Analysis of Salivary Cytokines in Retinopathy of Prematurity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Salivary Collection
2.3. Patient Grouping and Data Collection
2.4. Cytokine Analysis
2.5. Statistics
3. Results
3.1. Patient Enrollment
3.2. Patient Demographics
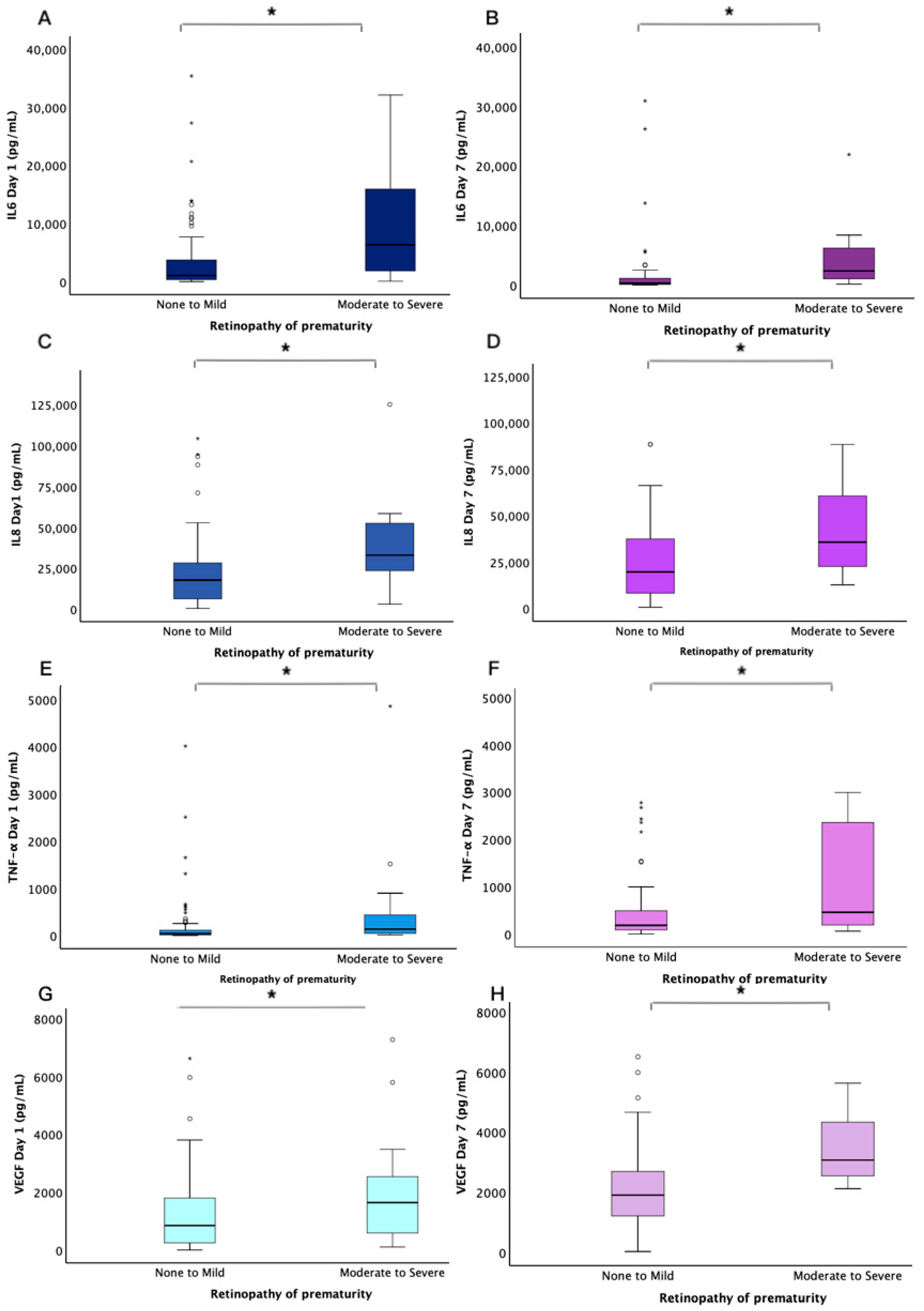
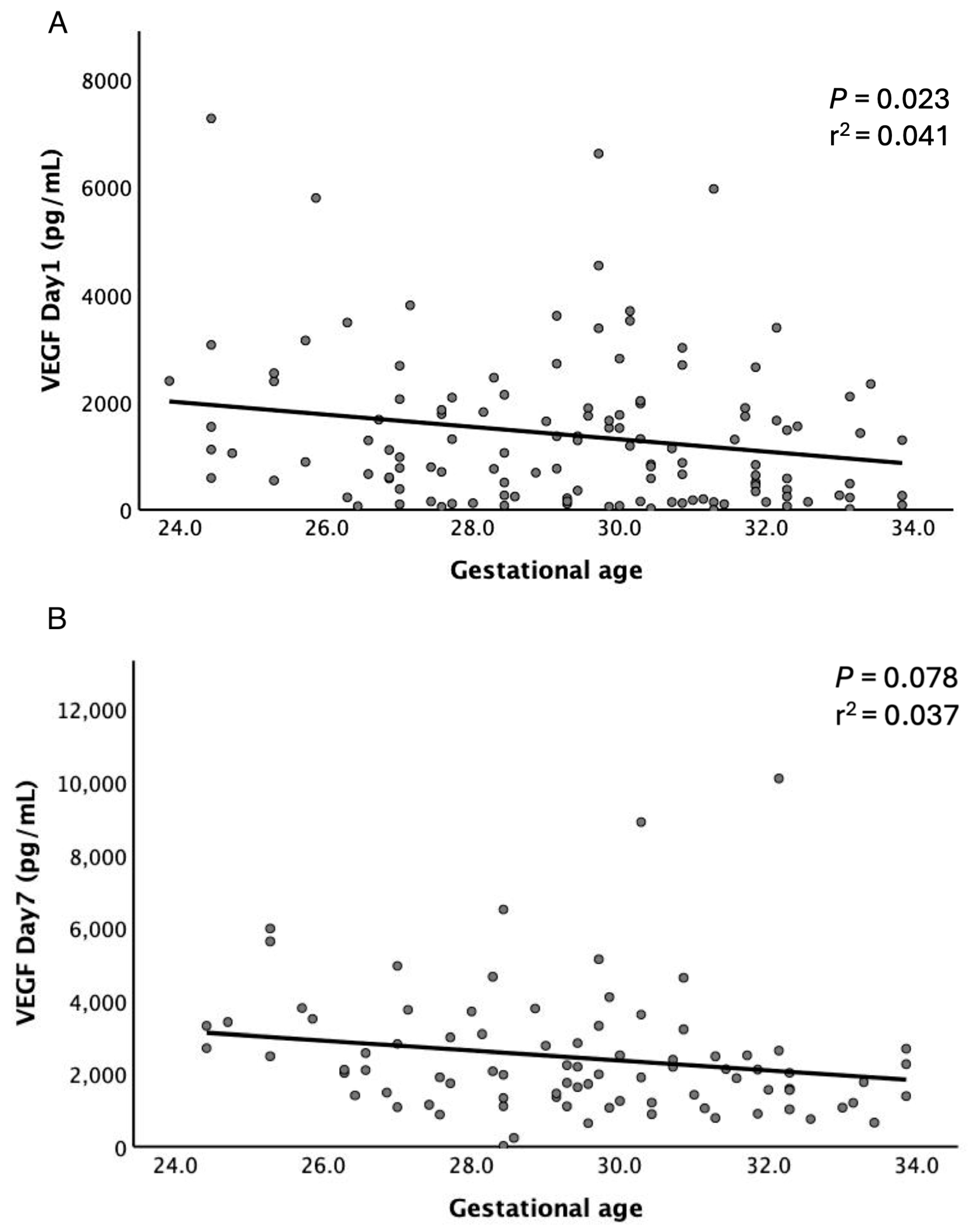
3.3. Salivary Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solebo, A.L.; Teoh, L.; Rahi, J. Epidemiology of blindness in children. Arch. Dis. Child. 2017, 102, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Fevereiro-Martins, M.; Marques-Neves, C.; Guimarães, H.; Bicho, M. Retinopathy of prematurity: A review of pathophysiology and signaling pathways. Surv. Ophthalmol. 2023, 68, 175–210. [Google Scholar] [CrossRef] [PubMed]
- Chan-Ling, T.; Gole, G.A.; Quinn, G.E.; Adamson, S.J.; Darlow, B.A. Pathophysiology, screening and treatment of ROP: A multi-disciplinary perspective. Prog. Retin. Eye Res. 2018, 62, 77–119. [Google Scholar] [CrossRef]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef]
- Fierson, W.M. American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar]
- Sonmez, K.; Drenser, K.A.; Capone, A., Jr.; Trese, M.T. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 2008, 115, 1065–1070.e1. [Google Scholar] [CrossRef]
- Sato, T.; Kusaka, S.; Shimojo, H.; Fujikado, T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009, 116, 1599–1603. [Google Scholar] [CrossRef]
- Sato, T.; Kusaka, S.; Shimojo, H.; Fujikado, T. Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 2009, 116, 2165–2169. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, L.; Zou, Y.; Peng, L.; Wang, Y.; Li, T.; Tang, S. Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS 2014, 122, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Morken, T.S.; Fichorova, R.N.; VanderVeen, D.K.; Allred, E.N.; Dammann, O.; Leviton, A.; ELGAN Study Neonatology and Ophthalmology Committees. Systemic inflammation-associated proteins and retinopathy of prematurity in infants born before the 28th week of gestation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6419–6428. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, B.; Wang, Z.; Zou, J.; Jia, Y.; Yoshida, S.; Zhou, Y. Novel potential biomarkers for retinopathy of prematurity. Front. Med. 2022, 9, 840030. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal complement activation and inflammation in the pathogenesis of retinopathy of prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Woo, S.J.; Park, J.Y.; Hong, S.; Kim, Y.M.; Park, Y.H.; Lee, Y.E.; Park, K.H. Inflammatory and angiogenic mediators in amniotic fluid are associated with the development of retinopathy of prematurity in preterm infants. Invest. Ophthalmol. Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef]
- Woo, S.J.; Park, K.H.; Lee, S.Y.; Ahn, S.J.; Ahn, J.; Park, K.H.; Oh, K.J.; Ryu, A. The relationship between cord blood cytokine levels and perinatal factors and retinopathy of prematurity: A gestational age-matched case-control study. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3434–3439. [Google Scholar] [CrossRef]
- Park, Y.J.; Woo, S.J.; Kim, Y.M.; Hong, S.; Lee, Y.E.; Park, K.H. Immune and inflammatory proteins in cord blood as predictive biomarkers of retinopathy of prematurity in preterm infants. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3813–3820. [Google Scholar] [CrossRef]
