Optimization of Postural Control, Balance, and Mobility in Children with Cerebral Palsy: A Randomized Comparative Analysis of Independent and Integrated Effects of Pilates and Plyometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethics
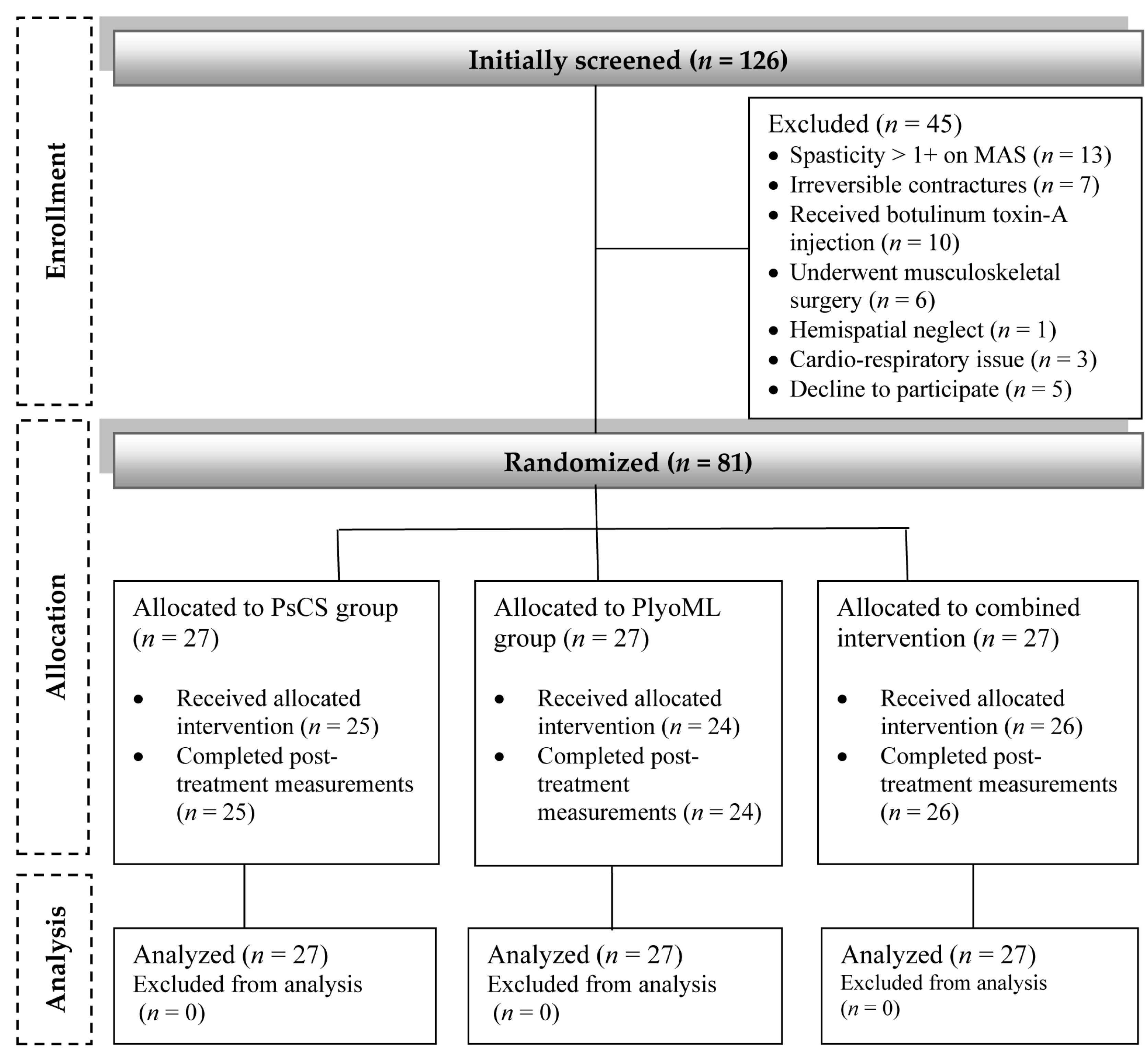
2.2. Participants
2.2.1. Sample Size Determination
2.2.2. Randomization Procedure
2.3. Outcome Measures
2.3.1. Postural Control
2.3.2. Balance and Mobility
2.4. Interventions
2.4.1. Pilates-Based Core Strengthening
2.4.2. Plyometric-Based Muscle Loading
2.4.3. Combined Intervention
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Novak, I.; Hines, M.; Goldsmith, S.; Barclay, R. clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics 2012, 130, e1285–e1312. [Google Scholar] [CrossRef]
- Gulati, S.; Sondhi, V. Cerebral Palsy: An Overview. Indian J. Pediatr. 2018, 85, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Østensjø, S.; Carlberg, E.B.; Vøllestad, N.K. Motor impairments in young children with cerebral palsy: Relationship to gross motor function and everyday activities. Dev. Med. Child Neurol. 2004, 46, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, R.K.; Alhowimel, A.; Alotaibi, M.; Abdrabo, M.S.; Elshafey, M.A. Accommodating variable-resistance exercise enhance weight-bearing/gait symmetry and balance capability in children with hemiparetic cerebral palsy: A parallel-group, single-blinded randomized clinical trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 378–386. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Carlberg, E.B. Postural Control: A Key Issue in Developmental Disorders; Mac Keith Press: London, UK, 2010. [Google Scholar]
- Bigongiari, A.; e Souza, F.D.A.; Franciulli, P.M.; Neto, S.E.R.; Araujo, R.C.; Mochizuki, L. Anticipatory and compensatory postural adjustments in sitting in children with cerebral palsy. Hum. Mov. Sci. 2011, 30, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Girolami, G.L.; Shiratori, T.; Aruin, A.S. Anticipatory postural adjustments in children with hemiplegia and diplegia. J. Electromyogr. Kinesiol. 2011, 21, 988–997. [Google Scholar] [CrossRef]
- Nashner, L.M.; Shumway-Cook, A.; Marin, O. Stance posture control in select groups of children with cerebral palsy: Deficits in sensory organization and muscular coordination. Exp. Brain Res. 1983, 49, 393–409. [Google Scholar] [CrossRef]
