Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases
Abstract
1. Introduction
2. Methods
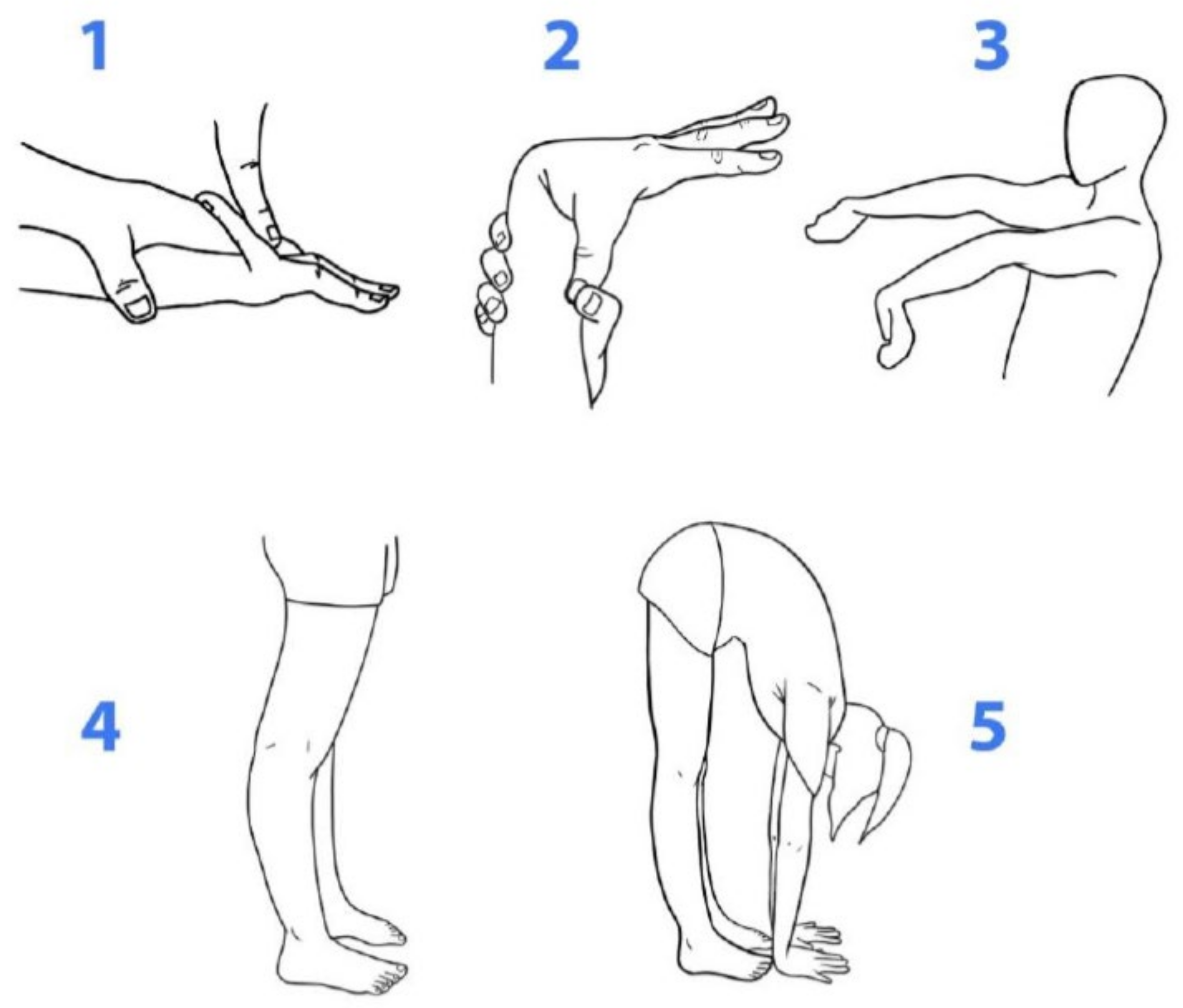
2.1. Data-Collection Instrument
2.2. Analysis of GJH Based on BS
2.3. Statistical Analysis
3. Results
3.1. Characteristics of JH Considering Beighton Variables and Distribution by Sex
3.2. Characteristics of GJH Based on BS and Distribution by Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 International Classification of the Ehlers-Danlos Syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Castori, M. Deconstructing and reconstructing joint hypermobility on an evo-devo perspective. Rheumatology 2021, 60, 2537–2544. [Google Scholar] [CrossRef]
- Guinchat, V.; Baeza-Velasco, C.; Bulbena, A.; Castori, M. Neurodevelopmental, neuropsychiatric and psychosocial correlates of joint hypermobility and related disorders. Front. Psychiatry 2022, 13, 1109515. [Google Scholar] [CrossRef]
- Castori, M.; Tinkle, B.; Levy, H.; Grahame, R.; Malfait, F.; Hakim, A. A framework for the classification of joint hypermobility and related conditions. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Grahame, R. Joint hypermobility: Emerging disease or illness behaviour? Clin. Med. 2013, 13 (Suppl. S6), s50–s52. [Google Scholar] [CrossRef]
- Castori, M.; Colombi, M. Generalized joint hypermobility, joint hypermobility syndrome and Ehlers-Danlos syndrome, hypermobility type. Am. J. Med. Genet. C Semin. Med. Genet. 2015, 169, 1–5. [Google Scholar] [CrossRef]
- Malfait, F.; Hakim, A.J.; De Paepe, A.; Grahame, R. The genetic basis of the joint hypermobility syndromes. Rheumatology 2006, 45, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.J.; Sahota, A. Joint hypermobility and skin elasticity: The hereditary disorders of connective tissue. Clin. Dermatol. 2006, 24, 521–533. [Google Scholar] [CrossRef]
