Brimonidine Eye Drops within the Reach of Children: A Possible Foe
Abstract
1. Introduction
2. Case Presentation
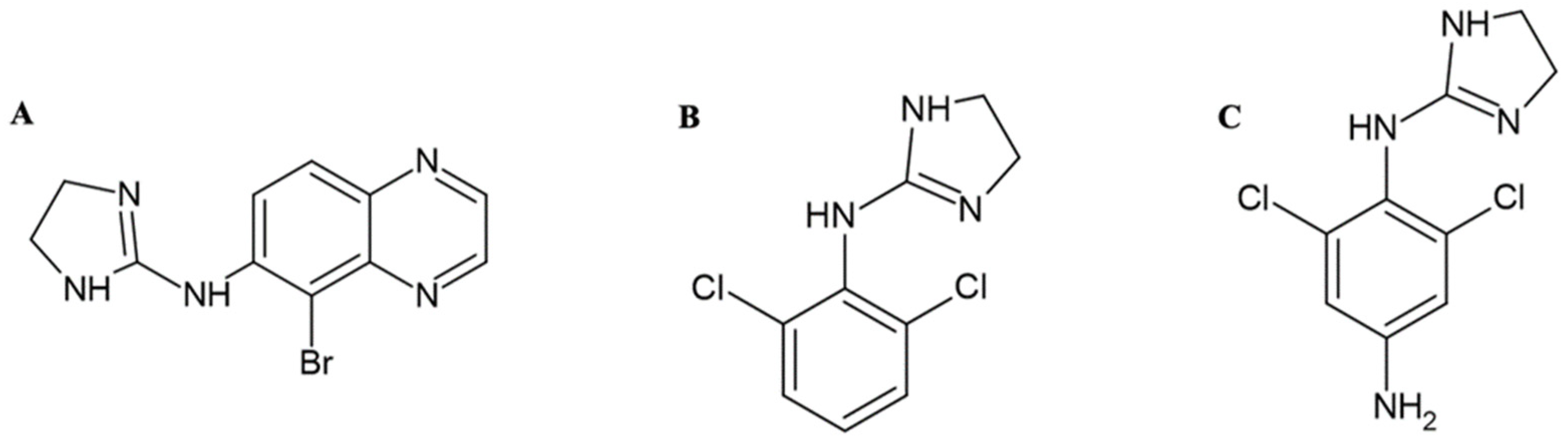
3. Biochemical Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, L.B. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert. Opin. Pharmacother. 2000, 1, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Lusthaus, J.A.; Goldberg, I. Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expert. Opin. Drug Saf. 2017, 16, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004, 82, 887. [Google Scholar] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, V.V.; Chan, M.P.Y.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2016, 100, 86. [Google Scholar] [CrossRef]
- Mukesh, B.N.; McCarty, C.A.; Rait, J.L.; Taylor, H.R. Five-year incidence of open-angle glaucoma: The visual impairment project. Ophthalmology 2002, 109, 1047. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 1965. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Mikelberg, F.S.; Balaszi, A.G.; LeBlanc, R.P.; Lesk, M.R.; Trope, G.E.; Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch. Ophthalmol. 2008, 126, 1030. [Google Scholar] [CrossRef]
- Friedman, D.S.; Wolfs, R.C.W.; O’Colmain, B.J.; Klein, B.E.; Taylor, H.R.; West, S.; Leske, M.C.; Mitchell, P.; Congdon, N.; Kempen, J.; et al. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol. 2004, 122, 532. [Google Scholar]
- Daubert, G.P. Is brimonidine ophthalmic a safe therapy for infants? Clin. Pharm. Ther. 2006, 31, 289–292. [Google Scholar] [CrossRef]
- Greenfield, D.S.; Liebmann, J.M.; Ritch, R. Brimonidine: A new alpha2-adrenoreceptor agonist for glaucoma treatment. J. Glaucoma. 1997, 6, 250–258. [Google Scholar] [CrossRef]
- Toris, C.B.; Gleason, M.L.; Camras, C.B.; Yablonski, M.E. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch. Ophthalmol. 1995, 113, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B.; Camras, C.B.; Yablonski, M.E. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am. J. Ophthalmol. 1999, 128, 8–14. [Google Scholar] [CrossRef]
- Rangan, C.; Everson, G.; Cantrell, F.L. Central alpha-2 adrenergic eye drops: Case series of 3 pediatric systemic poisonings. Pediatr. Emerg. Care 2008, 24, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.J.; Cope, J.; Nischal, K.K. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye 2004, 18, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Shagalov, D.R.; Taylor, D.; Schleichert, R.; Weiss, J.; Weiss, E. Association of central nervous system depression with topical brimonidine when used for hemostasis: A serious adverse event. JAMA Dermatol. 2017, 153, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Montague, A.J.; Bangh, S.; Cole, J.B. Bradycardia in a child after using brimonidine as toothpaste. JAMA Dermatol. 2017, 153, 330–331. [Google Scholar] [CrossRef]
- Lai Becker, M.; Huntington, N.; Woolf, A.D. Brimonidine tartrate poisoning in children: Frequency, trends, and use of naloxone as an antidote. Pediatrics 2002, 123, e305–e311. [Google Scholar] [CrossRef] [PubMed]
- Berlin, R.J.; Lee, U.T.; Samples, J.R.; Rich, L.F.; Tang-Liu, D.D.; Sing, K.A.; Steiner, R.D. Ophthalmic drops causing coma in an infant. J. Pediatr. 2001, 138, 441–443. [Google Scholar] [CrossRef]
- Juzych, M.S. Alpha-2 agonists in glaucoma therapy. In Textbook of Ocular, Pharmacology; Lippincott-Raven: Philadelphia, PA, USA, 1997. [Google Scholar]
- Fudemberg, S.J.; Batiste, C.; Katz, L.J. Efficacy, safety, and current applications of brimonidine. Expert. Opin. Drug Saf. 2008, 7, 795–799. [Google Scholar] [CrossRef]
