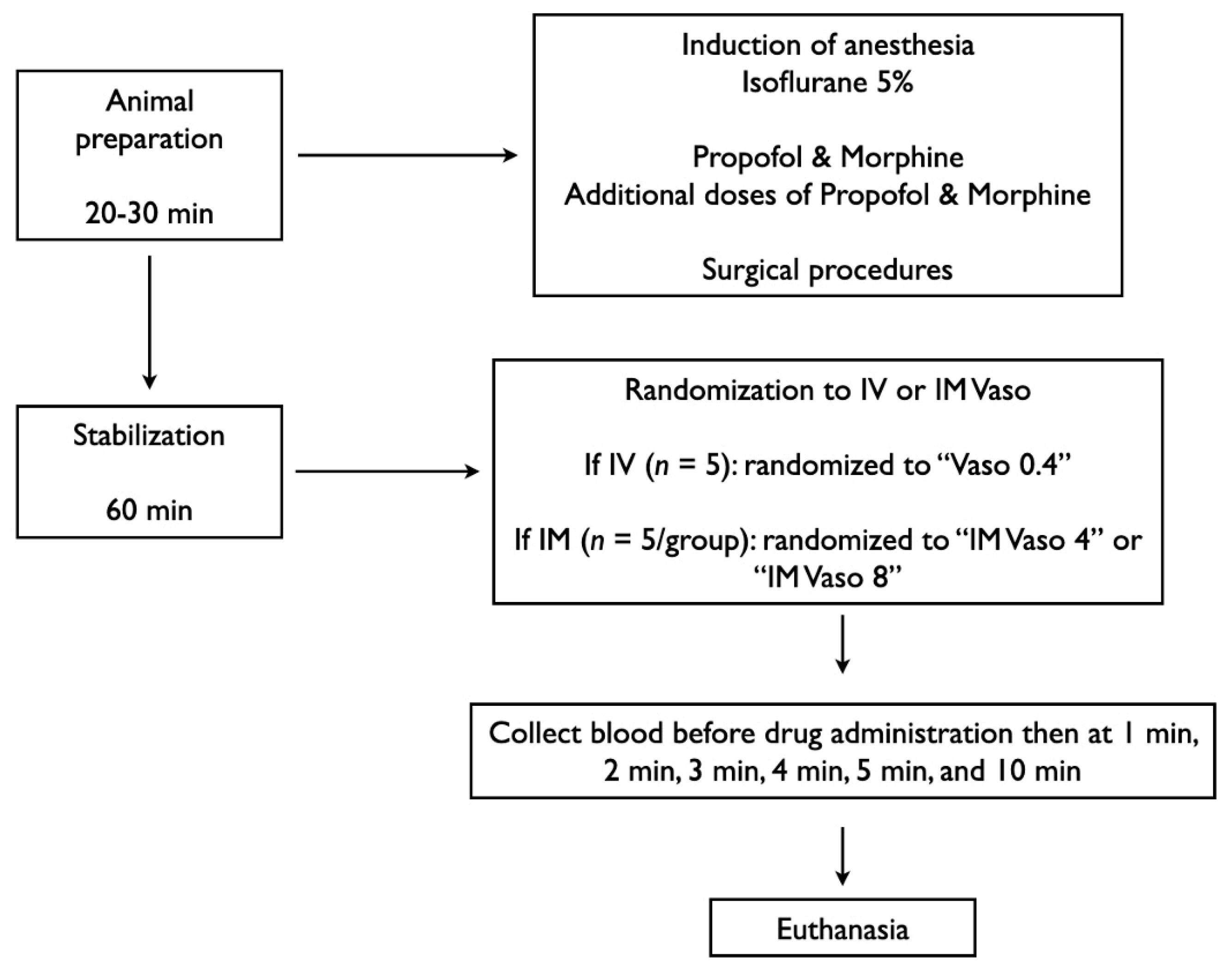
2.4. Animal Preparation
2.5. Hemodynamic Parameters
2.6. Cerebral Perfusion
2.7. Experimental Protocol
2.8. Data Collection and Analysis
3. Results
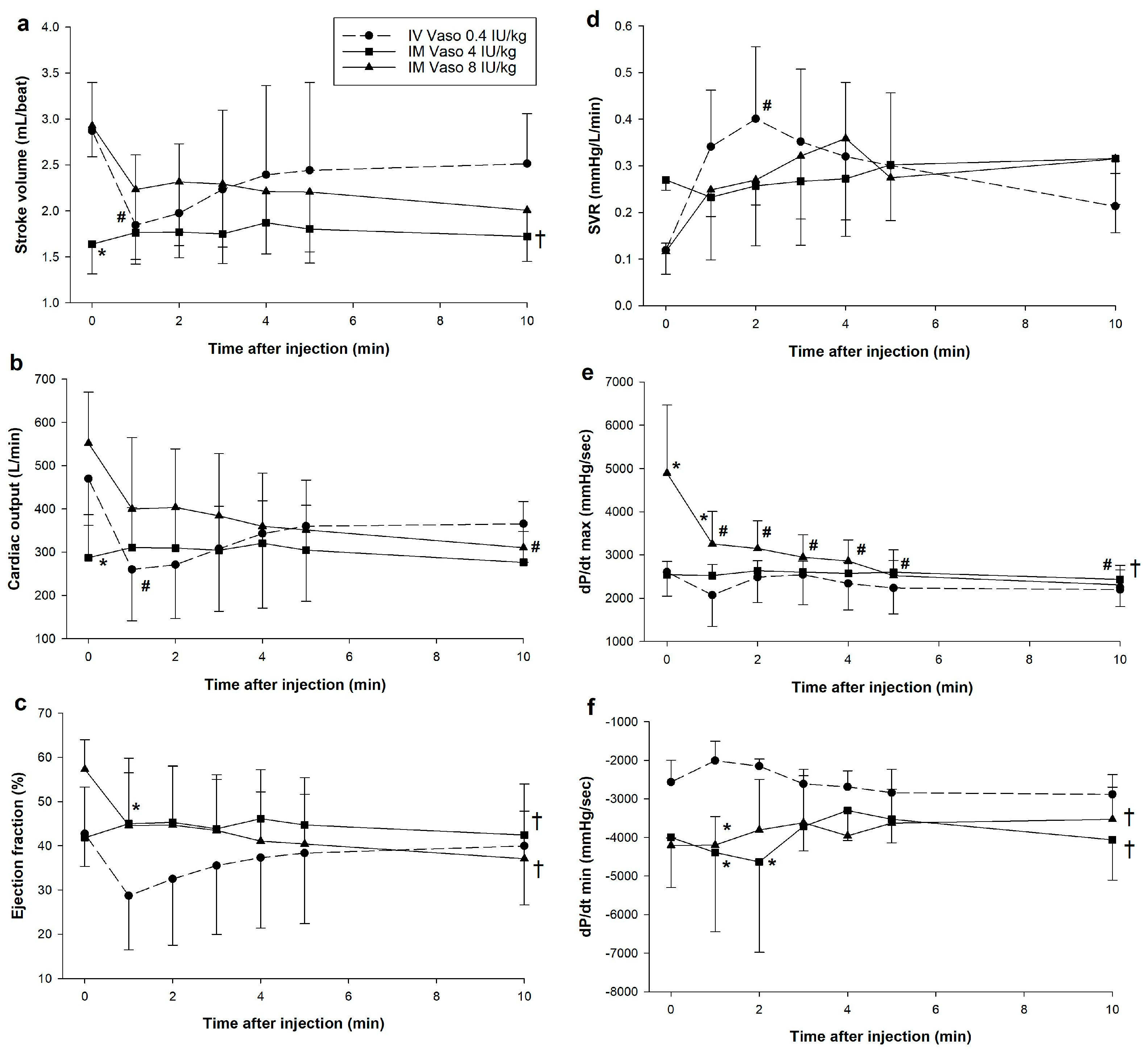
3.1. Hemodynamic Changes
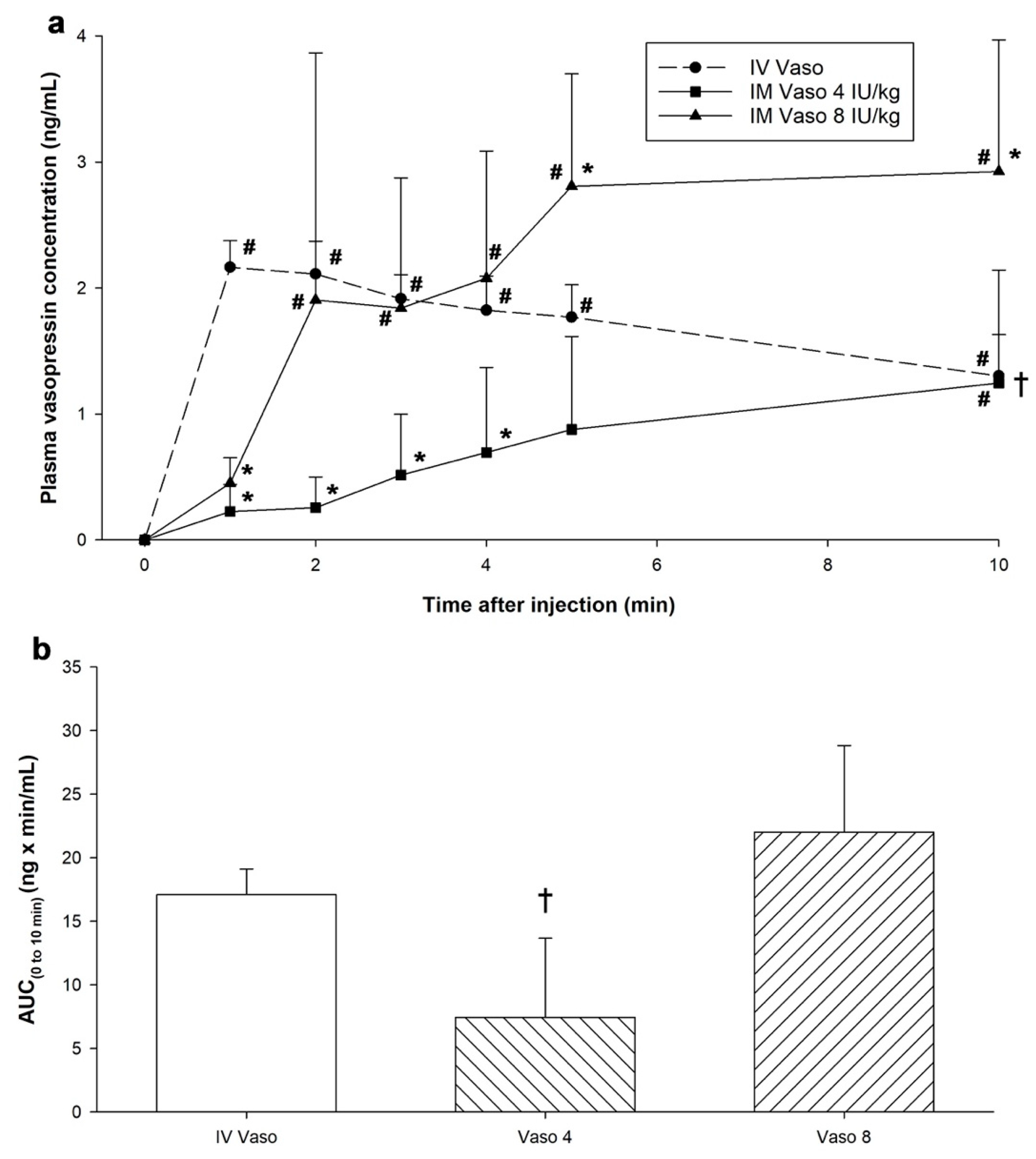
3.2. Changes in Plasma Vasopressin Concentrations
3.3. Pharmacokinetic Parameters
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Hidalgo, C.; Schmölzer, G.M. Chest Compressions in the Delivery Room. Children 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.C.; Passarella, M.; Raymond, T.T.; Lorch, S.A.; Ades, A.; Foglia, E.E. Epidemiology and Outcomes of Infants after Cardiopulmonary Resuscitation in the Neonatal or Pediatric Intensive Care Unit from a National Registry. Resuscitation 2021, 165, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Aziz, K.; Escobedo, M.B.; Kapadia, V.S.; Kattwinkel, J.; Perlman, J.M.; Simon, W.M.; Weiner, G.M.; Zaichkin, J.G. Part 13: Neonatal Resuscitation. Circulation 2015, 132, S543–S560. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 Practice Parameter Update. Ann. Allergy Asthma Immunol. 2024, 132, 124–176. [Google Scholar] [CrossRef]
- Wenzel, V.; Krismer, A.C.; Arntz, H.R.; Sitter, H.; Stadlbauer, K.H.; Lindner, K.H. A Comparison of Vasopressin and Epinephrine for Out-of-Hospital Cardiopulmonary Resuscitation. N. Engl. J. Med. 2004, 350, 105–113. [Google Scholar] [CrossRef]
- Mann, K.; Berg, R.A.; Nadkarni, V. Beneficial Effects of Vasopressin in Prolonged Pediatric Cardiac Arrest: A Case Series. Resuscitation 2002, 52, 149–156. [Google Scholar] [CrossRef]