- Lowes, L.P.; Westcott, S.L.; Palisano, R.J.; Effgen, S.K.; Orlin, M.N. Muscle force and range of motion as predictors of standing balance in children with cerebral palsy. Phys. Occup. Ther. Pediatr. 2004, 24, 57–77. [Google Scholar] [CrossRef] [PubMed]
- de Graaf-Peters, V.B.; Blauw-Hospers, C.H.; Dirks, T.; Bakker, H.; Bos, A.F.; Hadders-Algra, M. Development of postural control in typically developing children and children with cerebral palsy: Possibilities for intervention? Neurosci. Biobehav. Rev. 2007, 31, 1191–1200. [Google Scholar] [CrossRef]
- Adıguzel, H.; Elbasan, B. Effects of modified pilates on trunk, postural control, gait and balance in children with cerebral palsy: A single-blinded randomized controlled study. Acta Neurol. Belg. 2022, 122, 903–914. [Google Scholar] [CrossRef]
- Abd-Elfattah, H.M.; Galal, D.; Aly, M.I.E.; Aly, S.M.; Elnegamy, T.E. Effect of Pilates Exercises on Standing, Walking, and Balance in Children with Diplegic Cerebral Palsy. Ann Rehabil. Med. 2022, 46, 45–52. [Google Scholar] [CrossRef]
- Elnaggar, R.K. Effects of plyometric exercises on muscle-activation strategies and response-capacity to balance threats in children with hemiplegic cerebral palsy. Physiother. Theory Pract. 2022, 38, 1165–1173. [Google Scholar] [CrossRef]
- Elnaggar, R.K.; Alqahtani, B.A.; Alsubaie, S.F.; Mohamed, R.R.; Elbanna, M.F. Stretch-shortening cycle exercises can efficiently optimize gait-symmetry and balance capabilities in children with unilateral cerebral palsy: A randomized controlled trial. NeuroRehabilitation 2021, 49, 139–149. [Google Scholar] [CrossRef]
- dos Santos, A.N.; Serikawa, S.S.; Rocha, N.A.C.F. Pilates improves lower limbs strength and postural control during quite standing in a child with hemiparetic cerebral palsy: A case report study. Dev. Neurorehabilit. 2016, 19, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Kolt, G.S.; Bialocerkowski, A. Defining Pilates exercise: A systematic review. Complement. Ther. Med. 2012, 20, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Salzberg, C.L.; Stevenson, D.A. A systematic review: Plyometric training programs for young children. J. Strength Cond. Res. 2011, 25, 2623–2633. [Google Scholar] [PubMed]
