Prevalence of Somatic Symptoms and Somatoform Disorders among a German Adolescent Psychiatric Inpatient Sample
Abstract
1. Introduction
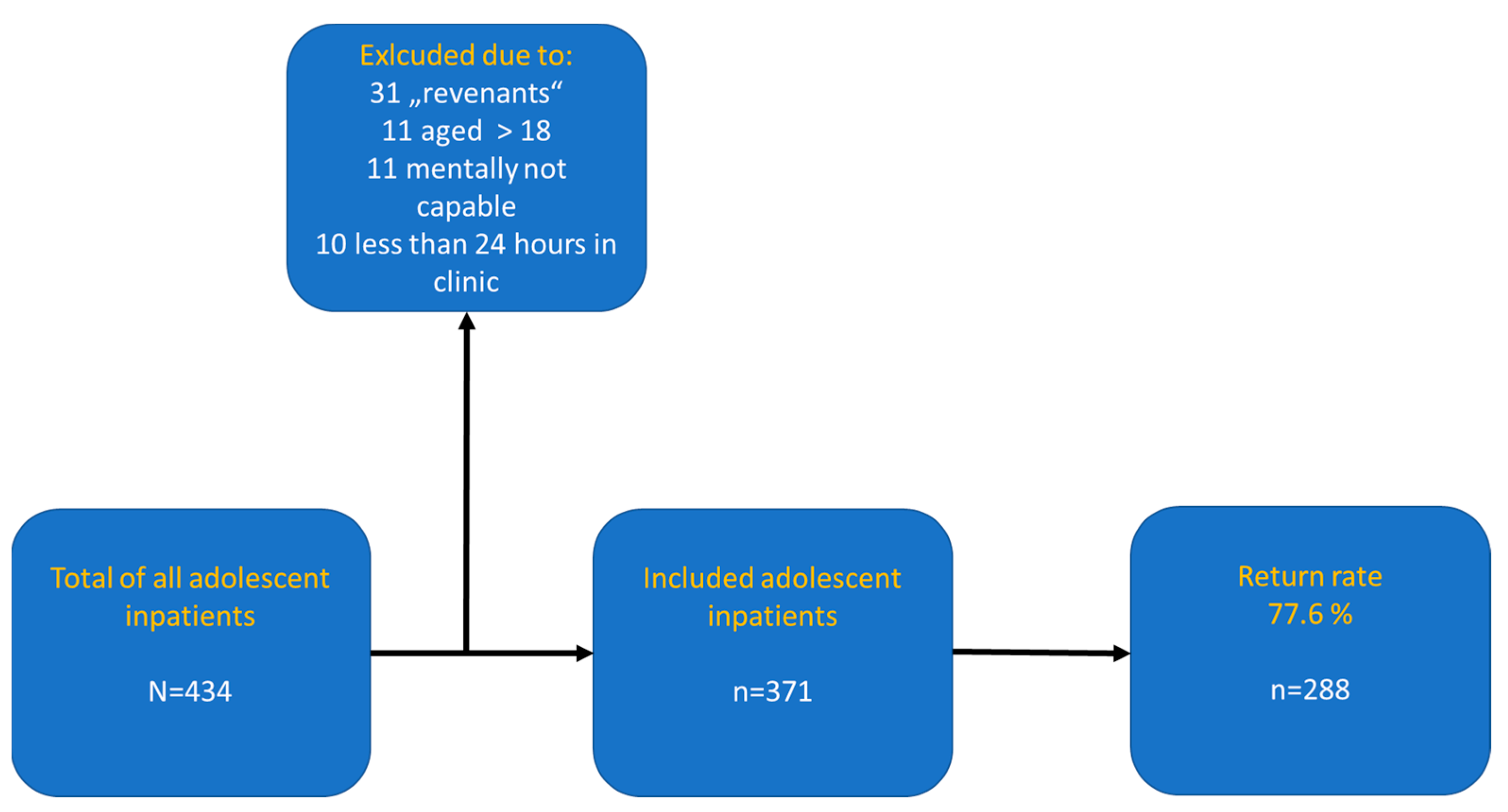
2. Materials and Methods
2.1. Design and Procedure
2.2. Measures
2.2.1. Somatoform Symptoms and Somatoform Disorder
2.2.2. Depressive Symptoms
2.2.3. Anxiety
2.3. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Somatic Symptoms: Frequencies of Single Somatic Symptoms and Number of Symptoms
3.3. Somatic Symptoms: Screening for Somatoform Disorders (SD) in Children and Adolescents [35]
3.4. Correlations and Multiple Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creed, F.H.; Davies, I.; Jackson, J.; Littlewood, A.; Chew-Graham, A.; Tomenson, B.; Macfarlane, G.; Barsky, A.; Katon, W.; McBeth, J. The epidemiology of multiple somatic symptoms. J. Psychosom. Res. 2012, 72, 311–317. [Google Scholar] [CrossRef]
- Saps, M.; Seshadri, R.; Sztainberg, M.; Schaffer, G.; Marshall, B.M.; Di Lorenzo, C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J. Pediatr. 2009, 154, 322–326. [Google Scholar] [CrossRef]
- Herzog, A.; Shedden-Mora, M.C.; Jordan, P.; Löwe, B. Duration of untreated illness in patients with somatoform disorders. J. Psychosom. Res. 2018, 107, 1–6. [Google Scholar] [CrossRef]
- Lieb, R.; Pfister, H.; Mastaler, M.; Wittchen, H.-U. Somatoform syndromes and disorders in a representative population sample of adolescents and young adults: Prevalence, comorbidity and impairments. Acta Psychiatr. Scand. 2000, 101, 194–208. [Google Scholar] [CrossRef]
- Vesterling, C.; Schütz-Wilke, J.; Bäker, N.; Bolz, T.; Eilts, J.; Koglin, U.; Rademacher, A.; Goagoses, N. Epidemiology of somatoform symptoms and disorders in childhood and adolescence: A systematic review and meta-analysis. Health Soc. Care Community 2023, 2023, 6242678. [Google Scholar] [CrossRef]
- Tomasson, K.; Kent, D.; Coryell, W. Somatization and conversion disorders: Comorbidity and demographics at presentation. Acta Psychiatr. Scand. 1991, 84, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Murphy, M. Somatoform and personality disorders: Syndromal comorbidity and overlapping developmental pathways. J. Psychosom. Res. 1995, 39, 403–427. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; WHO: Genève, Switzerland, 1993.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Dimsdale, J.; Sharma, N.; Sharpe, M. What do physicians think of somatoform disorders? Psychosomatics 2011, 52, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K. Functional somatic symptoms in childhood and adolescence. Curr. Opin. Psychiatry 2013, 26, 485–492. [Google Scholar] [CrossRef]
- Malas, N.; Ortiz-Aguayo, R.; Giles, L.; Ibeziako, P. Pediatric somatic symptom disorders. Curr. Psychiatry Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.; Court, A.; Sawyer, S.M. Somatic symptom and related disorders in a tertiary paediatric hospital: Prevalence, reach and complexity. Eur. J. Pediatr. 2021, 180, 1267–1275. [Google Scholar] [CrossRef]
