Abstract
SARS-CoV-2 serology may be helpful to retrospectively understand infection dynamics in specific settings including kindergartens. We assessed SARS-CoV-2 seroprevalence in individuals connected to kindergartens in Berlin, Germany in September 2021. Children, staff, and household members from 12 randomly selected kindergartens were interviewed on COVID-19 history and sociodemographic parameters. Blood samples were collected on filter paper. SARS-CoV-2 anti-S and anti-N antibodies were assessed using Roche Elecsys. We assessed seroprevalence and the proportion of so far unrecognized SARS-CoV-2 infections. We included 277 participants, comprising 48 (17.3%) kindergarten children, 37 (13.4%) staff, and 192 (69.3%) household members. SARS-CoV-2 antibodies were present in 65.0%, and 52.7% of all participants were vaccinated. Evidence of previous infection was observed in 16.7% of kindergarten children, 16.2% of staff, and 10.4% of household members. Undiagnosed infections were observed in 12.5%, 5.4%, and 3.6%, respectively. Preceding infections were associated with facemask neglect. In conclusion, two-thirds of our cohort were SARS-CoV-2 seroreactive in September 2021, largely as a result of vaccination in adults. Kindergarten children showed the highest proportion of non-vaccine-induced seropositivity and an increased proportion of previously unrecognized SARS-CoV-2 infection. Silent infections in pre-school children need to be considered when interpreting SARS-CoV-2 infections in the kindergarten context.
Keywords:
kindergarten; daycare; SARS-CoV-2; COVID-19; antibody; seroprevalence; pre-school children; Germany 1. Introduction
The role of children in SARS-CoV-2 transmission and their potential as reservoirs has extensively been discussed [1,2,3,4,5,6,7,8]. COVID-19 infections in young children tend to be mild or asymptomatic [8,9], and are severely underdiagnosed [10,11,12]. This partially explains why infection dynamics in the kindergarten context remain incompletely understood [13]. This context encompasses preschool children, their families and educational staff, each with distinct patterns of interpersonal interaction, vulnerability and risk of infection. The kindergarten environment presents unique challenges due to the widespread absence of routine virus screening and the difficulties associated with implementing mask-wearing or maintaining physical distance among young children [7,14]. Seroprevalence within families is higher when adult family members are index cases compared to when children are the index cases [15]. On the other hand, increased odds of infection have been observed in preschool educators as compared to teachers working with older children illustrating that infectious risk might also depend on child age and/or behavior [16]. Widespread vaccinations have further complicated the disentanglement of transmission routes and risk factors. Nevertheless, information on infection risks as reflected by infection prevalence, on potential pathways and risk factors as well as on manifestation in young children remain crucial for the preparedness in the face of potential resurgences or emerging pathogens. We aimed to provide descriptive data in this regard. Therefore, in the present study, we assessed (i) SARS-CoV-2 seroprevalence and (ii) the proportion of so far unrecognized SARS-CoV-2 infections in Berlin kindergarten children, kindergarten staff, and connected household members in September 2021. We further assessed (iii) associated factors with unrecognized SARS-CoV-2 infections and preceding infections.
2. Materials and Methods
This study analyzes cross-sectional seroprevalence data collected in Berlin in September 2021. Community incidence of SARS-CoV-2 infection in the preceding months varied greatly (Figure 1). The alpha variant (B.1.1.7) was replaced by the delta variant (B.1.617.2) starting in June 2021, reaching 99% by September 2021 [17]. Kindergartens were closed in the second lockdown in Germany between December 2020 and March 2021. This closure continued in Berlin until May 2021, except for children of essential workers such as health care professionals [18,19]. After reopening, kindergartens continued to follow distancing and hygiene rules. Wearing a facemask was mandatory only for contact between adults (staff and parents), but not for contact with and among children. In Germany, the first vaccinations against SARS-CoV-2 were available for adults from December 2020 following a prioritization scheme. From June 2021, children aged ≥12 years also had the opportunity to get vaccinated [20]. By September 2021, 68% of the German population had received at least one vaccination, whereas among children and adolescents aged 12–17 years, 59% were not yet vaccinated [21]. Vaccination against SARS-CoV-2 was not recommended for children of kindergarten age.