- Seiberl, W.; Hahn, D.; Power, G.A.; Fletcher, J.R.; Siebert, T. Editorial: The Stretch-Shortening Cycle of Active Muscle and Muscle-Tendon Complex: What, Why and How It Increases Muscle Performance? Front. Physiol. 2021, 12, 693141. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.; Riemann, B.L. Current Concepts of Plyometric Exercises for the Lower Extremity. In Return to Sport after ACL Re-construction and Other Knee Operations; Springer: Berlin/Heidelberg, Germany, 2019; pp. 277–304. [Google Scholar]
- Elnaggar, R.K.; Diab, R.H.; Alghadier, M.; Azab, A.R. Block-sequence of plyometric and balance training is superior to the alternating-sequence for enhancing motor function in children with hemiplegic cerebral palsy: A comparative randomized clinical trial. Isokinet. Exerc. Sci. 2022, 30, 373–383. [Google Scholar] [CrossRef]
- Elnaggar, R.K.; Elbanna, M.F.; Mahmoud, W.S.; Alqahtani, B.A. Plyometric exercises: Subsequent changes of weight-bearing symmetry, muscle strength and walking performance in children with unilateral cerebral palsy. J. Musculoskelet. Neuronal. Interact. 2019, 19, 507–515. [Google Scholar]
- Elnaggar, R.K.; Mahmoud, W.S.; Alsubaie, S.F.; El-Nabie, W.A.A. Effectiveness of a Multi-Modal Exercise Program Incorporating Plyometric and Balance Training in Children with Hemiplegic Cerebral Palsy: A Three-Armed Randomized Clinical Trial. Phys. Occup. Ther. Pediatr. 2022, 42, 113–129. [Google Scholar] [CrossRef]
- Elnaggar, R.K.; Alghadier, M.; Abdrabo, M.S.; Abonour, A.A. Effect of a structured aqua-plyometric exercise program on postural control and functional ability in children with hemiparetic cerebral palsy: A two-arm randomized controlled trial. NeuroRehabilitation 2022, 51, 247–258. [Google Scholar] [CrossRef]
- Kloubec, J.A. Pilates for improvement of muscle endurance, flexibility, balance, and posture. J. Strength Cond. Res. 2010, 24, 661–667. [Google Scholar] [CrossRef]
- Heinecke, M. Review of Literature: Neuromuscular Adaptations to Plyometrics. Int. J. Strength Cond. 2021, 1. Available online: https://journal.iusca.org/index.php/Journal/article/view/53 (accessed on 1 January 2024).
- World Medical Association. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 23 June 2022).