- Burton, C.; Fink, P.; Henningsen, P.; Löwe, B.; Rief, W.; EURONET-SOMA Group. Functional somatic disorders: Discussion paper for a new common classification for research and clinical use. BMC Med. 2020, 18, 34. [Google Scholar] [CrossRef]
- Dhossche, D.; van der Steen, F.; Ferdinand, R. Somatoform disorders in children and adolescents: A comparison with other internalizing disorders. Ann. Clin. Psychiatry 2002, 14, 23–31. [Google Scholar] [CrossRef]
- Mullick, M.S.I. Somatoform Disorders in Children and Adolescents. Bangladesh Med. Res. Counc. Bull. 2002, 28, 112–122. [Google Scholar]
- Barkmann, C.; Braehler, E.; Schulte-Markwort, M.; Richterich, A. Chronic somatic complaints in adolescents: Prevalence, predictive validity of the parent reports, and associations with social class, health status, and psychosocial distress. Soc. Psychiatry Psychiatr. Epidemiol. 2011, 46, 1003–1011. [Google Scholar] [CrossRef]
- Janssens, K.A.; Klis, S.; Kingma, E.M.; Oldehinkel, A.J.; Rosmalen, J.G. Predictors for persistence of functional somatic symptoms in adolescents. J. Pediatr. 2014, 164, 900–905. [Google Scholar] [CrossRef]
- Kelly, C.; Molcho, M.; Doyle, P.; Gabhainn, S.N. Psychosomatic symptoms among school children. Int. J. Adolesc. Med. Health 2010, 22, 227–233. [Google Scholar] [CrossRef]
- Rask, C.U.; Olsen, E.M.; Elberling, H.; Christensen, M.F.; Ørnbøl, E.; Fink, P.; Thomsen, P.H.; Skovgaard, A.M. Functional somatic symptoms and associated impairment in 5-7-year-old children: The Copenhagen Child Cohort 2000. Eur. J. Epidemiol. 2009, 24, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kronijnenberg, A.Y.; Uiterwaal, C.S.P.M.; Kimpen, J.L.L.; van der Hoeven, J.; Buitelaar, J.K.; de Graeff-Meeder, E.R. Children with unexplained chronic pain: Substantial impairment in everyday life. Arch. Dis. Child. 2005, 90, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.M.; Wiersma, M.; Cisneros, A.; Huth, A. Children and adolescents with medically unexplained symptoms: A systematic review of the literature. Am. J. Fam. Ther. 2019, 47, 183–197. [Google Scholar] [CrossRef]
- Dorn, L.D.; Campo, J.C.; Thato, S.; Dahl, R.E.; Lewin, D.; Ramamurti, C. Psychological comorbidity and stress reactivity in children with recurrent abdominal pain and anxiety disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2003, 42, 66–75. [Google Scholar] [CrossRef]
- Dufton, L.M.; Dunn, M.J.; Compas, B.E. Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J. Pediatr. Psychol. 2009, 34, 176–186. [Google Scholar] [CrossRef]
- Garber, J.; Zeman, J.; Walker, L.S. Recurrent abdominal pain in children: Psychiatric diagnoses and parental psychopathology. J. Am. Acad. Child. Adolesc. Psychiatry 1990, 29, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.; Robertson, M.; Dewey, D. Psychological correlates of depression in children with recurrent abdominal pain. J. Pediatr. Psychol. 2006, 31, 956–966. [Google Scholar] [CrossRef]