- Vinekar, A.; Nair, A.P.; Sinha, S.; Vaidya, T.; Chakrabarty, K.; Shetty, R.; Ghosh, A.; Sethu, S. Tear fluid angiogenic factors: Potential noninvasive biomarkers for retinopathy of prematurity screening in preterm infants. Invest. Ophthalmol. Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef]
- Byrne, M.L.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Walsh, K.A.; Laughton, K.; Waloszek, J.M.; Woods, M.J.; Trinder, J.; Allen, N.B. Acute phase protein and cytokine levels in serum and saliva: A comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav. Immun. 2013, 34, 164–175. [Google Scholar] [CrossRef]
- Slavish, D.C.; Graham-Engeland, J.E.; Smyth, J.M.; Engeland, C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2015, 44, 253–269. [Google Scholar] [CrossRef]
- Chen, I.L.; Huang, H.C.; Ou-Yang, M.C.; Chen, F.S.; Chung, M.Y.; Chen, C.C. A novel method to detect bacterial infection in premature infants: Using a combination of inflammatory markers in blood and saliva. J. Microbiol. Immunol. Infect. 2020, 53, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Su, T.Y.; Chen, I.L.; Yeh, T.F.; Yu, H.R.; Hsu, Y.L.; Hung, C.H.; Huang, H.C. Salivary cytokine - A non-invasive predictor for bronchopulmonary dysplasia in premature neonates. Cytokine 2021, 148, 155616. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Evans, P.D.; Ng, S.H.; Chong, A.M.; Siu, O.T.; Chan, C.L.; Ho, S.M.; Ho, R.T.; Chan, P.; Chan, C.C. Optimism, positive affectivity, and salivary cortisol. Br. J. Health Psychol. 2005, 10, 467–484. [Google Scholar] [CrossRef] [PubMed]
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Hong, H.F.; Lee, H.J.; Ko, J.H.; Park, J.H.; Park, J.Y.; Choi, C.W.; Yoon, C.H.; Ahn, S.J.; Park, K.H.; Woo, S.J.; et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J. Neuroinflamm. 2014, 11, 87. [Google Scholar]