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Arab, T.K.; Jalaie, S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: Limb and muscle group effect. NeuroRehabilitation 2008, 23, 231–237. [Google Scholar] [CrossRef]
- Balance System SD. Operation/Service Manual 950-441. Shirly; Biodex Medical Systems, Inc.: New York, NY, USA, 2010. [Google Scholar]
- A Howe, J.; Inness, E.L.; Venturini, A.; I Williams, J.; Verrier, M.C. The Community Balance and Mobility Scale-a balance measure for individuals with traumatic brain injury. Clin. Rehabil. 2006, 20, 885–895. [Google Scholar] [CrossRef]
- Quinn, A.; O’regan, M.; Horgan, F. Psychometric evaluation of the functional walking test for children withcerebral palsy. Disabil. Rehabil. 2011, 33, 2397–2403. [Google Scholar] [CrossRef]
- Zaino, C.A.; Marchese, V.G.; Westcott, S.L. timed up and down stairs test: Preliminary reliability and validity of a new measure of functional mobility. Pediatr. Phys. Ther. 2004, 16, 90–98. [Google Scholar] [CrossRef]
- Coman, C. The Effect of a Pilates-based Core Exercise Group on Balance and Gait in Ambulant Children with Cerebral Palsy: A Randomised Control Trial. Master’s Thesis, Royal College of Surgeons in Ireland, University of Medicine and Health Sciences, Dublin, Ireland, 2020. [Google Scholar]
- Faigenbaum, A.D.; Kraemer, W.J.; Blimkie, C.J.R.; Jeffreys, I.; Micheli, L.J.; Nitka, M.; Rowland, T.W. Youth resistance training: Updated position statement paper from the national strength and conditioning association. J. Strength Cond. Res. 2009, 23, S60–S79. [Google Scholar] [CrossRef]
- Anderson, B.D.; Spector, A. Introduction to Pilates-based rehabilitation. Orthop. Phys. Ther. Clin. N. Am. 2000, 9, 395–410. [Google Scholar]
- Elshafey, M.A.; Abdrabo, M.S.; Elnaggar, R.K. Effects of a core stability exercise program on balance and coordination in children with cerebellar ataxic cerebral palsy. J. Musculoskelet. Neuronal. Interact. 2022, 22, 172–178. [Google Scholar]
- Johnson, B.A.; Salzberg, C.; MacWilliams, B.A.; Shuckra, A.L.; D’Astous, J.L. Plyometric training: Effectiveness and optimal duration for children with unilateral cerebral palsy. Pediatr. Phys. Ther. 2014, 26, 169–179. [Google Scholar] [CrossRef]
- Ramachandran, A.K.; Singh, U.; Ramirez-Campillo, R.; Clemente, F.M.; Afonso, J.; Granacher, U. Effects of Plyometric Jump Training on Balance Performance in Healthy Participants: A Systematic Review with Meta-Analysis. Front. Physiol. 2021, 12, 730945. [Google Scholar] [CrossRef]
- Seo, B.-D.; Shin, H.-S.; Yoon, J.-D.; Han, D.-W. The effect of lower extremity plyometric training on the proprioception and postural stability of collegiate soccer players with postural instability. Korean J. Sport Biomech. 2010, 20, 1–12. [Google Scholar] [CrossRef][Green Version]
- Eddens, L.; van Someren, K.; Howatson, G. The Role of Intra-Session Exercise Sequence in the Interference Effect: A Systematic Review with Meta-Analysis. Sports Med. 2018, 48, 177–188. [Google Scholar] [CrossRef]
