Late Preterm Newborns: Breastfeeding and Complementary Feeding Practices
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
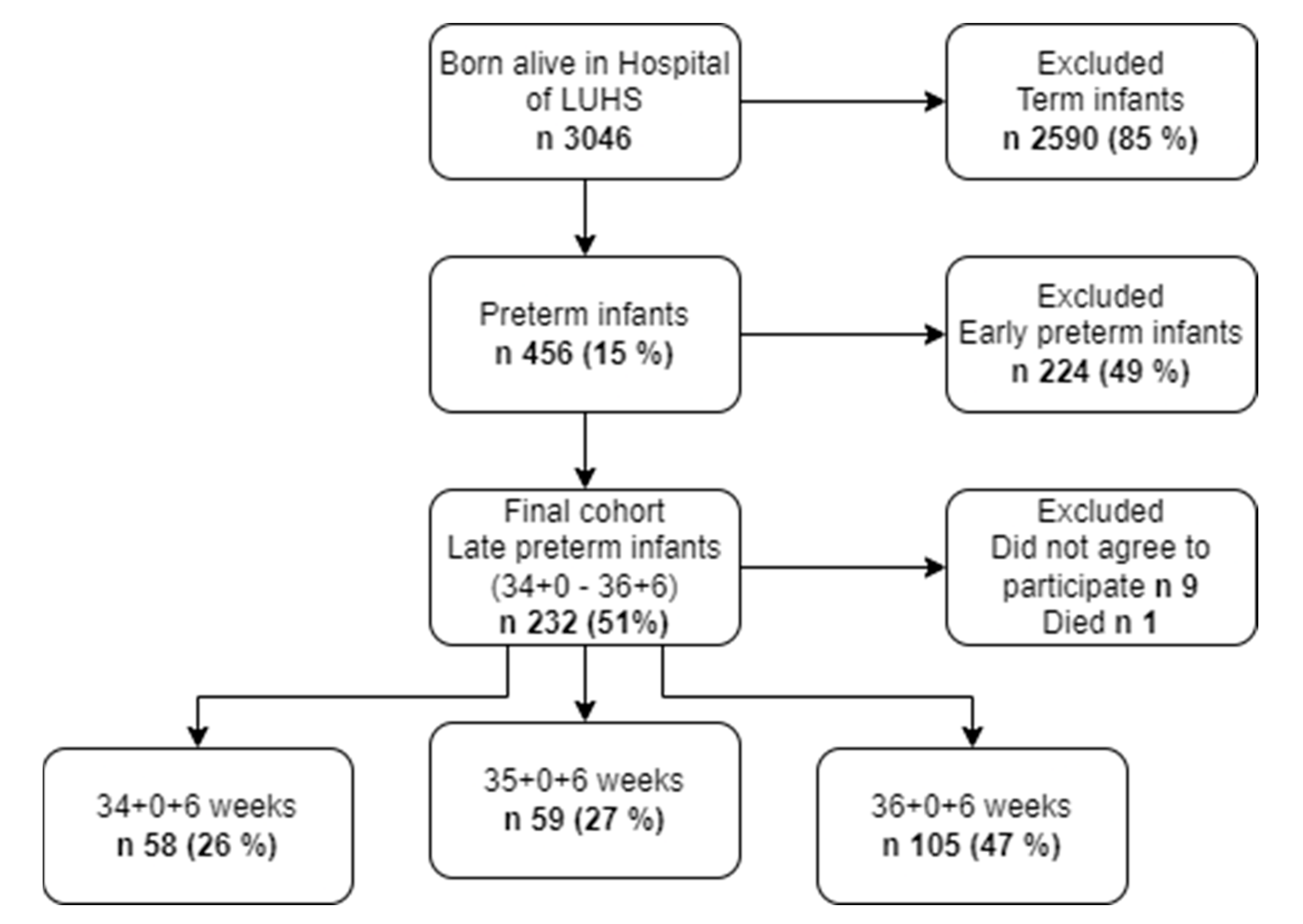
2.2. Sample
2.3. Data Collection
- Exclusively breastfed (including expressed mother’s milk).