- Knook, L.M.E.; Konijenberg, A.Y.; van der Hoeven, J.; Kimpen, J.L.L.; Buitelaar, J.K.; van Enegeland, H.; de Graeff-Meeder, E.R. Psychiatric disorders in children and adolescents presenting with unexplained chronic pain: What is the prevalence and clinical relevancy? Eur. Child. Adolesc. Psychiatry 2011, 20, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Skrove, M.; Rommundstad, P.; Indredavik, M.S. Chronic multisite pain in adolescent girls and boys with emotional and behavioral problems: The Young-HUNT study. Eur. Child. Adolesc. Psychiatry 2015, 24, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Laessle, R. Physical complaints in girls with major depression—A controlled study. Psychiatry Res. 2014, 218, 98–100. [Google Scholar] [CrossRef]
- Bailey, B.N.; Delaney-Black, V.; Hannigan, J.H.; Ager, J.; Sokol, R.J.; Covington, C.Y. Somatic complaints in children and community violence exposure. J. Dev. Behav. Pediatr. 2005, 26, 341–348. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Riddle, M.A.; Davies, M. Somatic symptoms in children and adolescents with anxiety disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2006, 45, 1179–1187. [Google Scholar] [CrossRef]
- Livingston, R.; Taylor, J.L.; Crawford, L. A study of somatic complaints and psychiatric diagnosis in children. J. Am. Acad. Child. Adolesc. Psychiatry 1988, 27, 185–187. [Google Scholar] [CrossRef]
- Masi, G.; Favilla, L.; Millepiedi, S.; Mucci, M. Somatic Symptoms in children and adolescents referred for emotional and behavioral disorders. Psychiatry 2000, 63, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Mangerud, W.L.; Bjerkeset, O.; Lydersen, S.; Indredavik, M.S. Chronic pain and pain-related disability across psychiatric disorders in a clinical adolescent sample. BMC Psychiatry 2013, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Quinn, C.; Lenz, K.; Pfeiffer, E.; Lehmkuhl, U. Screening for somatoform disorders in children and adolescents. Psychology 2014, 5, 1629–1637. [Google Scholar] [CrossRef][Green Version]
- Rief, W.; Hiller, W. SOMS—Das Screening für Somatoforme Störungen Manual zum Fragebogen; 4. vollst. überarb. u. neu norm. Aufl. Bern; Huber: Bern, Switzerland, 2008. [Google Scholar]
- Diepgen, T.L. (Ed.) Grundwissen Epidemiologie, Medizinische Biometrie und Medizinische Informatik; Verlag Hans Huber, Hogrefe: Bern, Switzerland, 2008. [Google Scholar]
- Stiensmeier-Pelster, J.; Braune-Krickau, M.; Schürmann, M.; Duda, K. Depressions-Inventar für Kinder und Jugendliche (DIKJ), 3rd ed.; Hogrefe: Göttingen, Germany, 2014. [Google Scholar]
- Döpfner, M.; Görtz-Dorten, A.; Lehmkuhl, G. Diagnostik-System für Psychische Störungen Nach ICD-10 und DSM-5 für Kinder und Jugendliche–III (DISYPS-III); Hogrefe: Göttingen, Germany, 2017. [Google Scholar]