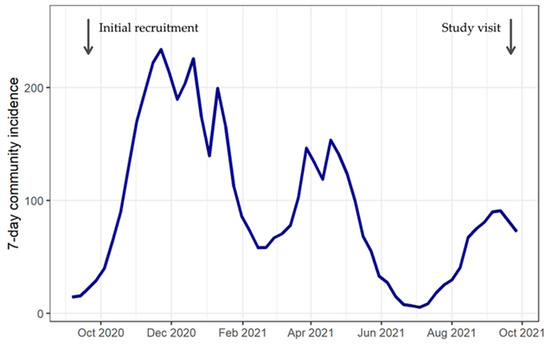
Figure 1.
Community incidence in Berlin, 2020–2021.
This study was conducted as part of the Berlin Corona School Study (BECOSS) with an initial recruitment phase in September 2020, as previously described [3]. Twelve kindergartens were randomly selected out of the more than 2700 facilities in Berlin. This selection process involved dividing the city districts into three strata according to socioeconomic status. Within each stratum, two districts were chosen, and within each district, two kindergartens were randomly selected. Per kindergarten, 20 children (referred to as “index children”) and five staff members were recruited, if possible, from one care group. Household members of recruited children and staff were also invited to participate [3]. This cohort formed the basis for participation in our data collection in September 2021. Only participants with a negative antibody result in September 2020 were eligible for our antibody analysis in September 2021.
In September 2021, study teams visited each facility to collect samples and data. Kindergarten children, staff, and household members were interviewed on present symptoms of COVID-19. Finger-prick blood samples were collected on filter paper (Bio-Sample Card, Ahlstrom Munksjö, Bärenstein, Germany). Reverse transcriptase–polymerase chain reaction (Roche COBAS SARS-CoV-2 test) was used to determine SARS-CoV-2 infection. Additionally, participants were asked to fill in an electronic questionnaire using the Research Electronic Data Capture (REDCap) tool [22] on further parameters including sex, age, sociodemographic background, contact to positive cases, protection behavior, pre-existing medical conditions, medication, SARS-CoV-2 infection, and vaccination status. Parents answered the questionnaire for kindergarten children.
Antibodies were determined using Roche Elecsys® Anti-SARS-CoV-2 test kits. For that, 3 × 3.2 mm discs were punched from dried blood spots and eluted for 1 h at 37 °C with 300 rpm shaking (MIUlab ES-60E, Hangzhou Miu Instruments, Hangzhou, China). As described elsewhere, antibodies were measured on cobas e801 modules (Roche, Mannheim, Germany) [23]. We considered only those participants for analysis who provided a blood sample of sufficient volume and had a confirmed antibody result [23].
We tested for both anti-S antibodies (detectable after infection and/or vaccination), and anti-N antibodies (detectable only after infection) [24,25], and categorized the antibody results in three groups: (i) negative, i.e., negative for anti-S and anti-N; (ii) vaccinated, i.e., only anti-S positive; and (iii) previously infected, i.e., positive for anti-N, or anti-S and anti-N. Since the vaccine was only approved for age groups 12 years and older, we assumed that children below 12 years of age showing only anti-S antibodies had previously been infected and already lost anti-N. This phenotype is plausible for mild infections where anti-N is dropping fast, or in case the anti-N reaction was delayed and had not yet taken place when the sample was taken [24,25]. For older participants with anti-S antibodies only (possibly vaccinated), we used the information from the questionnaires (SARS-CoV-2 infection and vaccination status) to identify individuals with a previous infection and lacking anti-N. These were also classified as previously infected. We categorized individuals as having undergone an undiagnosed infection if they were allocated to the serological group of “previously infected” but had not experienced a SARS-CoV-2 infection, according to their questionnaire. Participants were grouped as kindergarten children, staff, and household members and according to age for analysis. We calculated proportions for categorical variables and median (range) for continuous variables. To assess the associated factors with unrecognized SARS-CoV-2 infection and with preceding infections, we calculated crude odds ratios (OR) using cross-tabulation, and the corresponding 95% confidence intervals. Data were processed and analyzed using IBM SPSS Statistics 28.0.1.0 [26].
3. Results
3.1. Characteristics of Study Participants
Out of 580 BECOSS participants in September 2021, 546 had a seronegative antibody test result from the initial study visit one year earlier [3] and therefore fulfilled the eligibility criteria. In September 2021, 169 of those 546 individuals rejected antibody testing and thus did not provide a second blood sample. Of the 377 samples obtained, 71 had limited evaluability (mostly insufficient blood volume) and were excluded. For 29 participants, no information about infection history was provided. Hence, our final dataset included 277 participants with confirmed antibody tests and questionnaire information on infection history. Among those, 48 (17.3%) were kindergarten children, 37 (13.4%) were kindergarten staff, and 192 (69.3%) were household members (Table 1). All tested negative for SARS-CoV-2 using rt-PCR.

