Evaluating the Effectiveness of Enoxaparin in Treating Pediatric Arterial Thrombosis in Saudi Arabia
Abstract
1. Introduction
2. Method
2.1. Data Collection
2.2. Diagnosis and Treatment of CAT
2.3. Statistical Methodology
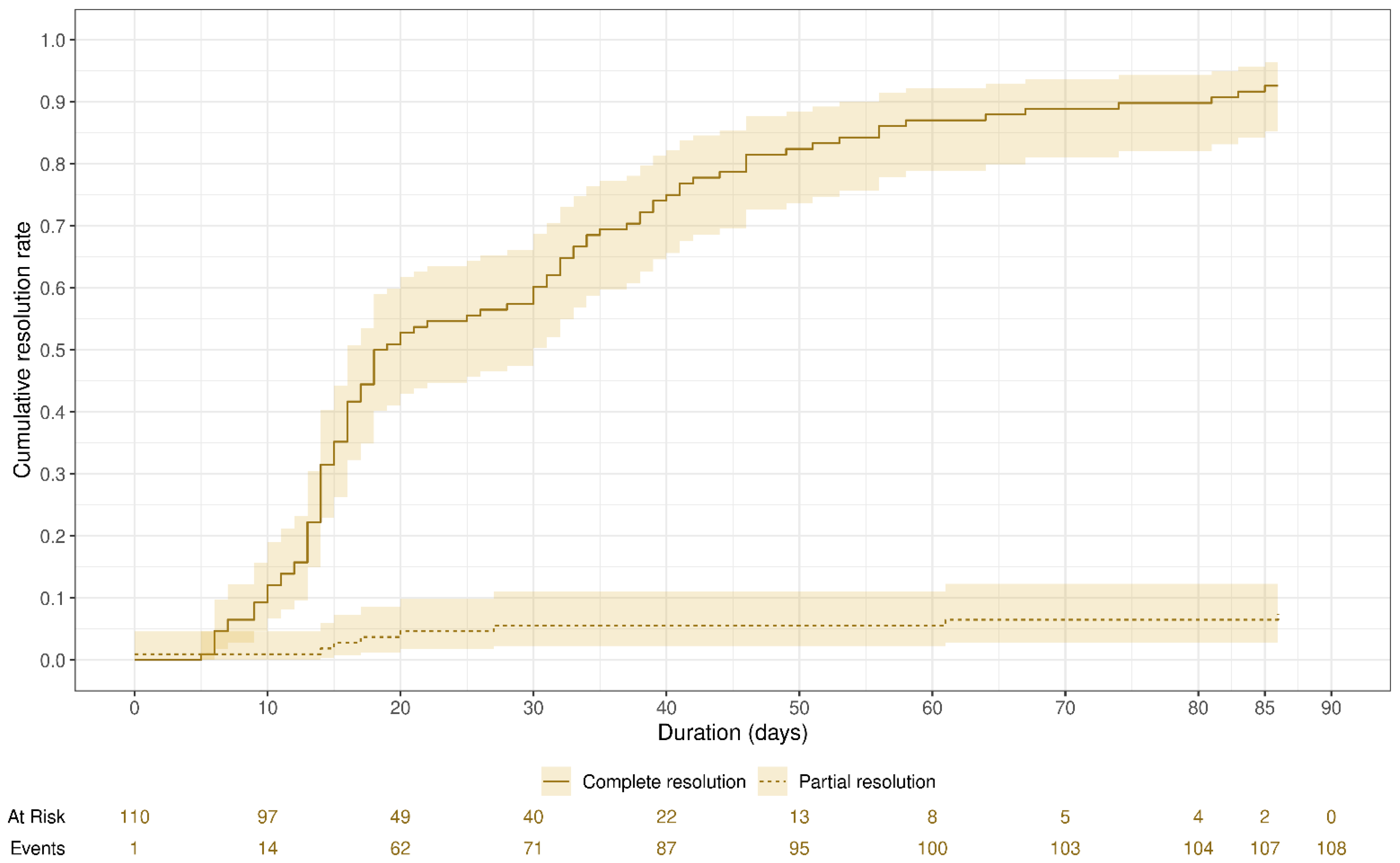
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumann Kreuziger, L.; Jaffray, J.; Carrier, M. Epidemiology, diagnosis, prevention and treatment of catheter-related thrombosis in children and adults. Thromb. Res. 2017, 157, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Robinson, V.; Achey, M.A.; Nag, U.P.; Reed, C.R.; Pahl, K.S.; Greenberg, R.G.; Clark, R.H.; Tracy, E.T. Thrombosis in infants in the neonatal intensive care unit: Analysis of a large national database. J. Thromb. Haemost. 2021, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D.O. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008, 140, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Albisetti, M. Treatment of arterial thrombosis in children: Methods and mechanisms. Thromb. Res. 2018, 169, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Goldenberg, N.; Bonduel, M.; Revel-Vilk, S.; Amankwah, E.; Albisetti, M. Catheter-Related Arterial Thrombosis in Neonates and Children: A Systematic Review. Thromb. Haemost. 2018, 118, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Crameri, O.; Brotschi, B.; Achini, F.; Rizzi, M.; Albisetti, M. Treatment of Catheter-Related Arterial Thrombosis in Children: A 15-Year Single- Center Experience. J. Pediatr. 2021, 239, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.T.; Anderson, V.; Desai, S.B.; Arunachalam, A.; Ahmed, M.; Diaz, R. Patient Characteristics and Treatment Outcomes of Symptomatic Catheter-Related Arterial Thrombosis in Infants: A Retrospective Cohort Study. J. Pediatr. 2021, 231, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bontadelli, J.; Moeller, A.; Schmugge, M.; Schraner, T.; Kretschmar, O.; Bauersfeld, U.; Bernet-Buettiker, V.; Albisetti, M. Enoxaparin therapy for arterial thrombosis in infants with congenital heart disease. Intensive Care Med. 2007, 33, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, E.; Agarwal, S.; Stanek, J.; Sankar, A.; Kerlin, B.A.; Rodriguez, V. Pediatric arterial thrombosis: A single-institution cohort study of patient characteristics and thrombosis outcomes. Pediatr. Blood Cancer. 2024, 71, e30756. [Google Scholar] [CrossRef] [PubMed]