- Essau, C.A.; Conradt, J.; Petermann, F. Prevalence, comorbidity and psychosocial impairment of somatoform disorders in adolescents. Psychol. Health Med. 1999, 4, 169–180. [Google Scholar] [CrossRef]
- Fritz, G.K.; Fritsch, S.; Hagino, O. Somatoform disorders in children and adolescents: A review of the past 10 years. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Rushkin, V.; Schwab-Stone, M. A longitudinal study of somatic complaints in urban adolescents: The role of internalizing psychopathology and somatic complaints. J. Youth Adolesc. 2014, 43, 834–845. [Google Scholar] [CrossRef]
- Bohman, H.; Jonsson, U.; von Knorring, A.-L.; von Knorring, L.; Päären, A.; Olsson, G. Somatic symptoms as a marker for severity in adolescent depression. Acta Paediatr. 2010, 99, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Oelkers-Ax, R.; Parzer, P.; Haffner, J.; Brunner, R.; Resch, F.; Kaess, M. The association of self-injurious behaviour and suicide attempts with recurrent idiopathic pain in adolescents: Evidence from a population-based study. Child. Adolesc. Psychiatry Ment. Health 2015, 9, 32. [Google Scholar] [CrossRef]
- Knook, L.M.; Lijmer, J.G.; Konijnenberg, A.Y.; Taminiau, B.; van Engeland, H. The course of chronic pain with and without psychiatric disorders: A 6-year follow-up study from childhood to adolescence and young adulthood. J. Clin. Psychiatry 2012, 73, 3893. [Google Scholar] [CrossRef]
- olde Hartman, T.C.; Borghuis, M.S.; Lucassen, P.L.B.J.; van der Laar, F.A.; Speckens, A.E.; van Weel, C. Medically unexplained symptoms, somatisation disorder and hypohondriasis: Course and prognosis. J. Psychosom. Res. 2009, 66, 363–377. [Google Scholar] [CrossRef]
- Bohmann, H.; Låftman, S.B.; Cleland, N.; Lundberg, M.; Päären, A.; Jonsson, U. Somatic symptoms in adolescence as a predictor of severe mental illness in adulthood: A long-term community-based follow-up study. Child. Adolesc. Psychiatry Ment. Health 2018, 12, 42. [Google Scholar] [CrossRef]
- Bohman, H.; Jonsson, U.; Päären, A.; von Knorring, L.; Olsson, G.; von Knorring, A.L. Prognostic significance of functional somatic symptoms in adolescence: A 15-year community-based follow-up study of adolescents with depression compared with healthy peers. BMC Psychiatry 2012, 12, 90. [Google Scholar] [CrossRef]
- Johnson, B. Somatic symptom disorders in children. BMH Med. J. 2017, 4, 55–61. [Google Scholar]
- Jungmann, S.M.; Witthöft, M. Medically unexplained symptoms in children and adolescents: Illness-related self-concept and parental symptom evaluations. J. Behav. Ther. Exp. Psychiatry 2020, 68, 101565. [Google Scholar] [CrossRef]
- Ask, H.; Waaktaar, T.; Seglem, K.B.; Torgersen, S. Common etiological sources of anxiety, depression and somatic complaints in adolescents: A multiple rater twin study. J. Abnorm. Child. Psychol. 2016, 44, 101–114. [Google Scholar] [CrossRef]