Table 1.
Characteristics of study participants, Berlin, September 2021.
The kindergarten children had a median age of 5 years; more than half were male. Staff were largely female with a median age of 49 years. Household members consisted of pre-school children (siblings) (n = 16; median age, 3 years; range, 2–6), school children (n = 44; median age, 9 years; range, 7–18), and adults (n = 132; median age, 40 years; range, 24–79). Further characteristics are shown in Table 1. Leading signs and symptoms stated to have occurred within the preceding 24 h were headache, rhinitis, and cough, with the latter two occurring more common in daycare children (Table 1). No signs of severe acute disease were observed at presentation.
3.2. SARS-CoV-2 Antibodies
SARS-CoV-2 antibodies were detected in 65.0% (180/277) of the participants (Table 2). This combined 52.7% of individuals categorized as vaccinated and 12.3% as previously infected. Looking at the subgroups, 81.1% of staff and 60.4% of household members were considered as vaccinated. Categorization as previously infected applied to 16.7% of kindergarten children, 16.2% of staff, and 10.4% of household members.

Table 2.
Anti-SARS-CoV-2 antibody prevalence in kindergarten children, kindergarten staff and household members, Berlin, September 2021.
Undiagnosed infections were found in 5.4% of all participants (Table 2). This figure was higher among kindergarten children (12.5%) as compared to household members (3.6%, crude OR 3.8; 95% confidence interval (CI), 1.2–11.8; Table 2). Grouping all children under 12 years of age (index kindergarten children and siblings from the household group), i.e., those ineligible for SARS-CoV-2 vaccination, the proportion of undiagnosed infections was almost five times higher (11.0%, 11/100) than in older participants (2.3%, 4/177; crude OR 5.3; 95% CI, 1.7–17.3). At the same time, among those categorized as previously infected, 44.1% (15/34) did not recall an episode of infection. This proportion was 75% (6/8) in kindergarten children, 33.3% (2/6) in staff, and 35% (7/20) in household members; or 64.7% (11/17) in children under 12 years and 23.5% (4/17) in participants aged 12 years or above (crude OR 6.0; 95% CI, 1.3–26.7).
Lastly, comparing individuals with serological and questionnaire-based evidence for preceding infection (n = 34) with those lacking such evidence (n = 243), the self-reported neglect of wearing a facemask was associated with preceding infection (crude OR, 3.7; 95%CI, 1.4–9.7; 57.9%, 11/19 vs. 27.3%, 45/165).
4. Discussion
In the present study assessing SARS-CoV-2 serostatus in a Berlin kindergarten environment in September 2021, two in three participants were SARS-CoV-2 seropositive, which was largely a result of vaccination among adults. Yet, one in six kindergarten children had non-vaccine-induced antibodies. The proportion of undiagnosed infections was highest in kindergarten children, and almost five times higher in children below 12 years of age than in persons aged 12 years or older. Facemask neglect was associated with previous SARS-CoV-2 infection.
Our identified SARS-CoV-2 seroprevalence of 16.7% in kindergarten children exceeds findings from other, mostly earlier studies in Germany. In a Bavarian screening for type 1 diabetes (Fr1da) during the second pandemic wave (September 2020–February 2021), a seroprevalence of 3.9% was observed in children aged 1–10 years [10,11]. In another Bavarian study in September 2021, seroprevalence was 11.8% in children 1–17 years [27]. Similar seroprevalences were found in children <18 years in Western Germany between June 2020 and February 2021 [12,28], or among >10,000 children admitted to hospitals across Germany in spring 2021 [29]. Regarding kindergarten staff, our identified seroprevalence of 16.2% exceeds results from France, where a multicenter study in 22 kindergartens showed a seroprevalence of 6.8% among kindergarten staff between March and May 2020 [30]. A study on transmission in daycare facilities in Germany showed that transmission was more likely to occur in households than in institutions [31]. In a meta-analysis from 2020, adults had a higher risk of infection than children in the same households [32]. This is supported by another meta-analysis from 2022, which shows that children do not dominate household transmission [33].