- Syx, D.; De Wandele, I.; Rombaut, L.; Malfait, F. Hypermobility, the Ehlers-Danlos syndromes and chronic pain. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S107), 116–122. [Google Scholar] [PubMed]
- Lamari, N.M.; Chueire, A.G.; Cordeiro, J.A. Analysis of joint mobility patterns among preschool children. Sao Paulo Med. J. 2005, 123, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Remvig, L.; Jensen, D.V.; Ward, R.C. Are diagnostic criteria for general joint hypermobility and benign joint hypermobility syndrome based on reproducible and valid tests? A review of the literature. J. Rheumatol. 2007, 34, 798–803. [Google Scholar] [PubMed]
- Rikken-Bultman, D.G.; Wellink, L.; van Dongen, P.W. Hypermobility in two Dutch school populations. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 73, 189–192. [Google Scholar] [CrossRef]
- Lamari, N.M.; Lamari, M.M. Characterization of brazilian children with joint hypermobility. Int. J. Physiatry 2016, 2, 11. [Google Scholar] [CrossRef]
- Demmler, J.C.; Atkinson, M.D.; Reinhold, E.J.; Choy, E.; Lyons, R.A.; Brophy, S.T. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: A national electronic cohort study and case-control comparison. BMJ Open 2019, 9, e031365. [Google Scholar] [CrossRef] [PubMed]
- Lamari, M.M.; Lamari, N.M.; Araujo-Filho, G.M.; Medeiros, M.P.; Pugliesi Marques, V.R.; Pavarino, É.C. Psychosocial and Motor Characteristics of Patients With Hypermobility. Front. Psychiatry 2022, 12, 787822. [Google Scholar] [CrossRef]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef]