- Kwinta, P.; Bik-Multanowski, M.; Mitkowska, Z.; Tomasik, T.; Pietrzyk, J.J. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1467–1475. [Google Scholar] [CrossRef]
- Yenice, O.; Cerman, E.; Ashour, A.; Firat, R.; Haklar, G.; Sirikci, O.; Akman, I.; Kazokoglu, H. Serum erythropoietin, insulin-like growth factor 1, and vascular endothelial growth factor in etiopathogenesis of retinopathy of prematurity. Ophthalmic Surg. Lasers Imaging Retina 2013, 44, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Cakir, U.; Tayman, C.; Yucel, C.; Ozdemir, O. Can IL-33 and endocan be new markers for retinopathy of prematurity? Comb. Chem. High. Throughput Screen. 2019, 22, 41–48. [Google Scholar] [CrossRef]
- Vannay, A.; Dunai, G.; Bányász, I.; Szabó, M.; Vámos, R.; Treszl, A.; Hajdú, J.; Tulassay, T.; Vásárhelyi, B. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr. Res. 2005, 57, 396–398. [Google Scholar] [CrossRef]
- Cooke, R.W.; Drury, J.A.; Mountford, R.; Clark, D. Genetic polymorphisms and retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1712–1715. [Google Scholar] [CrossRef]
- Silveira, R.C.; Fortes Filho, J.B.; Procianoy, R.S. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.N.; Aygun, A.D.; Godekmerdan, A.; Kurt, A.; Dogan, Y.; Yilmaz, E. Serum IL-1beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Mediators Inflamm. 2007, 2007, 31397. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, P.; Gomez-Lopez, N.; Yoon, B.H.; et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J. Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral. Biol. Craniofac Res. 2016, 6, 66–75. [Google Scholar] [CrossRef]