- Seitz, L.B.; Haff, G.G. Factors Modulating Post-Activation Potentiation of Jump, Sprint, Throw, and Upper-Body Ballistic Performances: A Systematic Review with Meta-Analysis. Sports Med. 2016, 46, 231–240. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Baggish, A.L. Exercise dose in clinical practice. Circulation 2016, 133, 2297–2313. [Google Scholar] [CrossRef]
- Abt, G.; Boreham, C.; Davison, G.; Jackson, R.; Nevill, A.; Wallace, E.; Williams, M. Power, precision, and sample size estimation in sport and exercise science research. J. Sports Sci. 2020, 38, 1933–1935. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; Moran, J.; Chaabene, H.; Granacher, U.; Behm, D.G.; García-Hermoso, A.; Izquierdo, M. Methodological characteristics and future directions for plyometric jump training research: A scoping review update. Scand. J. Med. Sci. Sports 2020, 30, 983–997. [Google Scholar] [CrossRef]
- Ben Kibler, W.; Press, J.; Sciascia, A. The role of core stability in athletic function. Sports Med. 2006, 36, 189–198. [Google Scholar] [CrossRef]
| PsCS Group (n = 27) | PlyoML Group (n = 27) | Combined Group (n = 27) | p- Value | |
|---|---|---|---|---|
| Age, year | 15.19 ± 1.84 | 14.78 ± 1.45 | 15.56 ± 1.63 | 0.23 ‡ |
| Gender (b/g), n (%) | 18 (66.7)/9 (33.3) | 16 (59.3)/11 (40.7) | 20 (74.1)/7 (25.9) | 0.56 § |
| Side affected (RT/LT), n (%) | 8 (29.6)/19 (70.4) | 6 (22.2)/21 (77.8) | 4 (14.8)/23 (85.2) | 0.48 § |
| MAS level (1/1+), n (%) | 14 (51.9)/13 (48.1) | 12 (44.4)/15 (55.6) | 16 (59.3)/11 (40.7) | 0.59 § |
| GMFCS level (I/II), n (%) | 15 (55.6)/12 (44.4) | 17 (63)/10 (37) | 19 (70.4)/8 (29.6) | 0.58 § |
| Height, cm | 155.6 ± 11.1 | 154.7 ± 9.3 | 156.6 ± 10.3 | 0.79 ‡ |
| Weight, Kg | 52.30 ± 7.58 | 51.81 ± 6.43 | 54.37 ± 7.29 | 0.38 ‡ |
| Body mass index, Kg/m2 | 21.51 ± 1.41 | 21.59 ± 1.24 | 22.11 ± 1.45 | 0.22 ‡ |
| PsCS Group (n = 27) | PlyoML Group (n = 27) | Combined Group (n = 27) | Interaction Effect | ||
|---|---|---|---|---|---|
| p-Value | η2Partial | ||||
| Directional LoS—backward | |||||
| Pre | 39.92 ± 4.47 | 40.52 ± 5.51 | 42.10 ± 4.99 | 0.003 * | 0.14 |
| Post | 42.74 ± 6.52 | 43.81 ± 4.92 | 49.15 ± 6.55 | ||
| p-value | 0.004 * | 0.002 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.49 (0.14–0.86) | 0.61 (0.22–1.03) | 1.18 (0.76–1.65) | ||
| Directional LoS—forward | |||||
| Pre | 42.52 ± 4.47 | 42.81 ± 4.45 | 43.33 ± 6.95 | <0.001 * | 0.23 |
| Post | 44.48 ± 6.15 | 45.11 ± 5.75 | 51.92 ± 7.81 | ||
| p-value | 0.014 * | 0.007 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.35 (0.07–0.65) | 0.43 (0.12–0.77) | 1.13 (0.63–1.68) | ||
| Directional LoS—paretic | |||||
| Pre | 45.41 ± 8.35 | 45.15 ± 9.09 | 48.52 ± 11.19 | 0.004 * | 0.13 |
| Post | 48.26 ± 7.10 | 48.56 ± 9.12 | 57.59 ± 8.50 | ||
| p-value | 0.023 * | 0.0006 * | 0.0001 * | ||
| Hedges’ g (95% CI) | 0.36 (0.05–0.68) | 0.36 (0.16–0.59) | 0.89 (0.44–1.37) | ||
| Directional LoS—non-paretic | |||||
| Pre | 53.37 ± 5.76 | 52.48 ± 5.34 | 54.93 ± 6.44 | 0.024 * | 0.09 |
| Post | 56.67 ± 7.64 | 57.63 ± 6.48 | 63.11 ± 8.45 | ||
| p-value | 0.006 * | 0.0005 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.47 (0.14–0.83) | 0.84 (0.37–1.35) | 1.06 (0.63–1.54) | ||
