Feasibility of Achieving Nutritional Adequacy in Critically Ill Children with Critical Neurological Illnesses (CNIs)?—A Quaternary Hospital Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutrition Assessment
2.2. Nutrition Support
3. Results
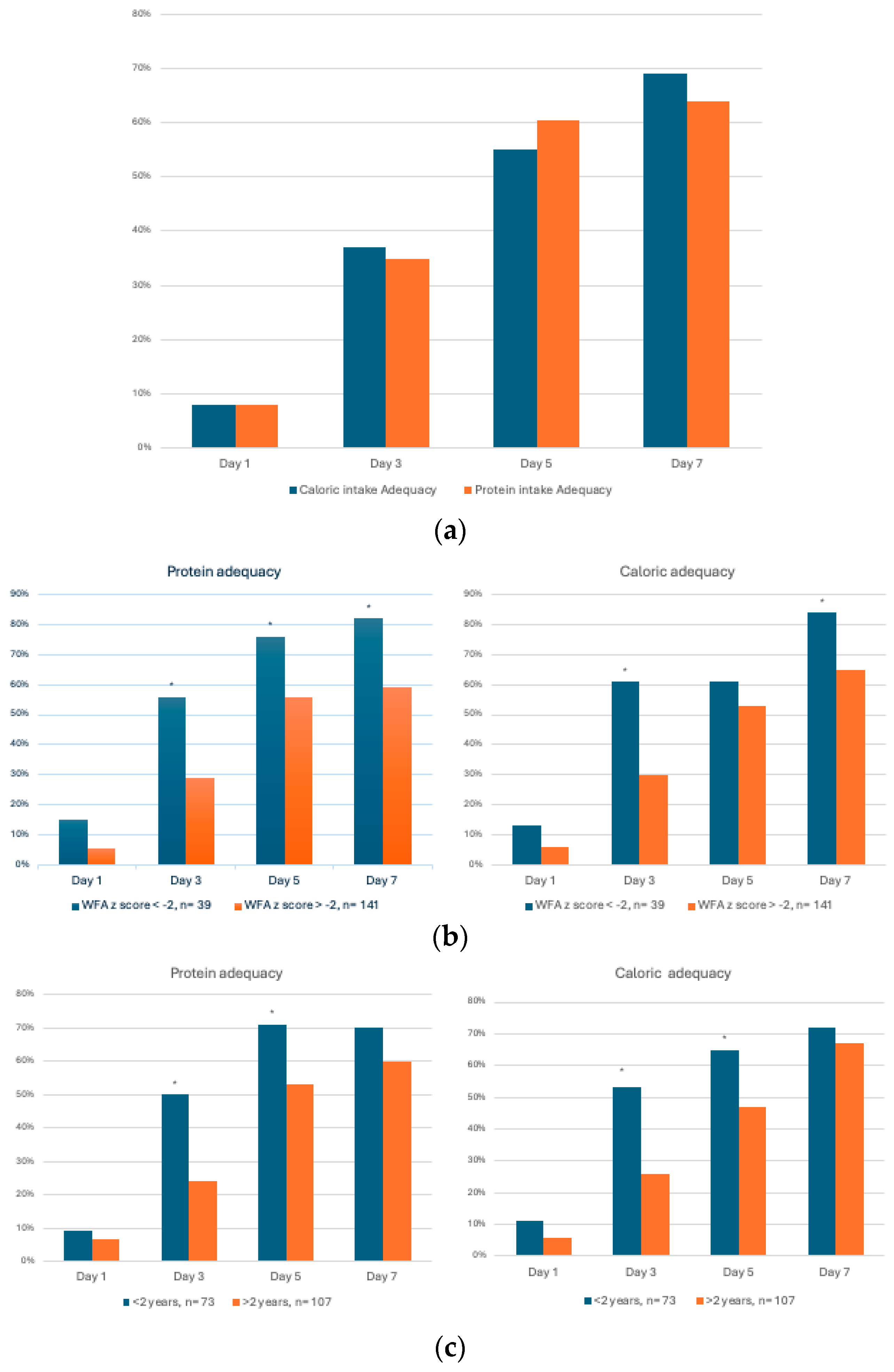
3.1. Nutritional Assessment
3.2. Nutrition Support
- (a)
- Nutrition support in underweight vs. non-underweight patients
- (b)
- Protein and caloric adequacy by age
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albert, B.D.; Martinez, E.E. Challenges and advances in nutrition for the critically ill child. Curr. Opin. Crit. Care 2022, 28, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Bechard, L.J.; Zurakowski, D.; Duggan, C.P.; Heyland, D.K. Adequate enteral protein intake is inversely associated with 60-d mortality in critically ill children: A multicenter, prospective, cohort study. Am. J. Clin. Nutr. 2015, 102, 199–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, N.M.; Bechard, L.J.; Cahill, N.; Wang, M.; Day, A.; Duggan, C.P.; Heyland, D.K. Nutritional practices and their relationship to clinical outcomes in critically ill children—An international multicenter cohort study. Crit. Care Med. 2012, 40, 2204–2211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [PubMed]
- Malakouti, A.; Sookplung, P.; Siriussawakul, A.; Philip, S.; Bailey, N.; Brown, M.; Farver, K.; Zimmerman, J.J.; Bell, M.J.; Vavilala, M.S. Nutrition support and deficiencies in children with severe traumatic brain injury. Pediatr. Crit. Care Med. 2012, 13, e18–e24. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, M.; Lintvedt, A.; Kogelschatz, B.; Heinze, E.; Parker, J.L.; Ferguson, K.; Rosner, E.; Boville, B.; Leimanis-Laurens, M.L. Daily Nutritional Intake of Pediatric Patients (N = 64) on Extracorporeal Membrane Oxygenation from 2018 to 2022: A Single-Center Report. Nutrients 2023, 15, 3221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zivick, E.; Kilgallon, K.; Cheifetz, I.M. Challenges of Nutrition Support in Pediatric Patients Requiring Extracorporeal Membrane Oxygenation. ASAIO J. 2024, 70, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Carpenter, D.; Keller, G.; Morgan, S.; Irving, S.Y. Enteral Nutrition in the PICU: Current Status and Ongoing Challenges. J. Pediatr. Intensive Care 2015, 4, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.C.; Hauschild, D.B.; Barbosa, E.; Bresolin, N.L.; Kawai, K.; Mehta, N.M.; Moreno, Y.M.F. Undernutrition at PICU Admission Is Predictor of 60-Day Mortality and PICU Length of Stay in Critically Ill Children. J. Acad. Nutr. Diet. 2020, 120, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Moreno, Y.; Ventura, J.; Oliveira, L.; Silveira, T.; Hauschild, D. Undernutrition in critically ill children. Pediatr. Med. 2020, 3, 22. [Google Scholar] [CrossRef]