- Gutierrez-Corrales, A.; Campano-Cuevas, E.; Castillo-Dali, G.; Torres-Lagares, D.; Gutierrez-Perez, J.L. Ability of salivary biomarkers in the prognostic of systemic and buccal inflammation. J. Clin. Exp. Dent. 2017, 9, e716–e722. [Google Scholar] [CrossRef]
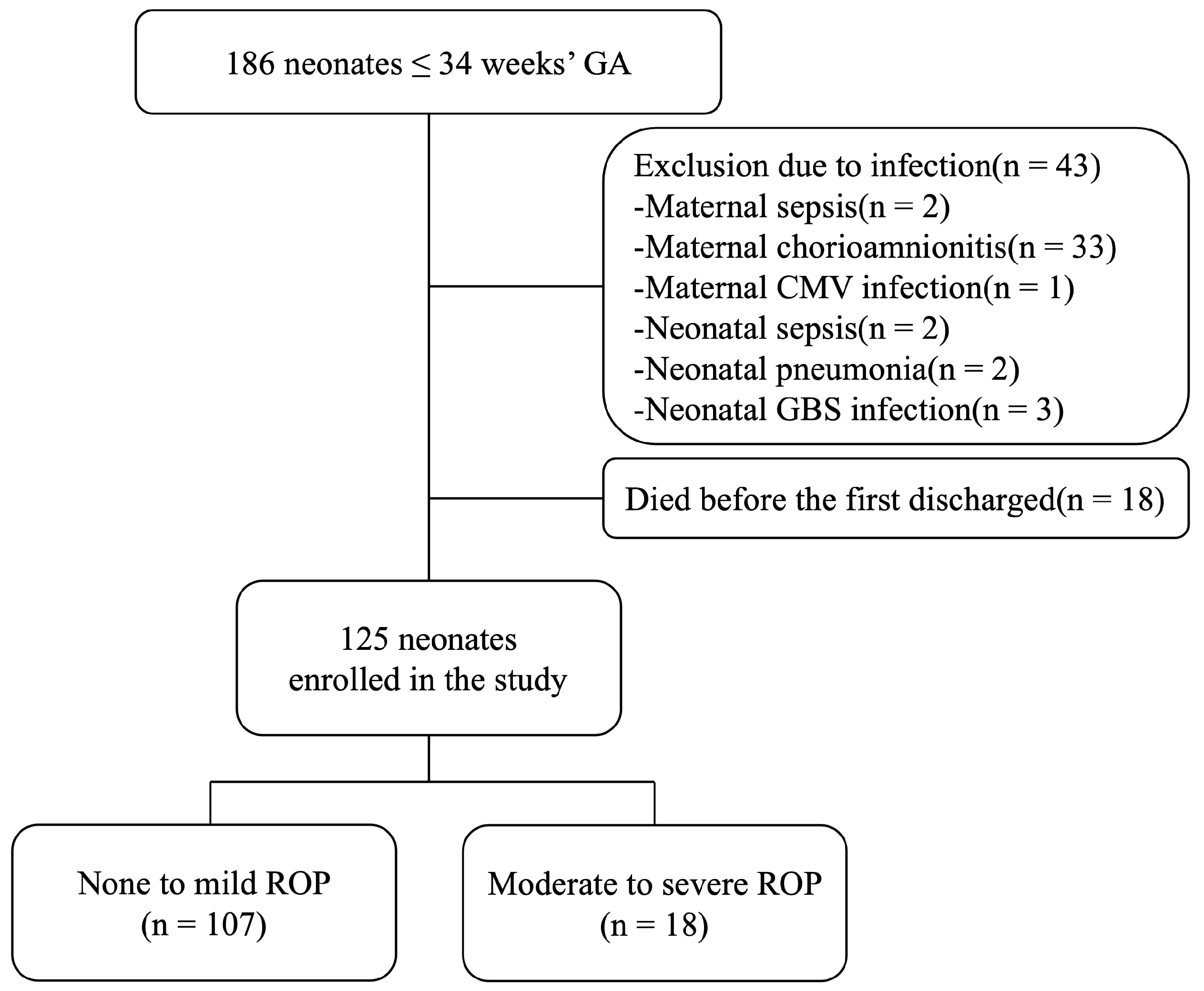
| Characteristics | None to Mild ROP (n= 107) | Moderate to Severe ROP (n= 18) | p |
|---|---|---|---|
| GA (weeks) | 30.0 (28.3–31.7) | 26.4 (24.4–27.8) | <0.001 |
| BW (gm) | 1280 (970–1600) | 790 (703–1038) | <0.001 |
| Sex (F/M) | 48/59 | 10/8 | 0.451 |
| Delivery mode (N/C) | 40/67 | 9/9 | 0.434 |
| A/S 1 | 6.0 (4.7–7.0) | 5.0 (2.0–6.3) | 0.054 |
| A/S 5 | 8.0 (7.0–9.0) | 7.0 (6.0–8.0) | 0.065 |
| G | 2.0 (1.0–3.0) | 1.5 (1.0–3.0) | 0.572 |
| p | 1.0 (1.0–2.0) | 1.0 (1.0–1.3) | 0.166 |
| SBP (mmHg) | 51.0 (45.0–55.0) | 50.5 (41.5–56.3) | 0.888 |
| DBP (mmHg) | 27.0 (24.0–32.0) | 29.0 (22.0–35.0) | 0.547 |
| RR (times/min) | 47.0 (40.0–58.0) | 42.0 (31.8–57.0) | 0.195 |
| HR (times/min) | 149.0 (140.0–156.0) | 151.5 (138.0–158.0) | 0.877 |
| BT (°C) | 36.0 (35.2–36.5) | 35.9 (35.1–36.1) | 0.132 |
| Days of invasive ventilation | 3 (0–7.0) | 54 (4.3–76.5) | <0.001 |
| Surfactant administration | 61 (57) | 14 (77.8) | 0.078 |
| IVH | 28 (26.2) | 3 (16.7) | 0.558 |
| PVL | 6 (5.6) | 2 (11.1) | 0.324 |
| BPD | 55 (51.4) | 17 (94.4) | <0.001 |
| Maternal Condition | |||
| Preeclampsia | 17 (15.8) | 1 (5.5) | 0.467 |
| GDM | 2 (1.9) | 1 (5.5) | 0.547 |
| Antepartum fever | 5 (4.72) | 3 (18.8) | 0.089 |
| Hours of PROM | 0 (0–72) | 0 (0–3.8) | 0.221 |
| Antepartum antibiotic | 96 (89.7) | 18(100) | 0.363 |
| Antepartum steroid | 76 (71.0) | 16 (94.1) | 0.151 |
| Cytokines | ROP Severity | Univariable Median (IQR) | p | Multivariable OR (95% C.I.) | p * |
|---|---|---|---|---|---|