- Partial breastfeeding included infants that were fed both with breast milk and formula.
- Exclusively formula-fed infants.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Protecting, Promoting and Supporting Breastfeeding: The Baby-Friendly Hospital Initiative for Small, Sick and Preterm Newborns (2020) Who.int. Available online: https://www.who.int/publications/i/item/9789240005648 (accessed on 20 February 2024).
- ESPGHAN Committee on Nutrition; Lapillonne, A.; Bronsky, J.; Campoy, C.; Embleton, N.; Fewtrell, M.; Mis, N.F.; Gerasimidis, K.; Hojsak, I.; Hulst, J.; et al. Feeding the late and moderately preterm infant: A position paper of the European Society for Paediatric Gastroenterology, Hepatology and nutrition Committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Huff, K.; Rose, R.S.; Engle, W.A. Late preterm infants. Pediatr. Clin. North Am. 2019, 66, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, N.K. The nuts and bolts of breastfeeding: Anatomy and physiology of lactation. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.P.; Psaila, K.; Patterson, T. Non-nutritive sucking for increasing physiologic stability and nutrition in preterm infants. Cochrane Libr. 2016, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Bloomfield, F.H.; Harding, J.E. Nutrition in late preterm infants. Semin. Perinatol. 2019, 43, 151160. [Google Scholar] [CrossRef] [PubMed]
- Baby-Friendly Hospital Initiative (No Date) Unicef. Org. Available online: https://www.unicef.org/documents/baby-friendly-hospital-initiative (accessed on 20 February 2024).
- Kitano, N.; Nomura, K.; Kido, M.; Murakami, K.; Ohkubo, T.; Ueno, M.; Sugimoto, M. Combined effects of maternal age and parity on successful initiation of exclusive breastfeeding. Prev. Med. Rep. 2016, 3, 121–126. [Google Scholar] [CrossRef]
- Singh, J.; Scime, N.V.; Chaput, K.H. Association of Caesarean delivery and breastfeeding difficulties during the delivery hospitalization: A community-based cohort of women and full-term infants in Alberta, Canada. Canadian journal of public health. Rev. Can. De Sante Publique 2023, 114, 104–112. [Google Scholar] [CrossRef]
- Li, L.; Wan, W.; Zhu, C. Breastfeeding after a cesarean section: A literature review. Midwifery 2021, 103, 103117. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.R.; Anderson, G.C. Randomized controlled trial of very early mother-infant skin-to-skin contact and breastfeeding status. J. Midwifery Women’s Health 2007, 52, 116–125. [Google Scholar] [CrossRef]
- Handayani, K.D.; Irwanto Masturina, M.; Etika, R.; Harianto, A.; Sauer, P.J. Duration of breastfeeding in late preterm infants: Maternal and infant factors. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2021, 37, 795–802. [Google Scholar] [CrossRef]
- WHO Immediate KMC Study Group. Impact of continuous Kangaroo Mother Care initiated immediately after birth (iKMC) on survival of newborns with birth weight between 1.0 to <1.8 kg: Study protocol for a randomized controlled trial. Trials 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Juharji, H.; Albalawi, K.; Aldwaighri, M.; Almalki, A.; Alshiti, H.; Kattan, W.; Alqarni, M.; Alsulaimani, S.; AlShaikh, T.; Alsulaimani, F.; et al. Impact of breastfeeding on low birthweight infants, weight disorders in infants, and child development. Cureus 2022, 14, e32894. [Google Scholar] [CrossRef] [PubMed]
- Lrv.lt. Available online: https://sam.lrv.lt/uploads/sam/documents/files/Veiklos_sritys/visuomenes-sveikatos-prieziura/mityba-ir-fizinis-aktyvumas/LDD%20POZICIJA%20D%C4%96L%20K%C5%AADIKI%C5%B2%20MAITINIMO%202020%2011%2030.pdf (accessed on 21 February 2024).