- Amirjani, S.; Ahmadizadeh, N.; Behzad, A.; Dadashi-Noshahr, Y.; Vahdat Shariatpanahi, Z. Undernutrition and 60-day mortality in critically ill children with respiratory failure: A prospective cohort study. BMC Pediatr. 2023, 23, 271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taha, A.A.; Badr, L.; Westlake, C.; Dee, V.; Mudit, M.; Tiras, K.L. Effect of early nutritional support on intensive care unit length of stay and neurological status at discharge in children with severe traumatic brain injury. J. Neurosci. Nurs. 2011, 43, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Tavarez, T.; Roehl, K.; Koffman, L. Nutrition in the Neurocritical Care Unit: A New Frontier. Curr. Treat. Options Neurol. 2021, 23, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Neurosurgery 2019, 84, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Datta, S.; Ray, K.; Mukhopadhyay, D.K. Nutrient adequacy and its correlation in a sub-Himalayan region of West Bengal, India. J. Family Med. Prim. Care 2016, 5, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kulsum, A., Jr.; Lakshmi, J.A.; Prakash, J. Food intake and energy protein adequacy of children from an urban slum in Mysore, India—A qualitative analysis. Malays. J. Nutr. 2008, 14, 163–172. [Google Scholar] [PubMed]
- Chaudhury, R.N. Determinants of nutrient adequacy in a rural area of Bangladesh. Food Nutr. Bull. 1986, 8, 24–31. [Google Scholar] [CrossRef]
- Chou, J.H.; Roumiantsev, S.; Singh, R. PediTools Electronic Growth Chart Calculators: Applications in Clinical Care, Research, and Quality Improvement. J. Med. Internet Res. 2020, 22, e16204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schofield, W.N. Predicting basal metabolic rate, new standards, and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.N.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F.; American Society for Parenteral and Enteral Nutrition Board of Directors. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. JPEN J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Duggan, C.P. Nutritional deficiencies during critical illness. Pediatr. Clin. N. Am. 2009, 56, 1143–1160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Souza Menezes, F.; Leite, H.P.; Koch Nogueira, P.C. Malnutrition as an independent predictor of clinical outcome in critically ill children. Nutrition 2012, 28, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Campos-Miño, S.; Figueiredo-Delgado, A.; Zárate, P.; Zamberlan, P.; Muñoz-Benavides, E.; Coss-Bu, J.A.; Nutrition Committee; Latin American Society of Pediatric Intensive Care (SLACIP). Malnutrition and Nutrition Support in Latin American PICUs: The Nutrition in PICU (NutriPIC) Study. Pediatr. Crit. Care Med. 2023, 24, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Abera, E.G.; Sime, H. The prevalence of malnutrition among critically ill children: A systematic review and meta-analysis. BMC Pediatr. 