- Matok, I.; Vardi, A.; Augarten, A.; Efrati, O.; Leibovitch, L.; Rubinshtein, M.; Paret, G. Beneficial Effects of Terlipressin in Prolonged Pediatric Cardiopulmonary Resuscitation: A Case Series. Crit. Care Med. 2007, 35, 1161–1164. [Google Scholar] [CrossRef]
- McNamara, P.J.; Engelberts, D.; Finelli, M.; Adeli, K.; Kavanagh, B.P. Vasopressin Improves Survival Compared with Epinephrine in a Neonatal Piglet Model of Asphyxial Cardiac Arrest. Pediatr. Res. 2014, 75, 738–748. [Google Scholar] [CrossRef]
- Ramsie, M.; Cheung, P.-Y.; Lee, T.-F.; O’Reilly, M.; Schmölzer, G.M. Comparison of Various Vasopressin Doses to Epinephrine during Cardiopulmonary Resuscitation in Asphyxiated Neonatal Piglets. Pediatr. Res. 2023, 95, 1265–1272. [Google Scholar] [CrossRef]
- O’Reilly, M.; Lee, T.-F.; Cheung, P.-Y.; Schmölzer, G.M. Vasopressin versus Epinephrine during Neonatal Cardiopulmonary Resuscitation of Asphyxiated Post-Transitional Piglets. Resusc. Plus 2023, 15, 100427. [Google Scholar] [CrossRef]
- Pelletier, J.-S.; Dicken, B.; Bigam, D.; Cheung, P.-Y. Cardiac Effects of Vasopressin. J. Cardiovasc. Pharmacol. 2014, 64, 100. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Ramsie, M.; Cheung, P.-Y.; O’Reilly, M.; Lee, T.-F.; Schmölzer, G.M. Pharmacokinetic and Pharmacodynamic Evaluation of Various Vasopressin Doses and Routes of Administration in a Neonatal Piglet Model. Sci. Rep. 2024, 14, 23096. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; O’Reilly, M.; Labossiere, J.; Lee, T.-F.; Cowan, S.; Qin, S.; Bigam, D.L.; Cheung, P.-Y. Cardiopulmonary Resuscitation with Chest Compressions during Sustained Inflations: A New Technique of Neonatal Resuscitation That Improves Recovery and Survival in a Neonatal Porcine Model. Circulation 2013, 128, 2495–2503. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; O’Reilly, M.; Labossiere, J.; Lee, T.-F.; Cowan, S.; Nicoll, J.; Bigam, D.L.; Cheung, P.-Y. 3:1 Compression to Ventilation Ratio versus Continuous Chest Compression with Asynchronous Ventilation in a Porcine Model of Neonatal Resuscitation. Resuscitation 2014, 85, 270–275. [Google Scholar] [CrossRef]
- Cheung, P.-Y.; Gill, R.S.; Bigam, D.L. A Swine Model of Neonatal Asphyxia. J. Vis. Exp. 2011, 56, 3166. [Google Scholar] [CrossRef]
- Wagner, M.; Cheung, P.-Y.; Li, E.S.; Lee, T.-F.; Lu, M.; O’Reilly, M.; Olischar, M.; Schmölzer, G.M. Effects of Epinephrine on Hemodynamic Changes during Cardiopulmonary Resuscitation in a Neonatal Piglet Model. Pediatr. Res. 2018, 83, 897–903. [Google Scholar] [CrossRef]
- Shen, W.; Xu, X.; Lee, T.-F.; Schmölzer, G.; Cheung, P.-Y. The Relationship Between Heart Rate and Left Ventricular Isovolumic Relaxation During Normoxia and Hypoxia-Asphyxia in Newborn Piglets. Front. Physiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Hansen, M.; Schmicker, R.H.; Newgard, C.D.; Grunau, B.; Scheuermeyer, F.; Cheskes, S.; Vithalani, V.; Alnaji, F.; Rea, T.; Idris, A.H.; et al. Time to Epinephrine Administration and Survival From Nonshockable Out-of-Hospital Cardiac Arrest Among Children and Adults. Circulation 2018, 137, 2032–2040. [Google Scholar] [CrossRef]
- Malayan, S.A.; Ramsay, D.J.; Keil, L.C.; Reid, I.A. Effects of Increases in Plasma Vasopressin Concentration on Plasma Renin Activity, Blood Pressure, Heart Rate, and Plasma Corticosteroid Concentration in Conscious Dogs. Endocrinology 1980, 107, 1899–1904. [Google Scholar] [CrossRef]