| IL-6 (Day 1) | None to Mild Moderate to Severe | 1070 (351–3750) 6335 (1579–16,041) | 0.003 | 1.000 (1.000–1.000) | 0.430 |
| IL-8 (Day 1) | None to Mild Moderate to Severe | 17,640 (5888–28,356) 32,945 (22,144–52,679) | 0.001 | 1.000 (1.000–1.000) | 0.647 |
| TNF-α (Day 1) | None to Mild Moderate to Severe | 44.5 (14.6–113.8) 136.5 (44.2–454.8) | 0.007 | 1.000 (0.999–1.001) | 0.601 |
| VEGF (Day 1) | None to Mild Moderate to Severe | 845.0 (4.1–1814.4) 1638 (579–2667) | 0.077 | 1.00 (1.000–1.001) | 0.295 |
| IL-6 (Day 7) | None to Mild Moderate to Severe | 313 (115–1161) 2363 (977–7288) | 0.001 | 1.000 (1.000–1.000) | 0.037 |
| IL-8 (Day 7) | None to Mild Moderate to Severe | 19,625 (7788–39,148) 35,645 (21,506–67,234) | 0.015 | 1.000 (1.000–1.000) | 0.132 |
| TNF-α (Day 7) | None to Mild Moderate to Severe | 181.4 (82.8–496.1) 457.5 (135.4–2673.0) | 0.045 | 1.000 (1.000–1.000) | 0.255 |
| VEGF (Day 7) | None to Mild Moderate to Severe | 1902 (1207–2730) 3065 (2524–4641) | 0.001 | 1.000 (1.000–1.001) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-S.; Huang, H.-C.; Chen, I.-L. Analysis of Salivary Cytokines in Retinopathy of Prematurity. Children 2025, 12, 80. https://doi.org/10.3390/children12010080
Wu H-S, Huang H-C, Chen I-L. Analysis of Salivary Cytokines in Retinopathy of Prematurity. Children. 2025; 12(1):80. https://doi.org/10.3390/children12010080
Chicago/Turabian StyleWu, Hwa-Shiu, Hsin-Chun Huang, and I-Lun Chen. 2025. "Analysis of Salivary Cytokines in Retinopathy of Prematurity" Children 12, no. 1: 80. https://doi.org/10.3390/children12010080
APA StyleWu, H.-S., Huang, H.-C., & Chen, I.-L. (2025). Analysis of Salivary Cytokines in Retinopathy of Prematurity. Children, 12(1), 80. https://doi.org/10.3390/children12010080