- Glatz, A.C.; Keashen, R.; Chang, J.; Balsama, L.; Dori, Y.; Gillespie, M.J.; Giglia, T.M.; Raffini, L.; Rome, J.J. Outcomes using a clinical practice pathway for the management of pulse loss following pediatric cardiac catheterization. Catheter. Cardiovasc. Interv. 2015, 85, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P.; Chan, A.K.C.; Goldenberg, N.A.; Ichord, R.N.; Journeycake, J.M.; Nowak-Göttl, U.; Vesely, S.K. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e737S–e801S. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Value 1 | N |
|---|---|---|
| Age at diagnosis (months) | 3.0 (1.0, 5.0) | 111 |
| Gender | 111 | |
| Male | 64 (58%) | |
| Female | 47 (42%) | |
| Body weight (kg) | 3.70 (2.85, 5.15) | 111 |
| Catheter type | 108 | |
| Cardiac catheter | 94 (87%) | |
| Indwelling catheter | 14 (13%) | |
| Type of radiological test | 111 | |
| US | 111 (100%) | |
| Degree of occlusion | 111 | |
| Bilateral complete | 70 (63%) | |
| Bilateral partial | 35 (32%) | |
| Partial and Complete | 6 (5.4%) | |
| Lower limb thrombosis | 111 | |
| Femoral | 34 (31%) | |
| Iliac | 41 (37%) | |
| Both | 36 (32%) |
| Characteristic | Value 1 | N |
|---|---|---|
| Enoxaparin start dose (mg/kg) | 1.37 (1.00, 1.50) | 111 |
| Duration of enoxaparin therapy (days) | 20 (13, 43) | 111 |
| Duration of anticoagulant (days) | 22 (14, 45) | 111 |
| Hemoglobin level at the start of enoxaparin therapy (g/L) | 124 (111, 135) | 110 |
| Hemoglobin level at the end of enoxaparin therapy (g/L) | 122 (114, 132) | 68 |
| Platelet level at the start of enoxaparin therapy (per mL) | 250 (172, 338) | 110 |
| Platelet level at the end of enoxaparin therapy (per mL) | 441 (316, 610) | 67 |
| Resolution degree | 111 | |
| No resolution | 2 (1.8%) | |
| Partial resolution | 9 (8.1%) | |
| Complete resolution | 100 (90%) | |
| Time to resolution (days) | 18 (14, 37) | 109 |
| Characteristic | Value 1 | N |
|---|---|---|
| Bleeding | 111 | |
| No bleeding | 108 (97%) | |
| Minor bleeding | 1 (0.9%) | |
| Major bleeding | 0 (0%) | |
| Fatal bleeding | 0 (0%) | |
| Other types of bleeding | 2 (1.8%) | |