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the European society for paediatric gastroenterology, hepatology, and nutrition (ESPGHAN) committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Yilmaz, G.; Caylan, N.; Turgut, M.; Gokcay, G.; Oguz, M.M. Baby-led complementary feeding: Randomized controlled study. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2018, 60, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Bezze, E.; Colombo, L.; Rossetti, C.; Pesenti, N.; Roggero, P.; Sannino, P.; Muscolo, S.; Plevani, L.; Mosca, F. Complementary feeding practices in a cohort of Italian late preterm infants. Nutrients 2018, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.V. Infant and young child feeding. Port. J. Pediatr. 2020, 40, 194–195. [Google Scholar] [CrossRef]
- Vizzari, G.; Morniroli, D.; Tiraferri, V.; Macchi, M.; Gangi, S.; Consales, A.; Ceroni, F.; Cerasani, J.; Mosca, F.; Giannì, M.L. Postnatal growth of small for gestational age late preterm infants: Determinants of catch-up growth. Pediatr. Res. 2023, 94, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Libr. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Development of visceral and subcutaneous-abdominal adipose tissue in breastfed infants during first year of lactation. Nutrients 2021, 13, 3294. [Google Scholar] [CrossRef]
- Galipeau, R.; Dumas, L.; Lepage, M. Perception of not having enough milk and actual milk production of first-time breastfeeding mothers: Is there a difference? Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2017, 12, 210–217. [Google Scholar] [CrossRef]
- Evans, L.; Hilditch, C.; Keir, A. Are there interventions that improve breastfeeding and the use of breast milk in late preterm infants? J. Paediatr. Child Health 2019, 55, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Dib, S.; Kittisakmontri, K.; Wells, J.C.; Fewtrell, M. Interventions to improve breastfeeding outcomes in late preterm and early term infants. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2022, 17, 781–792. [Google Scholar] [CrossRef]
- Estalella, I.; San Millán, J.; Trincado, M.J.; Maquibar, A.; Martínez-Indart, L.; San Sebastián, M. Evaluation of an intervention supporting breastfeeding among late-preterm infants during in-hospital stay. Women Birth J. Aust. Coll. Midwives 2020, 33, e33–e38. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 34+0+6 | 35+0+6 | 36+0+6 | Total |
|---|---|---|---|---|
| Mothers | Av. (SD) or % (n) | |||
| Mother’s age | 31.1 years (SD 5.1) | 31 years (SD 5.9) | 31.6 years (SD 5.1) | 31.1 years (SD 5.4) |
| Parity | ||||
| Primiparous | 37.9% (n = 16) | 45.7% (n = 21) | 51.7% (n = 45) | 43.6% (n = 82) |
| Multiparous | 62.1% (n = 34) | 54.3% (n = 30) | 48.3% (n = 42) | 56.4% (n = 106) |
| Type of delivery | ||||
| Vaginal | 56% (n = 28) | 56.9% (n = 29) | 66.7% (n = 58) | 61.2% (n = 115) |
| Caesarean | 44% (n = 22) | 43.1% (n = 22) | 33.3% (n = 29) | 38.8% (n = 73) |
| Infants | Av. (SD) or % (n) | |||
| Singleton | 72.4% (n = 42) | 67.8% (n = 40) | 67.6% (n = 71) | 68.9% (n = 153) |
| Twins/triplets | 27.6% (n = 16) | 32.2% (n = 19) | 32.4% (n = 34) | 31.1% (n = 69) |
| Birth weight | 2331 g (SD 438.8) | 2400 g (SD 375.9) | 2735 g (SD 461) | 2545 g (SD 476.4) |
| Hypoxia at birth | 6.9% (n = 4) | 10.2% (n = 6) | 2.9% (n = 3) | 5.9% (n = 13) |
| Born without hypoxia | 93.1% (n = 54) | 89.9% (n = 53) | 97.1% (n = 102) | 94.1% (n = 209) |