Seroprevalence studies provide important data on asymptomatic and unrecognized infections. This is corroborated by the fact that we found 16.7% seroprevalence in kindergarten children as compared to a 3.2% PCR-based cumulative infection frequency in children <5 years of age according to Robert Koch-Institute data in September 2021 in Germany [34], suggesting under-diagnosing in this age group. More undiagnosed infections in kindergarten children compared to older age groups could be caused by the fact that children develop fewer symptoms during SARS-CoV-2 infection [9,35,36,37,38,39,40,41]. The proportion of asymptomatic infections in children in previous research varied between 15% in China [9], 59% in Western Germany [12,28], and 47% to 68% in Bavaria [10,11]. Of note, none of our study participants tested positive for SARS-CoV-2 using rt-PCR. Fecal shedding of SARS-CoV-2, partially persistent, has been described in a substantial proportion of infected children [42] and associated with transmission [43]. However, the extent to which the detection of viral RNA in the stool of children is actually associated with transmission remains unclear [10,41]. Regardless, diarrhea is most common in SARS-CoV-2 infected pediatric outpatients at kindergarten age, which could facilitate transmission [44]. For future epidemic events, kindergarten staff need to take asymptomatic pediatric infections into account and employ counteracting measures like building fixed, small contact groups and check regularly for infections through pooled PCR testing even if no symptoms are present.
Preceding infection was associated with never wearing a facemask, supporting the known protective effect [12,45,46,47,48,49]. Other studies also reported low socioeconomic status or migration background to influence seropositivity among children [28,50]. In the present study, further associations were not observed, likely also due to its limited sample size. Kindergarten staff showed the highest vaccination rate (81.1%) in our study. A reason could be that kindergarten staff were a prioritized group for vaccination and could get vaccinated from April 2021 onwards, while most of the younger adults (e.g., parents) needed to wait until June 2021 [20].
One strength of our study is the random selection of kindergartens throughout Berlin. We generated empirical data and did not base our findings on model-based simulated estimates. Connected household members were also invited into the study. We used robust laboratory methods and performed non-symptomatic screening. However, our study also has limitations. First, voluntary participation entails a selection bias; possibly, participants who were vaccinated or who assumed an undiagnosed previous infection were more likely to participate in this serological survey. Secondly, we used dried blood spots for serology because of the less invasive finger-prick sampling [23,51,52,53]. The quality of such results can be hampered depending on blood volume [23,53,54], but to avoid that, we only included samples with sufficient volume and confirmed results. Another limitation is that antibodies can decrease over time to an undetectable level [25,38,55,56], and among vaccinated participants aged 12 years and older, previous infections could be masked by a comparatively faster decrease in anti-N antibodies than anti-S antibodies [24]. However, we triangulated serostatus with questionnaire information to reduce this effect. Nevertheless, the number of undiagnosed infections within our group could be an underestimation especially among vaccinated adolescents and adults. Finally, our findings must be seen in the light of the highly transmissible Omicron variant in late 2021, with recent German studies showing substantially increased seroprevalence in young children of up to 70% by May 2022 [27,57].
5. Conclusions
By September 2021, two-thirds of our tested cohort were SARS-CoV-2 seroreactive, largely as a result of vaccination in adults. One in six kindergarten children showed non-vaccine-induced seropositivity for SARS-CoV-2 and the highest proportion of undiagnosed infections in our kindergarten-based cohort. The high proportion of unrecognized infections in pre-school children needs to be considered when interpreting SARS-CoV-2 infections in the kindergarten context.
Author Contributions
Conceptualization, F.P.M., S.T., J.S., T.K. and M.A.M.; methodology, T.K., R.R.-A. and A.W.; formal analysis, W.v.L., R.R.-A. and A.W.; investigation, S.T., F.P.M. and W.v.L.; data curation, J.B., F.P.M., W.v.L. and S.T.; writing—original draft preparation, J.B.; writing—review and editing, J.B., S.T., W.v.L., M.A.M., J.S., T.K., R.R.-A., A.W. and F.P.M.; supervision, F.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Senate of Berlin funded the study. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Institutional Review Board Statement
The ethics committee of Charité-Universitätsmedizin Berlin (EA2/091/20, 3 June 2020) reviewed the study. All procedures were in accordance with the Declaration of Helsinki as amended in 2013.
Informed Consent Statement
Written informed consent was obtained from all participants (by legal representatives in the case of minors).