- Bonvanie, I.J.; Kallesøe, K.H.; Janssens, K.A.; Schröder, A.; Rosmalen, J.G.; Rask, C.U. Psychological interventions for children with functional somatic symptoms: A systematic review and meta-analysis. J. Pediatr. 2017, 187, 272–281. [Google Scholar] [CrossRef]
- Kangas, M.; Hansen Kallasoe, K.; Rask, C.U. Functional somatic symptoms (FSS) in children and adolescents—Conceptual, measurement and treatment issues. Z. Psychol. 2020, 228, 81–92. [Google Scholar]
- Winding, T.N.; Andersen, J.H. Do negative childhood conditions increase the risk of somatic symptoms in adolescence?—A prospective cohort study. BMC Public. Health 2019, 19, 828. [Google Scholar] [CrossRef]
- Reigstad, B.; Jørgensen, K.; Wichstrøm, L. Pain in adolescent psychiatric patients. Child. Adolesc. Ment. Health 2006, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rosendal, M.; Olde Hartman, T.C.; Aamland, A.; Van der Horst, H.; Lucassen, P.; Budtz-Lilly, A.; Burton, C. “Medically unexplained” symptoms and symptom disorders in primary care: Prognosis-based recognition and classification. BMC Fam. Pract. 2017, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Gandhi, S.; Chen, S.; Vigod, S.; Fung, K.; De Souza, C.; Saab, H.; Kurdyak, P. Health care use and costs of children, adolescents, and young adults with somatic symptom and related disorders. JAMA Netw. Open 2020, 3, e2011295. [Google Scholar] [CrossRef] [PubMed]
| Total | Female | Male | Test Statistic | p | |
|---|---|---|---|---|---|
| F(1,287), X2(1) | |||||
| age in years, M(SEM) | 15.15 (0.11) | 15.39 (0.13) | 14.86 (0.18) | 19.70 | 0.015 |
| sex (n) | 288 | 161 | 127 | ||
| primary diagnosis (ICD-10) | n (%) | n (%) | n (%) | X2(8) = 66.73 | <0.001 *** |
| affective disorders (F31–F32) | 47 (16.3) | 28 (17.4) | 19 (15.0) | ||
| emotional disorders (F40–F42; F93) | 50 (17.4) | 35 (21.7) | 15 (11.8) | ||
| trauma-related disorders (F43) | 56 (19.4) | 37 (23.0) | 19 (15.0) | ||
| eating disorders (F50) | 24 (8.3) | 24 (14.9) | 0 (0.0) | ||
| autism spectrum disorders (F84) | 9 (3.1) | 1 (0.6) | 8 (6.3) | ||
| ADHD (F90.0 & F98.8) | 9 (3.1) | 3 (1.8) | 6 (4.7) | ||
| conduct disorders (F90.1, F91 & F92) | 60 (20.8) | 14 (8.7) | 46 (36.2) | ||
| somatoform disorders (F44 & F45) | 10 (3.5) | 9 (5.6) | 1 (0.8) | ||
| substance abuse disorders (F10–F19) | 23 (8.0) | 10 (6.2) | 13 (10.2) | ||
| quarter (year) | X2(3) = 4.95 | 0.176 | |||
| first quarter (February) | 84 (29.2) | 55 (34.2) | 29 (22.8) | ||
| second quarter (May) | 64 (22.2) | 35 (21.7) | 29 (22.8) | ||
| third quarter (August) | 68 (23.6) | 33 (20.5) | 35 (27.6) | ||
| fourth quarter (October) | 72 (25.0) | 38 (23.6) | 34 (26.8) | ||
| depression score/T-value: M(SEM) | 67.07 (0.73) | 69.89 (0.89) | 63.49 (1.14) | F(1,287) = 2908.03 | <0.001 *** |
| anxiety score/T-value: M(SEM) | 63.63 (0.49) | 65.68 (0.52) | 61.02 (0.83) | F(1,287) = 1541.47 | <0.001 *** |
| medical examinations | |||||
| physical examination (yes/no): n | 283/6 | 157/4 | 126/2 | X2(1) = 0.30 | 0.585 |