| Males | 62.1% (n = 36) | 45.8% (n = 27) | 49.5% (n = 52) | 61.2% (n = 115) |
| Respiratory support need | 51.7% (n = 30) | 35.6% (n = 21) | 18.1% (n = 19) | 31.5% (n = 70) |
| Type of Feeding | 34+0+6 | 35+0+6 | 36+0+6 | Total |
|---|---|---|---|---|
| % (n) | ||||
| 1 month of age | ||||
| Exclusive breastfeeding | 52.5% (n = 32) | 48.2% (n = 27) | 58.3% (n = 60) | 53.6% (n = 119) |
| Partial breastfeeding | 30.5% (n = 17) | 37.5% (n = 24) | 25.2% (n = 28) | 31.1% (n = 69) |
| Formula feeding | 17% (n = 10) | 14.3% (n = 8) | 16.6 (n = 16) | 15.3% (n = 34) |
| 3 months of age | ||||
| Exclusive breastfeeding | 37.3% (n = 22) | 51.8% (n = 29) | 44.7% (n = 46) | 43.7% (n = 97) |
| Partial breastfeeding | 25.4% (n = 14) | 25% (n = 18) | 20.4% (n = 23) | 24.8% (n = 55) |
| Formula feeding | 37.3% (n = 22) | 23.2% (n = 13) | 34.9% (n = 35) | 31.5% (n = 70) |
| 6 months of age | ||||
| Exclusive breastfeeding | 30.5% (n = 18) | 38.2% (n = 21) | 35% (n = 36) | 33.8% (n = 75) |
| Partial breastfeeding | 18.7% (n = 10) | 18.2% (n = 14) | 15.5% (n = 18) | 18.9% (n = 42) |
| Formula feeding | 50.8% (n = 30) | 43.6% (n = 24) | 49.5% (n = 51) | 47.3% (n = 105) |
| 12 months of age | ||||
| Exclusive breastfeeding | 25.9% (n = 15) | 34% (n = 18) | 28.2% (n = 29) | 27.9% (n = 62) |
| Partial breastfeeding | 1.7% (n = 1) | 1.9% (n = 1) | 4.8% (n = 7) | 4.1% (n = 9) |
| Formula feeding | 72.4% (n = 42) | 64.2% (n = 40) | 67% (n = 69) | 68.0% (n = 151) |
| 34+0+6 | 35+0+6 | 36+0+6 | |
|---|---|---|---|
| Mother’s age | |||
| Av. (SD) | 31.08 years (SD 5.13) | 30.96 years (SD 5.89) | 31.57 years (SD 5.06) |
| Correlation from breastfeeding initiation | No correlation (p = 0.22) | No correlation (p = 0.948) | No correlation (p = 0.48) |
| Correlation from breastfeeding duration | LPIs born to mothers aged 30–39 years were breastfed more than 3 months, p = 0.010 | No correlation (p = 0.51) | No correlation (p = 0.642) |
| Number of live births | |||
| Correlation from breastfeeding initiation | No correlation (p = 0.30) | No correlation (p = 0.55) | No correlation (p = 0.69) |
| Correlation from breastfeeding duration | No correlation (p > 0.05) | No correlation (p > 0.05) | Primiparous breastfed at approximately more than 6 months, p = 0.006 |
| Type of delivery | |||
| Correlation from breastfeeding initiation | Earlier breastfeeding occurred among vaginal delivered, p = 0.04 | No correlation (p = 0.169) | No correlation (p = 0.186) |
| Correlation from breastfeeding duration | Vaginally born LPIs breastfed > 3 months, p < 0.001 | Vaginally born LPIs breastfed > 3 months, p < 0.001 | Vaginally born LPIs breastfed > 3 months, p < 0.001 |
| Breastfeeding duration of older siblings | |||
| Correlation from breastfeeding duration | No correlation (p = 0.30) | No correlation (p = 0.130) | Longer prior breastfeeding linked to significantly prolonged overall breastfeeding, p = 0.000 |
| Prior reasons of weaning | |||
| Correlation from breastfeeding initiation | No correlation (p > 0.005) | No correlation (p > 0.005) | No correlation (p > 0.005) |
| Correlation from breastfeeding duration | No correlation (p > 0.005) | No correlation (p > 0.005) | No correlation (p > 0.005) |
| 34+0+6 | 35+0+6 | 36+0+6 | |
|---|---|---|---|
| Birth weight | |||
| Av. (SD) | 2331 g (SD 438.77) | 2400 g (SD 375.95) | 2735 g (SD 461) |