- Singh, H.; McKay, M.; Baldwin, J.; Nicholson, L.; Chan, C.; Burns, J.; Hiller, C.E. Beighton scores and cut-offs across the lifespan: Cross-sectional study of an Australian population. Rheumatology 2017, 56, 1857–1864. [Google Scholar] [CrossRef]
- Tofts, L.J.; Elliott, E.J.; Munns, C.; Pacey, V.; Sillence, D.O. The differential diagnosis of children with joint hypermobility: A review of the literature. Pediatr. Rheumatol. Online J. 2009, 7, 1. [Google Scholar] [CrossRef]
- Nicholson, L.L.; Simmonds, J.; Pacey, V.; De Wandele, I.; Rombaut, L.; Williams, C.M.; Chan, C. International Perspectives on Joint Hypermobility: A Synthesis of Current Science to Guide Clinical and Research Directions. J. Clin. Rheumatol. 2022, 28, 314–320. [Google Scholar] [CrossRef]
- Tofts, L.J.; Simmonds, J.; Schwartz, S.B.; Richheimer, R.M.; O’Connor, C.; Elias, E.; Engelbert, R.; Cleary, K.; Tinkle, B.T.; Kline, A.D.; et al. Pediatric joint hypermobility: A diagnostic framework and narrative review. Orphanet J. Rare Dis. 2023, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Lamari, N.; Beighton, P. Hypermobility in Medical Practice; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Jekel, J.F.; Elmore, J.G.; Katz, D.L. Epidemiologia, Bioestatística e Medicina Preventiva; Artmed: Porto Alegre, Brazil, 2002. [Google Scholar]
- Minitab, Inc. Minitab Statistical Software™; Release 16.2.2; Copyright©2010; Minitab, Inc.: State College, PA, USA, 2010. [Google Scholar]
- Walter, S.M.; Dai, Z.; Wang, K. Comorbidities of Rural Children and Adolescents with Migraine and without Migraine. Children 2023, 10, 1133. [Google Scholar] [CrossRef]