Data Availability Statement
Data are not stored publicly due to underage participants, but can be made available upon reasonable scientific request from the corresponding author.
Acknowledgments
We thank the children, kindergarten staff, and families for participation, and Charité—Universitätsmedizin Berlin and the Senate of Berlin for support.
Conflicts of Interest
Wieser is part of different studies on the subject of SARS-CoV-2 serology supported by different public and European funding partners. He has received personal compensation from Roche for consultations regarding SARS-CoV-2 serology and diagnostics. His laboratory received an e801 ELECSYS system for reduced rates for research purposes. Other reagents and support from other companies was also received, including reduced prices for Euroimmun reagents. Outside of the work, Kurth reported receiving research grants from the German Federal Joint Committee (G-BA) and the Federal Ministry of Health (BMG). He reported receiving personal compensation from Eli Lilly & Company, the BMJ, and Frontiers. The other authors declare no conflicts of interest with the specific matter of this manuscript.
References
- Gilliam, W.S.; Malik, A.A.; Shafiq, M.; Klotz, M.; Reyes, C.; Humphries, J.E.; Murray, T.; Elharake, J.A.; Wilkinson, D.; Omer, S.B. COVID-19 Transmission in US Child Care Programs. Pediatrics 2021, 147, e2020031971. [Google Scholar] [CrossRef]
- Ehrhardt, J.; Ekinci, A.; Krehl, H.; Meincke, M.; Finci, I.; Klein, J.; Geisel, B.; Wagner-Wiening, C.; Eichner, M.; Brockmann, S. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germany. Eurosurveillance 2020, 25, 2001587. [Google Scholar] [CrossRef]
- Thielecke, M.; Theuring, S.; van Loon, W.; Hommes, F.; Mall, M.A.; Rosen, A.; Böhringer, F.; von Kalle, C.; Kirchberger, V.; Kurth, T.; et al. SARS-CoV-2 infections in kindergartens and associated households at the start of the second wave in Berlin, Germany—A cross-sectional study. Eur. J. Public Health 2021, 31, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Children are unlikely to be the main drivers of the COVID-19 pandemic—A systematic review. Acta Paediatr. 2020, 109, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Bark, D.; Dhillon, N.; St-Jean, M.; Kinniburgh, B.; McKee, G.; Choi, A. SARS-CoV-2 transmission in kindergarten to grade 12 schools in the Vancouver Coastal Health region: A descriptive epidemiologic study. CMAJ Open 2021, 9, E810–E817. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Steul, K.; Walczok, A.; Gottschalk, R. Children and COVID-19-Data from mandatory reporting and results of contact person testing in daycare centers and schools in Frankfurt am Main, Germany, August–December 2020. Monatsschrift Kinderheilkd. 2021, 169, 322–334. [Google Scholar] [CrossRef]
- Götzinger, F.; Strenger, V. The Role of Children and Young People in the Transmission of SARS-CoV-2. Pediatr. Infect. Dis. J. 2022, 41, e172–e174. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared with Adults: A systematic review and meta-analysis. JAMA Pediatr. 2021, 175, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Hippich, M.; Holthaus, L.; Assfalg, R.; Zapardiel-Gonzalo, J.; Kapfelsperger, H.; Heigermoser, M.; Haupt, F.; Ewald, D.A.; Welzhofer, T.C.; Marcus, B.A.; et al. A Public Health Antibody Screening Indicates a 6-Fold Higher SARS-CoV-2 Exposure Rate than Reported Cases in Children. Med 2020, 2, 149–163.e4. [Google Scholar] [CrossRef]