| Number of missed refills | 110 | |
| 0 | 93 (85%) | |
| 1 | 6 (5.5%) | |
| 2 | 7 (6.4%) | |
| 3 | 3 (2.7%) | |
| 4 | 1 (0.9%) | |
| Anti-factor Xa level (U/mL) | 0.52 (0.42, 0.58) | 108 |
| Compliance * | 95 (89%) | 107 |
| Characteristic | Odds Ratio (OR) | 95% CI 1 | p-Value |
|---|---|---|---|
| Age (months) | 1.61 | 1.05, 2.46 | 0.028 |
| Gender | |||
| Female | - | - | |
| Male | 2.89 | 0.31, 26.8 | 0.4 |
| Body weight (kg) | 0.12 | 0.02, 0.71 | 0.019 |
| Catheter | |||
| Cardiac catheter | - | - | |
| Indwelling catheter | 47.2 | 2.95, 75.6 | 0.006 |
| Degree of occlusion | |||
| Bilateral complete | - | - | |
| Bilateral partial | 0.45 | 0.03, 6.13 | 0.6 |
| Partial and complete | 0.08 | 0.00, 79.0 | 0.5 |
| Lower limb thrombosis | |||
| Femoral | - | - | |
| Iliac | 6.46 | 0.41, 103 | 0.2 |
| Both | 1.00 | 0.05, 19.8 | >0.9 |
| Enoxaparin start dose | 0.78 | 0.02, 28.1 | 0.9 |
| Duration of enoxaparin therapy (days) | 0.98 | 0.83, 1.17 | 0.8 |
| Duration of anticoagulant (days) | 1.04 | 0.88, 1.24 | 0.6 |
| Time to resolution (days) | 1.00 | 0.97, 1.04 | >0.9 |
| Number of missed refills | 1.39 | 0.35, 5.57 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Meshary, M.; Alotaibi, A.; Alsagri, N.S.; AlZhrani, A.; Ardah, H.I.; Alnuhait, M.A. Evaluating the Effectiveness of Enoxaparin in Treating Pediatric Arterial Thrombosis in Saudi Arabia. Children 2024, 11, 1139. https://doi.org/10.3390/children11091139
Al-Meshary M, Alotaibi A, Alsagri NS, AlZhrani A, Ardah HI, Alnuhait MA. Evaluating the Effectiveness of Enoxaparin in Treating Pediatric Arterial Thrombosis in Saudi Arabia. Children. 2024; 11(9):1139. https://doi.org/10.3390/children11091139
Chicago/Turabian StyleAl-Meshary, Meshary, Abdulrahman Alotaibi, Nouf S. Alsagri, Asmaa AlZhrani, Husam I. Ardah, and Mohammed A. Alnuhait. 2024. "Evaluating the Effectiveness of Enoxaparin in Treating Pediatric Arterial Thrombosis in Saudi Arabia" Children 11, no. 9: 1139. https://doi.org/10.3390/children11091139
APA StyleAl-Meshary, M., Alotaibi, A., Alsagri, N. S., AlZhrani, A., Ardah, H. I., & Alnuhait, M. A. (2024). Evaluating the Effectiveness of Enoxaparin in Treating Pediatric Arterial Thrombosis in Saudi Arabia. Children, 11(9), 1139. https://doi.org/10.3390/children11091139