- Juul-Kristensen, B.; Schmedling, K.; Rombaut, L.; Lund, H.; Engelbert, R.H. Measurement properties of clinical assessment methods for classifying generalized joint hypermobility—A systematic review. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 116–147. [Google Scholar] [CrossRef] [PubMed]
- Lamari, N.M. Mobilidade Articular em Pré-Escolares: Um Estudo Exploratório. Master’s Thesis, Medical School of São José do Rio Preto, São José do Rio Preto, Brazil, 1994. [Google Scholar]
- Yazgan, P.; Geyikli, I.; Zeyrek, D.; Baktiroglu, L.; Kurcer, M.A. Is joint hypermobility important in prepubertal children? Rheumatol. Int. 2008, 28, 445–451. [Google Scholar] [CrossRef]
- Wolf, J.M.; Cameron, K.L.; Owens, B.D. Impact of joint laxity and hypermobility on the musculoskeletal system. J. Am. Acad. Orthop. Surg. 2011, 19, 463–471. [Google Scholar] [CrossRef]
- Birrell, F.N.; Adebajo, A.O.; Hazleman, B.L.; Silman, A.J. High prevalence of joint laxity in West Africans. Br. J. Rheumatol. 1994, 33, 56–59. [Google Scholar] [CrossRef]
- Clinch, J.; Deere, K.; Sayers, A.; Palmer, S.; Riddoch, C.; Tobias, J.H.; Clark, E.M. Epidemiology of generalized joint laxity (hypermobility) in fourteen-year-old children from the UK: A population-based evaluation. Arthritis. Rheum. 2011, 63, 2819–2827. [Google Scholar] [CrossRef]
- Piedimonte, C.; Penge, R.; Morlino, S.; Sperduti, I.; Terzani, A.; Giannini, M.T.; Colombi, M.; Grammatico, P.; Cardona, F.; Castori, M. Exploring relationships between joint hypermobility and neurodevelopment in children (4–13 years) with hereditary connective tissue disorders and developmental coordination disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 546–556. [Google Scholar] [CrossRef]