- Hippich, M.; Sifft, P.; Zapardiel-Gonzalo, J.; Böhmer, M.M.; Lampasona, V.; Bonifacio, E.; Ziegler, A.-G. A public health antibody screening indicates a marked increase of SARS-CoV-2 exposure rate in children during the second wave. Med 2021, 2, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, F.; Diebner, H.H.; Matenar, C.; Schlegtendal, A.; Spiecker, J.; Eitner, L.; Timmesfeld, N.; Maier, C.; Lücke, T. Longitudinal Rise in Seroprevalence of SARS-CoV-2 Infections in Children in Western Germany—A Blind Spot in Epidemiology? Infect. Dis. Rep. 2021, 13, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ruf, S.; Hommes, F.; van Loon, W.; Seybold, J.; Kurth, T.; Mall, M.A.; Mockenhaupt, F.P.; Theuring, S. A Retrospective Outbreak Investigation of a COVID-19 Case Cluster in a Berlin Kindergarten, November 2020. Int. J. Environ. Res. Public Health 2021, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Coffin, S.E.; Rubin, D. Yes, Children Can Transmit COVID, but We Need Not Fear. JAMA Pediatr. 2021, 175, 1110–1112. [Google Scholar] [CrossRef]
- Dunay, G.A.; Barroso, M.; Woidy, M.; Danecka, M.K.; Engels, G.; Hermann, K.; Neumann, F.S.; Paul, K.; Beime, J.; Escherich, G.; et al. Long-Term Antibody Response to SARS-CoV-2 in Children. J. Clin. Immunol. 2022, 43, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kindzierski, S.; van Loon, W.; Theuring, S.; Hommes, F.; Thombansen, E.; Böttcher, M.; Matthes, H.; Rössig, H.; Weiger, D.; Wiesmann, C.; et al. SARS-CoV-2 infection among educational staff in Berlin, Germany, June to December 2020. Eurosurveillance 2022, 27, 2100524. [Google Scholar] [CrossRef] [PubMed]
- RKI. Epidemiologisches Bulletin 10_2022: Robert Koch Institute Germany; 2022. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2022/Ausgaben/10_22.pdf?__blob=publicationFile (accessed on 9 November 2022).
- Bundesregierung. Infektionsschutzgesetz—Vierte Gesetz Zum Schutz Der Bevölkerung Bei Einer Epidemischen Lage Von Nationaler Tragweite Bundesgesetzblatt Jahrgang 2021 Teil I Nr. 18, ausgegeben zu Bonn am 22. April 2021. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/B/4_BevSchG_BGBL.pdf (accessed on 16 November 2022).
- LAGeSo LfGuSB. COVID-19 in Berlin, Fallzahlen und Indikatoren—Gesamtübersicht: Landesamt für Gesundheit und Soziales Berlin LAGeSo. 2022. Available online: https://daten.berlin.de/datensaetze/covid-19-berlin-fallzahlen-und-indikatoren-gesamt%C3%BCbersicht (accessed on 16 November 2022).
- RKI. Epidemiologisches Bulletin 23_2021: Robert Koch Institute Germany. 2021. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/23_21.pdf?__blob=publicationFile (accessed on 16 November 2022).
- RKI. Wöchentlicher Lagebericht Des Rki Zur Coronavirus-Krankheit-2019 (COVID-19) 38_2021: Robert Koch Institute Germany. 2021. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2021-09-30.pdf?__blob=publicationFile (accessed on 9 November 2022).
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Beyerl, J.; Rubio-Acero, R.; Castelletti, N.; Paunovic, I.; Kroidl, I.; Khan, Z.N.; Bakuli, A.; Tautz, A.; Oft, J.; Hoelscher, M.; et al. A dried blood spot protocol for high throughput analysis of SARS-CoV-2 serology based on the Roche Elecsys anti-N assay. EBioMedicine 2021, 70, 103502. [Google Scholar] [CrossRef] [PubMed]
- Scheiblauer, H.; Nübling, C.M.; Wolf, T.; Khodamoradi, Y.; Bellinghausen, C.; Sonntagbauer, M.; Esser-Nobis, K.; Filomena, A.; Mahler, V.; Maier, T.J.; et al. Antibody response to SARS-CoV-2 for more than one year—Kinetics and persistence of detection are predominantly determined by avidity progression and test design. J. Clin. Virol. 2022, 146, 105052. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Acero, R.; Castelletti, N.; Fingerle, V.; Olbrich, L.; Bakuli, A.; Wölfel, R.; Girl, P.; Müller, K.; Jochum, S.; Strobl, M.; et al. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-characterized Oligo-/Asymptomatic Patients. Infect. Dis. Ther. 2021, 10, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics for Windows, Version 28.0; IBM Corp.: Armonk, NY, USA, 2021. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 16 November 2022).