| Correlation from breastfeeding initiation | Breastfeeding initiation was earlier in newborns weighing > 2500 g, p = 0.032 | No correlation (p = 0.088) | Breastfeeding initiation was earlier in newborns weighing > 2500 g, p = 0.000 |
| Correlation from breastfeeding duration | No correlation (p = 0.604) | No correlation (p = 0.643) | No correlation (p = 0.698) |
| APGAR score | |||
| Correlation from breastfeeding initiation | No correlation (p = 0.296) | No correlation (p = 0.078) | No correlation (p = 0.355) |
| Correlation from breastfeeding duration | No correlation (p = 0.76) | No correlation (p = 0.894) | No correlation (p = 0.280) |
| Skin-to-skin contact after birth | |||
| Correlation from breastfeeding initiation | Skin-to-skin contact longer than 30 min caused earlier breastfeeding initiation, p = 0.000 | Skin-to-skin contact longer than 30 min caused earlier breastfeeding initiation, p = 0.048 | Skin-to-skin contact longer than 30 min caused earlier breastfeeding initiation, p = 0.01 |
| Correlation from breastfeeding duration | No correlation (p = 0.476) | No correlation (p = 0.398) | No correlation (p = 0.108) |
| Breastfeeding within 2 h after birth | |||
| Correlation from breastfeeding initiation | Was not performed | LPIs breastfed within 2 h after birth had successful continuation, p = 0.038 | LPIs breastfed within 2 h after birth had successful continuation, p = 0.000 |
| Correlation from breastfeeding duration | Was not performed | No correlation (p = 0.063) | No correlation (p = 0.066) |
| Respiratory support need | |||
| Correlation from breastfeeding initiation | No correlation (p = 0.130) | No correlation (p = 0.096) | LPIs with respiratory distress had delayed breastfeeding, p = 0.003 |
| Correlation from breastfeeding duration | No correlation (p = 0.30) | No correlation (p = 0.130) | No correlation (p = 0.280) |
| Rooming-in after birth | |||
| Av. (SD) | 3.96 days (SD 2.83) | 2.39 days (SD 2.46) | 1.48 days (SD 1.58) |
| Correlation from breastfeeding initiation | Earlier rooming-in promoted earlier breastfeeding initiation in LPIs, p > 0.001 | Earlier rooming-in promoted earlier breastfeeding initiation in LPIs, p > 0.001 | Earlier rooming-in promoted earlier breastfeeding initiation in LPIs, p > 0.001 |
| Correlation from breastfeeding duration | LPIs with early rooming-in breastfed for a longer duration, p > 0.001 | LPIs with early rooming-in breastfed for a longer duration, p > 0.001 | LPIs with early rooming-in breastfed for a longer duration, p > 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijokienė, I.; Žemaitienė, R.; Stonienė, D. Late Preterm Newborns: Breastfeeding and Complementary Feeding Practices. Children 2024, 11, 401. https://doi.org/10.3390/children11040401
Dijokienė I, Žemaitienė R, Stonienė D. Late Preterm Newborns: Breastfeeding and Complementary Feeding Practices. Children. 2024; 11(4):401. https://doi.org/10.3390/children11040401
Chicago/Turabian StyleDijokienė, Ieva, Raminta Žemaitienė, and Dalia Stonienė. 2024. "Late Preterm Newborns: Breastfeeding and Complementary Feeding Practices" Children 11, no. 4: 401. https://doi.org/10.3390/children11040401
APA StyleDijokienė, I., Žemaitienė, R., & Stonienė, D. (2024). Late Preterm Newborns: Breastfeeding and Complementary Feeding Practices. Children, 11(4), 401. https://doi.org/10.3390/children11040401