- Gensemer, C.; Burks, R.; Kautz, S.; Judge, D.P.; Lavallee, M.; Norris, R.A. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev. Dyn. 2021, 250, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Camerota, F.; Celletti, C.; Grammatico, P.; Padua, L. Ehlers-Danlos syndrome hypermobility type and the excess of affected females: Possible mechanisms and perspectives. Am. J. Med. Genet. A 2010, 152A, 2406–2408. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Camerota, F.; Celletti, C.; Danese, C.; Santilli, V.; Saraceni, V.M.; Grammatico, P. Natural history and manifestations of the hypermobility type Ehlers-Danlos syndrome: A pilot study on 21 patients. Am. J. Med. Genet. A 2010, 152A, 556–564. [Google Scholar] [CrossRef] [PubMed]
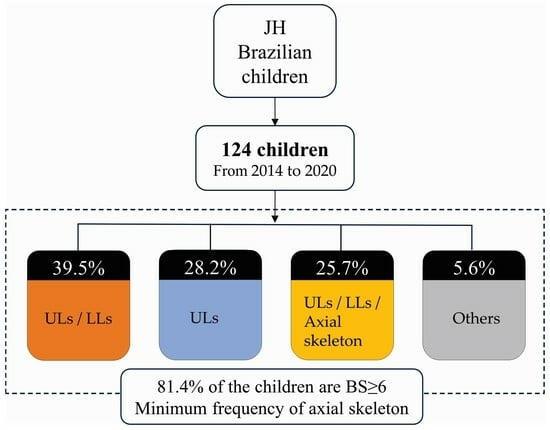
| Combination | Frequency (%) | Location |
|---|---|---|
| A, F, E, K | 33 (26.6%) | ULs/LLs |
| A, E, K | 8 (6.5%) | Uls/LLs |
| A, F, K | 6 (4.8%) | Uls/LLs |
| E, K | 2 (1.6%) | Uls/LLs |
| A, F, E | 21 (16.9%) | Uls |
| A, F | 10 (8.1%) | Uls |
| A, E | 4 (3.2%) | Uls |
| A, F, E, K, ATF | 20 (16.1%) | Uls/LLs/Axial skeleton |
| A, F, E, ATF | 7 (5.6%) | Uls/Axial skeleton |
| A, F, K, ATF | 5 (4.0%) | Uls/LLs/Axial skeleton |
| Others | 7 (5.6%) | - |
| Total | 124 (100%) |
| Beighton Variable | Hypermobility Present | p-Value (Fisher’s Exact Test) | |
|---|---|---|---|
| Female | Male | ||
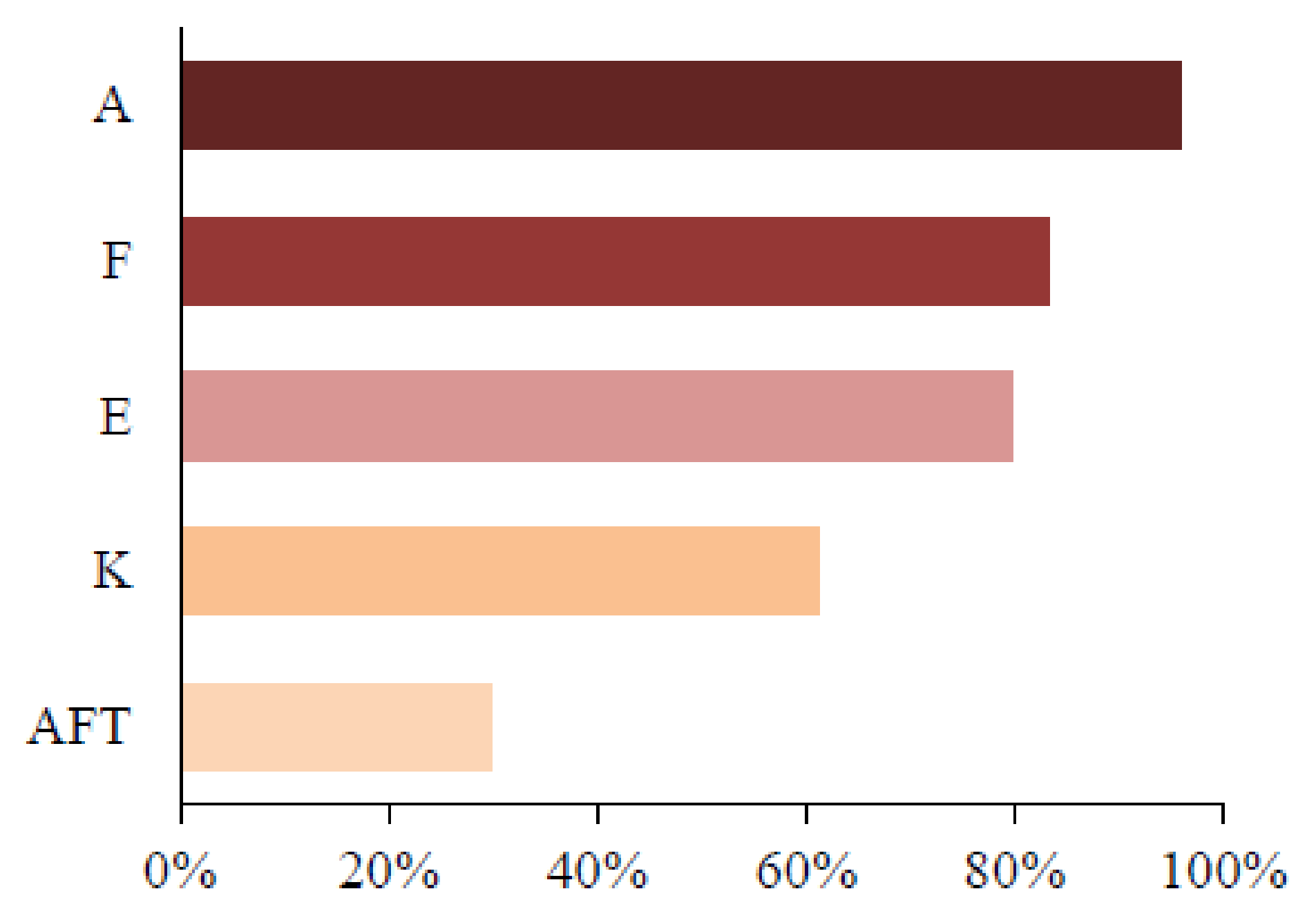
| F | 60 (81.1%) | 43 (86.0%) | 0.626 |
| A | 71 (95.9%) | 48 (96.0%) | 0.999 |
| E | 62 (83.8%) | 37 (74.0%) | 0.254 |
| K | 50 (67.6%) | 26 (52.0%) | 0.093 |
| ATF | 28 (37.8%) | 09 (18.0%) | 0.026 |
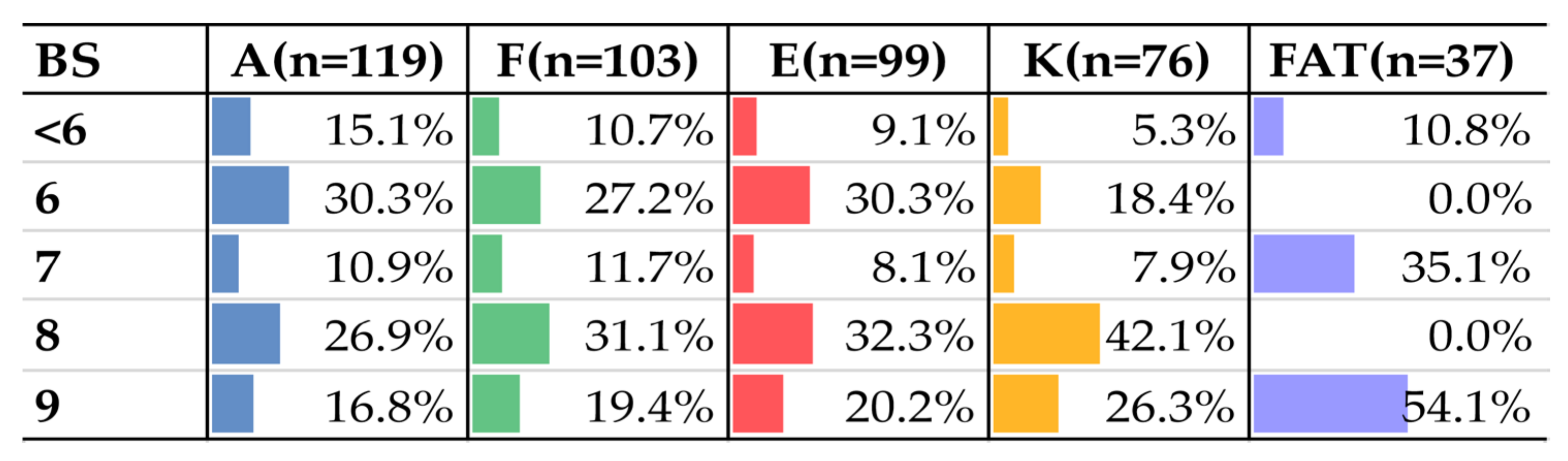
| BS | Overall | Female | Male |
|---|---|---|---|
| 2 | 2 (1.6%) | 1 (1.4%) | 1 (2.0%) |
| 3 | 2 (1.6%) | 1 (1.4%) | 1 (2.0%) |
| 4 | 17 (13.7%) | 8 (10.8%) | 9 (18.0%) |