| Overall LoS | |||||
| Pre | 45.31 ± 2.80 | 45.24 ± 3.39 | 47.21 ± 4.55 | <0.001 * | 0.29 |
| Post | 48.04 ± 3.96 | 48.78 ± 3.27 | 55.44 ± 4.39 | ||
| p-value | 0.0004 * | <0.001 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.77 (0.35–1.23) | 1.04 (0.61–1.50) | 1.79 (1.20–2.47) | ||
| PsCS Group (n = 27) | PlyoML Group (n = 27) | Combined Group (n = 27) | Interaction Effect | ||
|---|---|---|---|---|---|
| p-Value | η2Partial | ||||
| CB&M, /96 | |||||
| Pre | 62.35 ± 6.78 | 59.21 ± 5.79 | 61.22 ± 7.60 | <0.001 * | 0.25 |
| Post | 64.94 ± 5.47 | 66.73 ± 6.18 | 74.01 ± 8.91 | ||
| p-value | 0.022 * | <0.001 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.41 (0.06–0.77) | 1.22 (0.74–1.76) | 1.50 (0.92–2.15) | ||
| FWT, /23 | |||||
| Pre | 16.88 ± 2.37 | 17.10 ± 2.25 | 17.27 ± 2.33 | <0.001 * | 0.21 |
| Post | 17.49 ± 2.03 | 18.29 ± 1.90 | 20.57 ± 1.96 | ||
| p-value | 0.005 * | 0.032 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.27 (0.08–0.47) | 0.55 (0.04–1.10) | 1.49 (0.91–2.14) | ||
| TUDS, seconds | |||||
| Pre | 18.31 ± 4.10 | 19.10 ± 4.43 | 18.36 ± 4.31 | <0.001 * | 0.31 |
| Post | 16.17 ± 2.70 | 15.96 ± 3.14 | 11.78 ± 2.34 | ||
| p-value | 0.002 * | <0.001 * | <0.001 * | ||
| Hedges’ g (95% CI) | 0.59 (0.22–0.99) | 0.79 (0.45–1.18) | 1.84 (1.31–2.48) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnaggar, R.K.; Ramirez-Campillo, R.; Azab, A.R.; Alrawaili, S.M.; Alghadier, M.; Alotaibi, M.A.; Alhowimel, A.S.; Abdrabo, M.S.; Elbanna, M.F.; Aboeleneen, A.M.; et al. Optimization of Postural Control, Balance, and Mobility in Children with Cerebral Palsy: A Randomized Comparative Analysis of Independent and Integrated Effects of Pilates and Plyometrics. Children 2024, 11, 243. https://doi.org/10.3390/children11020243
Elnaggar RK, Ramirez-Campillo R, Azab AR, Alrawaili SM, Alghadier M, Alotaibi MA, Alhowimel AS, Abdrabo MS, Elbanna MF, Aboeleneen AM, et al. Optimization of Postural Control, Balance, and Mobility in Children with Cerebral Palsy: A Randomized Comparative Analysis of Independent and Integrated Effects of Pilates and Plyometrics. Children. 2024; 11(2):243. https://doi.org/10.3390/children11020243
Chicago/Turabian StyleElnaggar, Ragab K., Rodrigo Ramirez-Campillo, Alshimaa R. Azab, Saud M. Alrawaili, Mshari Alghadier, Mazyad A. Alotaibi, Ahmed S. Alhowimel, Mohamed S. Abdrabo, Mohammed F. Elbanna, Ahmed M. Aboeleneen, and et al. 2024. "Optimization of Postural Control, Balance, and Mobility in Children with Cerebral Palsy: A Randomized Comparative Analysis of Independent and Integrated Effects of Pilates and Plyometrics" Children 11, no. 2: 243. https://doi.org/10.3390/children11020243
APA StyleElnaggar, R. K., Ramirez-Campillo, R., Azab, A. R., Alrawaili, S. M., Alghadier, M., Alotaibi, M. A., Alhowimel, A. S., Abdrabo, M. S., Elbanna, M. F., Aboeleneen, A. M., & Morsy, W. E. (2024). Optimization of Postural Control, Balance, and Mobility in Children with Cerebral Palsy: A Randomized Comparative Analysis of Independent and Integrated Effects of Pilates and Plyometrics. Children, 11(2), 243. https://doi.org/10.3390/children11020243