| blood sample (yes/no): n | 269/19 | 151/9 | 118/10 | X2(1) = 0.55 | 0.457 |
| electrocardiogram (ECG) (yes/no): n | 85/204 | 53/1087 | 32/967 | X2(1) = 0.30 | 0.142 |
| magnetic resonance imaging (MRI) (yes/no): n | 22 (266) | 11/150) | 11 (116) | X2(1) = 0.34 | 0.562 |
| school | X2(11) = 14.71 | 0.005 * | |||
| elementary school and general (n) | 182 | 96 | 86 | ||
| grammar school (n) | 64 | 48 | 16 | ||
| free schools/Danish schools (n) | 10 | 4 | 6 | ||
| no school (n) | 28 | 12 | 16 | ||
| school for children with special needs (n) | 4 | 1 | 3 |
| Categories | Symptoms | Total 278 n (%) | Female 152 n (%) | Male 126 n (%) | Chi | p |
|---|---|---|---|---|---|---|
| Pain symptoms | headache | 186 (66.9) | 122 (80.2) | 64 (50.8) | 27.021 | <0.001 *** |
| stomach pain | 163 (58.6) | 109 (71.7) | 54 (42.9) | 23.647 | <0.001 *** | |
| back pain | 147 (52.9) | 83 (54.6) | 64 (50.8) | 0.402 | 0.548 | |
| joint pain | 91 (32.7) | 52 (34.2) | 31 (24.6) | 0.332 | 0.328 | |
| pain in legs/feet/arms/hands | 106 (38.1) | 62 (40.8) | 44 (34.9) | 1.006 | 0.324 | |
| chest pain | 103 (37.1) | 64 (42.1) | 39 (31.0) | 3.674 | 0.062 | |
| eurache | 55 (19.8) | 34 (22.4) | 21 (16.7) | 1.411 | 0.290 | |
| pain during urination | 19 (6.8) | 17 (11.2) | 2 (1.6) | 9.965 | 0.002 ** | |
| pain in or around the genital area | 8 (2.9) | 7 (4.6) | 1 (0.8) | 3.581 | 0.058 | |
| other pain | 22 (7.9) | 14 (9.2) | 8 (6.3) | 0.774 | 0.504 | |
| gastrointestinal | nausea | 149 (53.6) | 108 (71.1) | 41 (32.5) | 41.086 | <0.001 *** |
| symptoms | vomiting | 51 (18.3) | 36 (23.7) | 15 (11.9) | 6.381 | 0.012 |
| loss of appetite | 143 (51.4) | 99 (65.1) | 44 (34.9) | 25.172 | <0.001 *** | |
| diarrhoea | 52 (18.7) | 27 (17.8) | 25 (19.8) | 0.196 | 0.658 | |
| constipation | 42 (15.1) | 28 (18.4) | 14 (11.1) | 2.870 | 0.090 | |
| cardiorespiratory | lump in one’s throat | 75 (27.0) | 49 (32.2) | 26 (20.6) | 4.707 | 0.030 * |
| symptoms | coughing | 117 (42.1) | 63 (41.4) | 54 (42.9) | 0.056 | 0.813 |
| shortness of breath or sensation of suffocation | 99 (35.6) | 70 (46.1) | 29 (23.0) | 15.945 | <0.001 *** | |
| heart palpitations/heart flutter | 126 (45.3) | 77 (50.7) | 49 (38.9) | 3.851 | 0.050 | |
| tiredness | 189 (64.0) | 127 (83.6) | 62 (49.2) | 37.337 | <0.001 *** | |
| pseudeoneurological | paralysis, muscle weakness | 52 (18.7) | 37 (24.3) | 15 (11.9) | 7.008 | 0.008 ** |
| symptoms | numbness, pins and needles | 94 (33.8) | 62 (40.8) | 32 (25.4) | 7.294 | 0.007 ** |
| muscle twitching | 92 (33.1) | 55 (36.2) | 37 (29.4) | 1.447 | 0.229 | |
| heaviness in arms/legs | 67 (24.1) | 45 (29.6) | 22 (17.5) | 5.555 | 0.018 * | |
| gait disturbances/difficulties in walking | 80 (28.8) | 54 (35.5) | 26 (20.6) | 7.454 | 0.006 ** | |
| visual impairment/double vision | 38 (13.6) | 22 (14.5) | 10 (7.9) | 0.184 | 0.668 | |
| speech disorders, loss of voice, hoarseness | 56 (20.1) | 39 (25.7) | 17 (13.5) | 6.339 | 0.012 * | |