- Boccaccini, A.; Cavaterra, D.; Carnevale, C.; Tanga, L.; Marini, S.; Bocedi, A.; Lacal, P.M.; Manni, G.; Graziani, G.; Sbardella, D.; et al. Novel frontiers in neuroprotective therapies in glaucoma: Molecular and clinical aspects. Mol. Asp. Med. 2023, 94, 101225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chappa, A.K.; Proksch, J.W. A rapid and sensitive LC/MS/MS assay for the quantification of brimonidine in ocular fluids and tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 107–114. [Google Scholar] [CrossRef]
- del Amo, E.M.; Hammid, A.; Tausch, M.; Toropainen, E.; Sadeghi, A.; Valtari, A.; Puranen, J.; Reinisalo, M.; Ruponen, M.; Urtti, A.; et al. Ocular metabolism and distribution of drugs in the rabbit eye: Quantitative assessment after intracameral and intravitreal administrations. Int. J. Pharm. 2022, 613, 121361. [Google Scholar] [CrossRef]
- Giovannitti, J.A., Jr.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kaur, S.; Taneja, S.K.; Mandal, R. Review Article on Molecular Mechanism of Regulation of Hypertension by Macro-elements (Na, K, Ca and Mg), Micro-elements/Trace Metals (Zn and Cu) and Toxic Elements (Pb and As). Biol. Trace Elem. Res. 2024, 202, 1477–1502. [Google Scholar] [CrossRef]
- Jiménez-Rivera, C.A.; Figueroa, J.; Vázquez-Torres, R.; Vélez-Hernandez, M.E.; Schwarz, D.; Velásquez-Martinez, M.C.; Arencibia-Albite, F. Presynaptic inhibition of glutamate transmission by α2 receptors in the VTA. Eur. J. Neurosci. 2012, 35, 1406–1415. [Google Scholar] [CrossRef]
- Bielory, L.; Schoenberg, D. Emerging Therapeutics for Ocular Surface Disease. Curr. Allergy Asthma Rep. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Li, J.; Pi, J.; Qi, D.; Guo, P.; Li, N.; Wu, Y.; Liu, Z. Increasing efficacy and reducing systemic absorption of brimonidine tartrate ophthalmic gels in rabbits. Pharm. Dev. Technol. 2018, 23, 231–239. [Google Scholar] [CrossRef]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic side effects of eye drops: A pharmacokinetic perspective. Clin. Ophthalmol. 2016, 10, 2433–2441. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Zakariaei, Z.; Ghazaeian, M.; Jafari, R.; Ezoddin, N.; Yousefi Nouraee, H.; Navaeifar, M.R. Adverse effects of brimonidine eye drop in children: A case series. J. Clin. Pharm. Ther. 2021, 46, 1469–1472. [Google Scholar] [CrossRef]
- Navaeifar, M.R.; Alipour, A.; Shokooh, S.S. Accidental ingestion of local anesthetic solutions in children. J. Pediatrics Rev. 2019, 7, 169–176. [Google Scholar]
- Angelov, O.V.; Wiese, A.G.; Tang-Liu, D.D.; Acheampong, A.A.; Ismail, I.M.; Brar, B.S. Preclinical safety profile of brimonidine. Eur. J. Ophthalmol. 1996, 6, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Azarcon, C.P.; Santiago, D.E. Prolonged central nervous system and respiratory depression in preterm neonates after exposure to brimonidine tartrate and timolol maleate ophthalmic drops. GMS Ophthalmol. Cases 2020, 6, Doc25. [Google Scholar]
- Saghir, S.A.; Khan, S.A.; McCoy, A.T. Ontogeny of mammalian metabolizing enzymes in humans and animals used in toxicological studies. Crit. Rev. Toxicol. 2012, 42, 323–357. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, D.; Zucchelli, M.; Salladini, C.; Ballerini, P.; Rossi, C.; Aricò, M. Brimonidine Eye Drops within the Reach of Children: A Possible Foe. Children 2024, 11, 317. https://doi.org/10.3390/children11030317
Trotta D, Zucchelli M, Salladini C, Ballerini P, Rossi C, Aricò M. Brimonidine Eye Drops within the Reach of Children: A Possible Foe. Children. 2024; 11(3):317. https://doi.org/10.3390/children11030317
Chicago/Turabian StyleTrotta, Daniela, Mirco Zucchelli, Carmela Salladini, Patrizia Ballerini, Claudia Rossi, and Maurizio Aricò. 2024. "Brimonidine Eye Drops within the Reach of Children: A Possible Foe" Children 11, no. 3: 317. https://doi.org/10.3390/children11030317
APA StyleTrotta, D., Zucchelli, M., Salladini, C., Ballerini, P., Rossi, C., & Aricò, M. (2024). Brimonidine Eye Drops within the Reach of Children: A Possible Foe. Children, 11(3), 317. https://doi.org/10.3390/children11030317