- Montani, J.P.; Liard, J.F.; Schoun, J.; Möhring, J. Hemodynamic Effects of Exogenous and Endogenous Vasopressin at Low Plasma Concentrations in Conscious Dogs. Circ. Res. 1980, 47, 346–355. [Google Scholar] [CrossRef]
- Bronicki, R.A.; Acosta, S.; Savorgnan, F.; Flores, S.; Achuff, B.-J.; Loomba, R.; Ahmed, M.; Ghanayem, N.; Heinle, J.S.; Asadourian, V.; et al. The Acute Influence of Vasopressin on Hemodynamic Status and Tissue Oxygenation Following the Norwood Procedure. JTCVS Open 2022, 9, 217–224. [Google Scholar] [CrossRef]
- Nakano, J. Studies on the Cardiovascular Effects of Synthetic Vasopressin. J. Pharmacol. Exp. Ther. 1967, 157, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.A.; Segel, L.D. Attenuation of Vasopressin-Mediated Coronary Constriction and Myocardial Depression in the Hypoxic Heart. Circ. Res. 1990, 66, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.; Buyssens, L.; Stroe, M.; Valenzuela, A.; Allegaert, K.; Smits, A.; Annaert, P.; Mulder, A.; Carpentier, S.; Van Ginneken, C.; et al. The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics 2021, 13, 44. [Google Scholar] [CrossRef]

| IV Vaso 0.4 IU/kg (n = 5) | IM Vaso 4 IU/kg (n = 5) | IM Vaso 8 IU/kg (n = 5) | p-Value | |
|---|---|---|---|---|
| Age (days) | 2 (2–3) | 1 (1–2) | 3 (1–3) | 0.531 |
| Weight (kg) | 2.1 (1.8–2.3) | 1.9 (1.7–2) | 2 (1.9–2.1) | 0.611 |
| Sex (male/female) | 3/2 | 1/4 | 5/0 | 0.066 |
| Heart rate (bpm) | 160 (152–168) | 174 (148–181) | 183 (162–191) | 0.267 |
| Mean arterial pressure (mmHg) | 61 (58–66) | 61 (56–72) | 62 (60–75) | 0.604 |
| Systolic (mmHg) | 94 (83–102) | 80 (75–84) | 84 (72–94) | 0.561 |
| Diastolic (mmHg) | 41 (39–44) | 48 (41–49) | 55 (44–55) | 0.134 |
| Carotid blood flow (mL/kg/min) | 89 (52–101) | 50 (45–57) | 64 (38–72) | 0.310 |