| seizures | 42 (15.1) | 27 (17.8) | 15 (11.9) | 1.844 | 0.175 | |
| trembling | 107 (38.5) | 73 (48.0) | 34 (27.0) | 12.884 | <0.001 *** | |
| dizziness | 150 (54.0) | 108 (71.1) | 42 (33.3) | 39.453 | <0.001 *** | |
| loss of consciousness/fainting | 30 (10.8) | 22 (14.5) | 8 (6.3) | 4.724 | 0.030 * | |
| hearing difficulties buzzing/ringing in the ears | 77 (27.7) | 43 (28.3) | 34 (27.0) | 0.059 | 0.809 | |
| last week | point prevalence | 127 (45.7) | 88 (57.9) | 39 (31.0) | 20.153 | <0.001 *** |
| SOMS-CA | SOMS Score | |||||
|---|---|---|---|---|---|---|
| n | Neg: 114 | Pos: 164 | X2 | M | SEM | |
| diagnoses | n (%) | n (%) | 18.504 | p = 0.010 * | ||
| affective disorders | 16 (34.0) | 31 (66.0) | 4.4 | 0.28 | ||
| anxiety and emotional disorders | 16 (32.0) | 34 (68.0) | 4.5 | 0.33 | ||
| adjustment disorders and PTSD | 17 (30.4) | 39 (69.6) | 4.4 | 0.26 | ||
| eating disorders | 7 (29.2) | 17 (71.8) | 4.5 | 0.31 | ||
| developmental disorders | 5 (55.6) | 4 (44.4) | 3.2 | 0.83 | ||
| ADHD | 6 (66.67) | 3 (33.3) | 2.7 | 0.58 | ||
| ODD/CD | 35 (58.3) | 25 (41.7) | 3.0 | 0.27 | ||
| substance disorders | 12 (52.2) | 11 (47.8) | 3.0 | 0.35 | ||
| Total | 117 (40.5) | 172 (59.5) | 4.0 | 0.13 | ||
| diagnostic category | 18.105 | p < 0.001 *** | ||||
| internalizing disorders | 49 (32.0) | 104 (68.0) | 4.4 | 0.17 | ||
| externalizing disorders | 41 (59.4) | 28 (40.6) | 3.0 | 0.25 | ||
| eating disorders | 7 (29.2) | 17 (71.8) | 4.5 | 0.31 | ||
| other category | 17 (53.1) | 15 (46.9) | 3.1 | 0.33 | ||
| sex | 18.023 | p < 0.001 *** | ||||
| female | 45 (31.6) | 107 (68.4) | 4.5 | 0.15 | ||
| male | 71 (55.5) | 57 (44.5) | 3.2 | 0.20 | ||
| age group | 10.024 | p = 0.007 * | ||||
| 11–13 years | 51 (55.4) | 41 (44.6) | 3.4 | 0.22 | ||
| 14 & 15 years | 27 (33.3) | 54 (66.7) | 4.0 | 0.22 | ||
| 16 & 17 years | 38 (35.6) | 69 (64.4) | 4.3 | 0.20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremek, A.; Lindner, C.; Jung, M.; Calvano, C.; Munz, M. Prevalence of Somatic Symptoms and Somatoform Disorders among a German Adolescent Psychiatric Inpatient Sample. Children 2024, 11, 280. https://doi.org/10.3390/children11030280
Geremek A, Lindner C, Jung M, Calvano C, Munz M. Prevalence of Somatic Symptoms and Somatoform Disorders among a German Adolescent Psychiatric Inpatient Sample. Children. 2024; 11(3):280. https://doi.org/10.3390/children11030280
Chicago/Turabian StyleGeremek, Adam, Clemens Lindner, Martin Jung, Claudia Calvano, and Manuel Munz. 2024. "Prevalence of Somatic Symptoms and Somatoform Disorders among a German Adolescent Psychiatric Inpatient Sample" Children 11, no. 3: 280. https://doi.org/10.3390/children11030280
APA StyleGeremek, A., Lindner, C., Jung, M., Calvano, C., & Munz, M. (2024). Prevalence of Somatic Symptoms and Somatoform Disorders among a German Adolescent Psychiatric Inpatient Sample. Children, 11(3), 280. https://doi.org/10.3390/children11030280