2023, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Hulst, J.; Joosten, K.; Zimmermann, L.; Hop, W.; van Buuren, S.; Büller, H.; Tibboel, D.; van Goudoever, J. Malnutrition in critically ill children: From admission to 6 months after discharge. Clin. Nutr. 2004, 23, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.J.; Nieman Carney, L.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). J. Acad. Nutr. Diet. 2014, 114, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Serón-Arbeloa, C.; Puzo-Foncillas, J.; Garcés-Gimenez, T.; Escós-Orta, J.; Labarta-Monzón, L.; Lander-Azcona, A. A retrospective study about the influence of early nutritional support on mortality and nosocomial infection in the critical care setting. Clin. Nutr. 2011, 30, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Shoykhet, M.; Bell, M.J.; Wai, K. Nutritional Support for Pediatric Severe Traumatic Brain Injury. Front. Pediatr. 2022, 10, 904654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skillman, H.E.; Mehta, N.M. Nutrition therapy in the critically ill child. Curr. Opin. Crit. Care 2012, 18, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lui, A.; Kumar, K.K.; Grant, G.A. Management of Severe Traumatic Brain Injury in Pediatric Patients. Front. Toxicol. 2022, 4, 910972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kratochvíl, M.; Klučka, J.; Klabusayová, E.; Musilová, T.; Vafek, V.; Skříšovská, T.; Djakow, J.; Havránková, P.; Osinová, D.; Štourač, P. Nutrition in Pediatric Intensive Care: A Narrative Review. Children 2022, 9, 1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balakrishnan, B.; Flynn-O’Brien, K.T.; Simpson, P.M.; Dasgupta, M.; Hanson, S.J. Enteral Nutrition Initiation in Children Admitted to Pediatric Intensive Care Units After Traumatic Brain Injury. Neurocrit. Care 2019, 30, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jin, Z.; Cheng, Y.; Su, J.; Li, Z.; Jin, Z. Effect of Early Nutritional Assessment and Nutritional Support on Immune Function and Clinical Prognosis of Critically Ill Children. J. Healthc. Eng. 2022, 2022, 7100238, retracted in J. Healthc. Eng. 2022, 2022, 9786015. [Google Scholar] [CrossRef] [PubMed]
- Dietitian and nutrition-related practices and resources in Australian and New Zealand PICUs: A clinician survey. Aust. Crit. Care 2024, 37, 490–494. [CrossRef] [PubMed]