| 5 | 2 (1.6%) | 2 (2.7%) | 0 |
| 6 | 36 (29.0%) | 17 (23.0%) | 19 (38.0%) |
| 7 | 13 (10.5%) | 10 (13.5%) | 3 (6.0%) |
| 8 | 32 (25.8%) | 20 (27.0%) | 12 (24.0%) |
| 9 | 20 (16.1%) | 15 (20.3%) | 5 (10.0%) |
| Total | 124 | 74 | 50 |
| BS Range | Female | Male | p-Value (Fisher’s Exact Test) |
|---|---|---|---|
| ≥6 | 62 (83.8%) | 39 (78%) | 0.483 |
| ≥7 | 45 (60.8%) | 20 (40%) | 0.028 |
| ≥8 | 35 (47.3%) | 17 (34%) | 0.194 |
| 9 | 15 (20.3%) | 05 (10%) | 0.144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamari, M.M.; Lamari, N.M.; de Medeiros, M.P.; Giacomini, M.G.; Santos, A.B.; de Araújo Filho, G.M.; Goloni-Bertollo, E.M.; Pavarino, É.C. Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases. Children 2024, 11, 344. https://doi.org/10.3390/children11030344
Lamari MM, Lamari NM, de Medeiros MP, Giacomini MG, Santos AB, de Araújo Filho GM, Goloni-Bertollo EM, Pavarino ÉC. Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases. Children. 2024; 11(3):344. https://doi.org/10.3390/children11030344
Chicago/Turabian StyleLamari, Mateus Marino, Neuseli Marino Lamari, Michael Peres de Medeiros, Matheus Gomes Giacomini, Adriana Barbosa Santos, Gerardo Maria de Araújo Filho, Eny Maria Goloni-Bertollo, and Érika Cristina Pavarino. 2024. "Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases" Children 11, no. 3: 344. https://doi.org/10.3390/children11030344
APA StyleLamari, M. M., Lamari, N. M., de Medeiros, M. P., Giacomini, M. G., Santos, A. B., de Araújo Filho, G. M., Goloni-Bertollo, E. M., & Pavarino, É. C. (2024). Generalized Joint Hypermobility: A Statistical Analysis Identifies Non-Axial Involvement in Most Cases. Children, 11(3), 344. https://doi.org/10.3390/children11030344