- Ott, R.; Achenbach, P.; Ewald, D.A.; Friedl, N.; Gemulla, G.; Hubmann, M.; Kordonouri, O.; Loff, A.; Marquardt, E.; Sifft, P.; et al. SARS-CoV-2 Seroprevalence in Preschool and School-Age Children. Dtsch. Aerzteblatt Online 2022, 119, 765. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, F.; Diebner, H.H.; Matenar, C.; Schlegtendal, A.; Eitner, L.; Timmesfeld, N.; Maier, C.; Lücke, T. Seroconversion rate and socio-economic and ethnic risk factors for SARS-CoV-2 infection in children in a population-based cohort, Germany, June 2020 to February 2021. Eurosurveillance 2022, 27, 2101028. [Google Scholar] [CrossRef] [PubMed]
- Sorg, A.-L.; Bergfeld, L.; Jank, M.; Corman, V.; Semmler, I.; Goertz, A.; Beyerlein, A.; Verjans, E.; Wagner, N.; Von Bernuth, H.; et al. Cross-sectional seroprevalence surveys of SARS-CoV-2 antibodies in children in Germany, June 2020 to May 2021. Nat. Commun. 2022, 13, 3128. [Google Scholar] [CrossRef] [PubMed]
- Lachassinne, E.; de Pontual, L.; Caseris, M.; Lorrot, M.; Guilluy, C.; Naud, A.; Dommergues, M.-A.; Pinquier, D.; Wannepain, E.; Hausherr, E.; et al. SARS-CoV-2 transmission among children and staff in daycare centres during a nationwide lockdown in France: A cross-sectional, multicentre, seroprevalence study. Lancet Child Adolesc. Health 2021, 5, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Loss, J.; Wurm, J.; Varnaccia, G.; Schienkiewitz, A.; Iwanowski, H.; Loer, A.-K.M.; Allen, J.; Wess, B.; Rosario, A.S.; Damerow, S.; et al. Transmission of SARS-CoV-2 among children and staff in German daycare centres. Epidemiol. Infect. 2022, 150, e141. [Google Scholar] [CrossRef]
- Koh, W.C.; Naing, L.; Chaw, L.; Rosledzana, M.A.; Alikhan, M.F.; Jamaludin, S.A.; Amin, F.; Omar, A.; Shazli, A.; Griffith, M.; et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLoS ONE 2020, 15, e0240205. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tian, Y.; Zhang, L.; Shi, Y. The role of children in household transmission of COVID-19: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 266–275. [Google Scholar] [CrossRef] [PubMed]
- RKI. SurvStat@RKI Abfrage Der Meldedaten Nach Infektionsschutzgesetz (IfSG) Über Das Web: Robert Koch Institute Germany. 2022. Available online: https://www.rki.de/DE/Content/Infekt/SurvStat/survstat_node.html (accessed on 13 November 2022).
- Martins, M.M.; Prata-Barbosa, A.; da Cunha, A.J.L.A. Update on SARS-CoV-2 infection in children. Paediatr. Int. Child Health 2021, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Calò Carducci, F.I.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Thoon, K.C.; Chong, C.Y.; Maiwald, M.; Kam, K.Q.; Nadua, K.; Tan, N.W.; Yung, C.F. Comparative Analysis of Symptomatic and Asymptomatic SARS-CoV-2 Infection in Children. Ann. Acad. Med. Singap. 2020, 49, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.F.; Saffari, S.E.; Mah, S.Y.Y.; Tan, N.W.H.; Chia, W.-N.; Thoon, K.C.; Wang, L.-F. Analysis of Neutralizing Antibody Levels in Children and Adolescents Up to 16 Months After SARS-CoV-2 Infection. JAMA Pediatr. 2022, 176, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Hetrich, M.K.; Bin Na, Y.; Knoll, M.D.; Schappell, E.; Meece, J.; Hanson, E.; Tong, S.; Lee, J.S.; Veguilla, V.; et al. Assessment of Clinical and Virological Characteristics of SARS-CoV-2 Infection Among Children Aged 0 to 4 Years and Their Household Members. JAMA Netw. Open 2022, 5, e2227348. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Englund, J.A. How Do We Stop the Spread of SARS-CoV-2 in Young Children? JAMA Netw. Open 2022, 5, e2227357. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.C.; Howard-Jones, A.R.; Hsu, P.; Palasanthiran, P.; Gray, P.E.; McMullan, B.J.; Britton, P.N.; Bartlett, A.W. SARS-CoV-2 in children: Spectrum of disease, transmission and immunopathological underpinnings. Pathology 2020, 52, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Benvari, S.; Mahmoudi, S.; Mohammadi, M. Gastrointestinal viral shedding in children with SARS-CoV-2: A systematic review and meta-analysis. World J. Pediatr. 