- Beggs, M.R.; Ashkin, A.; Larsen, B.M.K.; Garros, D. Measuring Energy Requirements of Traumatic Brain Injury Patients in Pediatric Intensive Care With Indirect Calorimetry: A Comparison With Empiric Methods. Pediatr. Crit. Care Med. 2023, 24, e468–e475. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gburek, J.; Zhu, P.H.; Mansour, M.; Walding, D.; Coss-Bu, J.A. A methodological and clinical approach to measured energy expenditure in the critically ill pediatric patient. Front. Pediatr. 2022, 10, 1027358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| n = 1112 | Median (IQR) |
|---|---|
| Age (years) | 6.7 (1.9–13) |
| Weight (kg) | 22 (11.7–45.4) |
| Height (n = 1099) (cm) | 115 (83–147) |
| Medical diagnosis n (%) | 847 (76) |
| Surgical diagnosis n (%) | 265 (24) |
| PIM2 ROM % | 1 (0.82–3.7) |
| LOS MV (n = 385) (h) | 51 (20–153) |
| PICU LOS (days) | 2 (1–4) |
| Hospital LOS (days) | 5.7 (3.5–15) |
| WFA z score | −0.17 ± 2 (SD) |
| Prevalence of underweight, n (%) | 135 (12.14) |
| HFA z score | −0.9 ± 2 (SD) |
| Prevalence of chronic malnutrition, n (%) | 250 (22.5) |
| WFL/BMI z score | −0.45 ± 1.8 (SD) |
| Prevalence of acute malnutrition, n (%) | 88 (7.9) |
| Mortality, n (%) | 19 (1.70) |
| WFA > −2 n = 977 | WFA < −2 n = 135 | p Value | |
|---|---|---|---|
| Age (years) | 6.6 (2–13) | 8.9 (1–14) | 0.84 |
| Female gender n (%) | 446 (45.6) | 58 (43) | 0.56 |
| PIM2 ROM % | 1.03 (0.80–3.71) | 1.09 (0.83–3.75) | 0.5 |
| MV (h) | 47 (20–139) n = 331 | 98 (31–229) n = 54 | 0.0055 |
| PICU LOS (days) | 1.9 (1.03–3.8) | 2.9 (1.2–6.8) | 0.0008 |
| Hospital LOS (days) | 5.6 (3.4–14.7) | 6.6 (3.7–17.6) | 0.12 |
| Mortality, n (%) | n = 14 (1.4) | n = 5 (3.7) | 0.056 |
| Age < 2 Years Old n = 282 | Age > 2 Years Old n = 830 | p Value | |
|---|---|---|---|
| PIM2 ROM % | 1.23 (0.92–4.13) | 0.99 (0.80–3.47) | p < 0.0001 |
| MV (h) (n = 385) | 74 (28–225) (n = 120) | 43 (18–115) (n = 265) | p < 0.0005 |
| PICU LOS (days) | 2.6 (1.31–6.4) | 1.9 (1–3.7) | p < 0.0001 |
| Hospital LOS (days) | 7.2 (3.6–20.7) | 5.5 (3.44–13.71) | p < 0.005 |
| Underweight n (%) | 41 (14.5) | 94 (11.3) | p = 0.15 |
| Mortality, n (%) | 8 (2.84) | 11 (1.33) | p = 0.09 |
| n= 180 | Median (IQR) |
|---|---|
| Age (years) | 4.8 (0.8–12) |
| Female, n (%) | 70 (39) |
| Weight (kg) | 17 (8.8–35) |
| Height (cm) | 99 (69–136) |
| PIM2 ROM % | 3.7 (1–7) |
| MV (n =148) (h) | 170 (98–358) |
| ICU LOS (days) | 11 (7–20) |
| Hospital LOS (days) | 23 (14.5–42) |
| WFA z score | −0.59 ± 2.31 (SD) |
| Prevalence of underweight, n (%) | 39 (21.6) |
| Patients on ketogenic diet, n (%) | 7 (3.8) |
| Mortality, n (%) | 9 (5) |
| Evaluated before 48 h. n = 98 | Evaluated after 48 h. n = 82 | p Value | ||