| Cerebral oxygenation (%) | 46 (40–50) | 44 (42–49) | 49 (45–51) | 0.433 |
| pH | 7.44 (7.42–7.50) | 7.50 (7.49–7.56) | 7.53 (7.49–7.56) | 0.171 |
| PaCO2 (torr) | 35 (34–40) | 31 (31–32) | 32 (26–33) | 0.017 |
| PaO2 (torr) | 83 (64–91) | 74 (73–81) | 73 (70–74) | 0.608 |
| Base excess (mmol/L) | 0.7 (−0.200~4.9) | 3.8 (0.1~4.5) | 1.7 (1.2~3.8) | 0.911 |
| Lactate (mmol/L) | 3.31 (3.00–5.11) | 5.69 (4.33–6.28) | 5.70 (5.38–5.79) | 0.237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsie, M.; Cheung, P.-Y.; Hyderi, R.; Praveen, S.; Lee, T.-F.; O’Reilly, M.; Schmölzer, G.M. Effects of Intramuscular Vasopressin on Pharmacokinetics and Pharmacodynamics in Healthy Neonatal Piglets: A Dose–Response Study. Children 2025, 12, 1284. https://doi.org/10.3390/children12101284
Ramsie M, Cheung P-Y, Hyderi R, Praveen S, Lee T-F, O’Reilly M, Schmölzer GM. Effects of Intramuscular Vasopressin on Pharmacokinetics and Pharmacodynamics in Healthy Neonatal Piglets: A Dose–Response Study. Children. 2025; 12(10):1284. https://doi.org/10.3390/children12101284
Chicago/Turabian StyleRamsie, Marwa, Po-Yin Cheung, Raza Hyderi, Shrieya Praveen, Tze-Fun Lee, Megan O’Reilly, and Georg M. Schmölzer. 2025. "Effects of Intramuscular Vasopressin on Pharmacokinetics and Pharmacodynamics in Healthy Neonatal Piglets: A Dose–Response Study" Children 12, no. 10: 1284. https://doi.org/10.3390/children12101284
APA StyleRamsie, M., Cheung, P.-Y., Hyderi, R., Praveen, S., Lee, T.-F., O’Reilly, M., & Schmölzer, G. M. (2025). Effects of Intramuscular Vasopressin on Pharmacokinetics and Pharmacodynamics in Healthy Neonatal Piglets: A Dose–Response Study. Children, 12(10), 1284. https://doi.org/10.3390/children12101284