2022, 18, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Donà, D.M.; Minotti, C.; Costenaro, P.; Da Dalt, L.; Giaquinto, C. Fecal-Oral Transmission of SARS-CoV-2 In Children: Is it Time to Change Our Approach? Pediatr. Infect. Dis. J. 2020, 39, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Mahon, A.; Moss, A.; Rao, S. SARS-CoV-2 infection in children evaluated in an ambulatory setting during Delta and Omicron time periods. J. Med. Virol. 2023, 95, e28318. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Butler, J.C. Effectiveness of Mask Wearing to Control Community Spread of SARS-CoV-2. JAMA 2021, 325, 998–999. [Google Scholar] [CrossRef]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, M.; Gao, L.; Ahmed, M.A.; Uy, J.P.; Cheng, C.; Zhou, Q.; Sun, C. Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis. Am. J. Infect. Control. 2020, 49, 900–906. [Google Scholar] [CrossRef]
- Rader, B.; White, L.F.; Burns, M.R.; Chen, J.; Brilliant, J.; Cohen, J.; Shaman, J.; Brilliant, L.; Kraemer, M.U.G.; Hawkins, J.B.; et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: A cross-sectional study. Lancet Digit. Health 2021, 3, e148–e157. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637-20. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Reyes, M.M.; Daza, M.; Pacheco, A.; Meneses-Gil, M.X.; Galindo, M.; Catama, J.; Botero, L.S.; Muñoz, L.; Quinche, G.; Ospina, M.L. Seroprevalence of SARS-CoV-2 Antibodies in Children and Adolescents: Results from a Population-Based Survey in 10 Colombian Cities. Glob. Pediatr. Health 2022, 9, 2333794x221085385. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Kannan, K.; Cui, Y.; Merrill, L.; Petrick, L.M.; Meeker, J.D.; Fennell, T.R.; Faustman, E.M. The use of dried blood spots for characterizing children’s exposure to organic environmental chemicals. Environ. Res. 2021, 195, 110796. [Google Scholar] [CrossRef] [PubMed]
- Ostler, M.W.; Porter, J.H.; Buxton, O.M. Dried Blood Spot Collection of Health Biomarkers to Maximize Participation in Population Studies. J. Vis. Exp. 2014, e50973. [Google Scholar] [CrossRef]
- Prosperi, C.; Kaduskar, O.; Bhatt, V.; Hasan, A.Z.; Thangaraj, J.W.V.; Kumar, M.S.; Sabarinathan, R.; Kumar, S.; Duraiswamy, A.; Deshpande, G.R.; et al. Diagnostic Accuracy of Dried Blood Spots Collected on HemaSpot HF Devices Compared to Venous Blood Specimens to Estimate Measles and Rubella Seroprevalence. mSphere 2021, 6, e0133020. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Brown, B.; Brooks, T.; Semper, A.; Machin, N.; Linley, E.; Borrow, R.; Wyllie, D.; Taylor-Philips, S.; Jones, H.; et al. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys® high throughput immunoassay. J. Clin. Virol. 2021, 136, 104739. [Google Scholar] [CrossRef]
- Méndez-Echevarría, A.; Sainz, T.; Falces-Romero, I.; de Felipe, B.; Escolano, L.; Alcolea, S.; Pertiñez, L.; Neth, O.; Calvo, C. Long-Term Persistence of Anti-SARS-CoV-2 Antibodies in a Pediatric Population. Pathogens 2021, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Oygar, P.D.; Ozsurekci, Y.; Gurlevik, S.L.; Aykac, K.; Kukul, M.G.; Yayla, B.C.C.; Ilbay, S.; Karakaya, J.; Teksam, O.; Cengiz, A.B.; et al. Longitudinal Follow-up of Antibody Responses in Pediatric Patients with COVID-19 up to 9 Months After Infection. Pediatr. Infect. Dis. J. 2021, 40, e294–e299. [Google Scholar] [CrossRef] [PubMed]
- Engels, G.; Hecker, K.; Forster, J.; Toepfner, N.; Hick, E.; Pietsch, F.; Heuschmann, P.; Berner, R.; Härtel, C.; Kurzai, O.; et al. High seroprevalence of SARS-CoV-2 in preschool children in July 2022—A cross-sectional data collection in day-care centers. Dtsch. Arztebl. Int. 2022, 119, 771–772. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).