|---|---|---|---|---|
| Day 3 | Caloric adequacy (%) | 51 (8–93) | 21 (0–77) | 0.03 |
| Protein adequacy (%) | 45 (0–96) | 15 (0–69) | 0.02 | |
| Day 5 | Caloric adequacy (%) | 76 (11–125) | 57 (7–109) | 0.12 |
| Protein adequacy (%) | 90 (44–134) | 64 (16–116) | 0.0495 | |
| Day 7 | Caloric adequacy (%) | 103 (56–152) | 86 (26–116) | 0.007 |
| Protein adequacy (%) | 98 (48–136) | 72 (25–105) | 0.006 |
| Protein Adequacy ≥ 60% | Caloric Adequacy ≥ 60% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infection n = 41 | Seizure n = 57 | Neurovascular n = 32 | Other n = 50 | p Value | Infection n = 41 | Seizure n = 57 | Neurovascular n = 32 | Other n = 50 | p Value | |
| Day 1 | 9.7% (n = 4) | 3.5% (n = 2) | 3% (n = 1) | 14% (n = 7) | 0.14 | 9.5% (n = 4) | 3.5% (n = 2) | 3% (n = 1) | 14% (n = 7) | 0.2 |
| Day 3 | 31% (n = 13) | 35% (n = 20) | 22% (n = 7) | 46% (n = 23) | 0.15 | 36.5% (n = 15) | 38.5% (n = 22) | 28% (n = 9) | 42% (n = 21) | 0.1 |
| Day 5 | 56% (n = 23) | 63% (n = 36) | 56% (n = 18) | 64% (n = 32) | 0.8 | 51% (n = 21) | 67% (n = 38) | 50% (n = 16) | 48% (n = 24) | 0.16 |
| Day 7 | 65% (n = 27) | 61% (n = 35) | 56% (n = 18) | 72% (n = 36) | 0.48 | 73% (n = 30) | 64% (n = 37) | 65% (n = 21) | 74% (n = 37) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, M.; Knebusch, N.; Daughtry, J.; Fogarty, T.P.; Lam, F.W.; Orellana, R.A.; Lai, Y.-C.; Erklauer, J.; Coss-Bu, J.A. Feasibility of Achieving Nutritional Adequacy in Critically Ill Children with Critical Neurological Illnesses (CNIs)?—A Quaternary Hospital Experience. Children 2024, 11, 711. https://doi.org/10.3390/children11060711
Mansour M, Knebusch N, Daughtry J, Fogarty TP, Lam FW, Orellana RA, Lai Y-C, Erklauer J, Coss-Bu JA. Feasibility of Achieving Nutritional Adequacy in Critically Ill Children with Critical Neurological Illnesses (CNIs)?—A Quaternary Hospital Experience. Children. 2024; 11(6):711. https://doi.org/10.3390/children11060711
Chicago/Turabian StyleMansour, Marwa, Nicole Knebusch, Jennifer Daughtry, Thomas P. Fogarty, Fong Wilson Lam, Renan A. Orellana, Yi-Chen Lai, Jennifer Erklauer, and Jorge A. Coss-Bu. 2024. "Feasibility of Achieving Nutritional Adequacy in Critically Ill Children with Critical Neurological Illnesses (CNIs)?—A Quaternary Hospital Experience" Children 11, no. 6: 711. https://doi.org/10.3390/children11060711
APA StyleMansour, M., Knebusch, N., Daughtry, J., Fogarty, T. P., Lam, F. W., Orellana, R. A., Lai, Y.-C., Erklauer, J., & Coss-Bu, J. A. (2024). Feasibility of Achieving Nutritional Adequacy in Critically Ill Children with Critical Neurological Illnesses (CNIs)?—A Quaternary Hospital Experience. Children, 11(6), 711. https://doi.org/10.3390/children11060711





