Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Population
2.2. Data Collection
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Statistical Analysis
3. General Results
3.1. Clinical Features of Children with IgAV
3.2. Univariate Analysis
3.3. Multivariate Analysis
4. Results of the 25-Hydroxyvitamin D Status of IgAV Patients
4.1. Univariate Analysis
4.2. Multivariate Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Li, Y.; Wu, X. IgA vasculitis update: Epidemiology, pathogenesis, and biomarkers. Front. Immunol. 2022, 13, 921864. [Google Scholar] [CrossRef]
- Nüsken, E.; Weber, L.T. IgA vasculitis nephritis. Curr. Opin. Pediatr. 2022, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. A review of IgA vasculitis (Henoch-Schönlein purpura) past, present, and future. Med. Sci. Monit. 2024, 30, e943912. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, H.; Li, G.; Guan, W.; Zhang, T.; Zeng, Q.; Gong, Y.; Xu, H.; Sun, L. Severe gastrointestinal involvement in pediatric IgA vasculitis: A retrospective single-center cohort study in China. Front. Pediatr. 2023, 11, 1194214. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fu, R.; Peng, X.J.; Wang, Y.; Yin, T.T.; Deng, Y.Q. Comparative study on clinicopathological features and prognosis of IgA vasculitis nephritis and IgA nephropathy in children. BMC Pediatr. 2023, 23, 423. [Google Scholar] [CrossRef]
- Ruperto, N.; Ozen, S.; Pistorio, A.; Dolezalova, P.; Brogan, P.; Cabral, D.A.; Cuttica, R.; Khubchandani, R.; Lovell, D.J.; O’Neil, K.M.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical characterisation. Ann. Rheum. Dis. 2010, 69, 790–797. [Google Scholar] [CrossRef]
- Louie, C.Y.; Gomez, A.J.; Sibley, R.K.; Bass, D.; Longacre, T.A. Histologic features of gastrointestinal tract biopsies in IgA vasculitis (Henoch-Schönlein purpura). Am. J. Surg. Pathol. 2018, 42, 529–533. [Google Scholar] [CrossRef]
- De Rosa, G.; Pardeo, M.; Rigante, D. Current recommendations for the pharmacologic therapy in Kawasaki syndrome and management of its cardiovascular complications. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 301–308. [Google Scholar] [PubMed]
- Rigante, D.; Valentini, P.; Rizzo, D.; Leo, A.; De Rosa, G.; Onesimo, R.; De Nisco, A.; Angelone, D.F.; Compagnone, A.; Delogu, A.B. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol. Int. 2010, 30, 841–846. [Google Scholar] [CrossRef]
- Selvaskandan, H.; Cheung, C.K.; Muto, M.; Barratt, J. New strategies and perspectives on managing IgA nephropathy. Clin. Exp. Nephrol. 2019, 23, 577–588. [Google Scholar] [CrossRef]
- Xia, L.; Chen, M.; Zhang, H.; Zheng, X.; Bao, J.; Gao, J.; Zhu, C.; Sun, L.; Xia, H.; Zhang, X. Genome-wide association study of 7661 Chinese Han individuals and fine-mapping major histocompatibility complex identifies HLA-DRB1 as associated with IgA vasculitis. J. Clin. Lab. Anal. 2022, 36, e24457. [Google Scholar] [CrossRef]
- Atas, N.; Armagan, B.; Bodakci, E.; Satis, H.; Sari, A.; Bilge, N.S.Y.; Salman, R.B.; Yardımcı, G.K.; Babaoglu, H.; Guler, A.A.; et al. Familial Mediterranean fever is associated with a wide spectrum of inflammatory disorders: Results from a large cohort study. Rheumatol. Int. 2020, 40, 41–48. [Google Scholar] [CrossRef]
- Wang, Y.B.; Shan, N.N.; Chen, O.; Gao, Y.; Zou, X.; Wei, D.E.; Wang, C.X.; Zhang, Y. Imbalance of interleukin-18 and interleukin-18 binding protein in children with Henoch-Schönlein purpura. J. Int. Med. Res. 2011, 39, 2201–2208. [Google Scholar] [CrossRef]
- Sestan, M.; Srsen, S.; Kifer, N.; Sapina, M.; Batnozic Varga, M.; Ovuka, A.; Held, M.; Kozmar, A.; Frkovic, M.; Laskarin, G.; et al. Persistence and severity of cutaneous manifestations in IgA vasculitis is associated with development of IgA vasculitis nephritis in children. Dermatology 2022, 238, 340–346. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Xiang, R.; Luo, Y. Correlations of leukotriene B4 and 25-hydroxyvitamin D3 levels with disease severity in children with Henoch-Schonlein purpura. Clin. Lab. 2022, 68, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, W.P. The comparisons of vitamin D3 levels in IgA vasculitis across different subgroups and healthy children: A comparative study. Transl. Pediatr. 2023, 12, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Shi, M.F.; Chen, Y. Clinical effect of alfacalcidol in children with Henoch-Schönlein purpura: A prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front. Immunol. 2019, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Q.; Sun, S.; Tang, X.; Shen, W.; Liang, J.; Yao, G.; Geng, L.; Ding, S.; Chen, H.; et al. Factors associated with 25-hydroxyvitamin D level in Chinese hospitalized patients with systemic lupus erythematosus: A retrospective cohort study. Rheumatol. Int. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, S.; Andreoli, L.; Scarsi, M.; Zanola, A.; Dall’Ara, F.; Pizzorni, C.; Cutolo, M.; Airò, P.; Tincani, A. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.S.; Freitas, T.Q.; Bernardo, W.M.; Pereira, R.M.R. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine 2017, 96, e7024. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.S.; Jung, Y.K.; Lee, D.W. Relationship between vitamin D levels and intravenous immunoglobulin resistance in Kawasaki disease. Korean J. Pediatr. 2017, 60, 216–220. [Google Scholar] [CrossRef]
- Mahamid, M.; Agbaria, K.; Mahamid, A.; Nseir, W. Vitamin D linked to PFAPA syndrome. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 362–364. [Google Scholar] [CrossRef]
- Stagi, S.; Bertini, F.; Rigante, D.; Falcini, F. Vitamin D levels and effects of vitamin D replacement in children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Lim, I.S.; Choi, B.S.; Yi, D.Y. Analysis of seasonal tendencies in pediatric Henoch-Schönlein purpura and comparison with outbreak of infectious diseases. Medicine 2018, 97, e12217. [Google Scholar] [CrossRef] [PubMed]
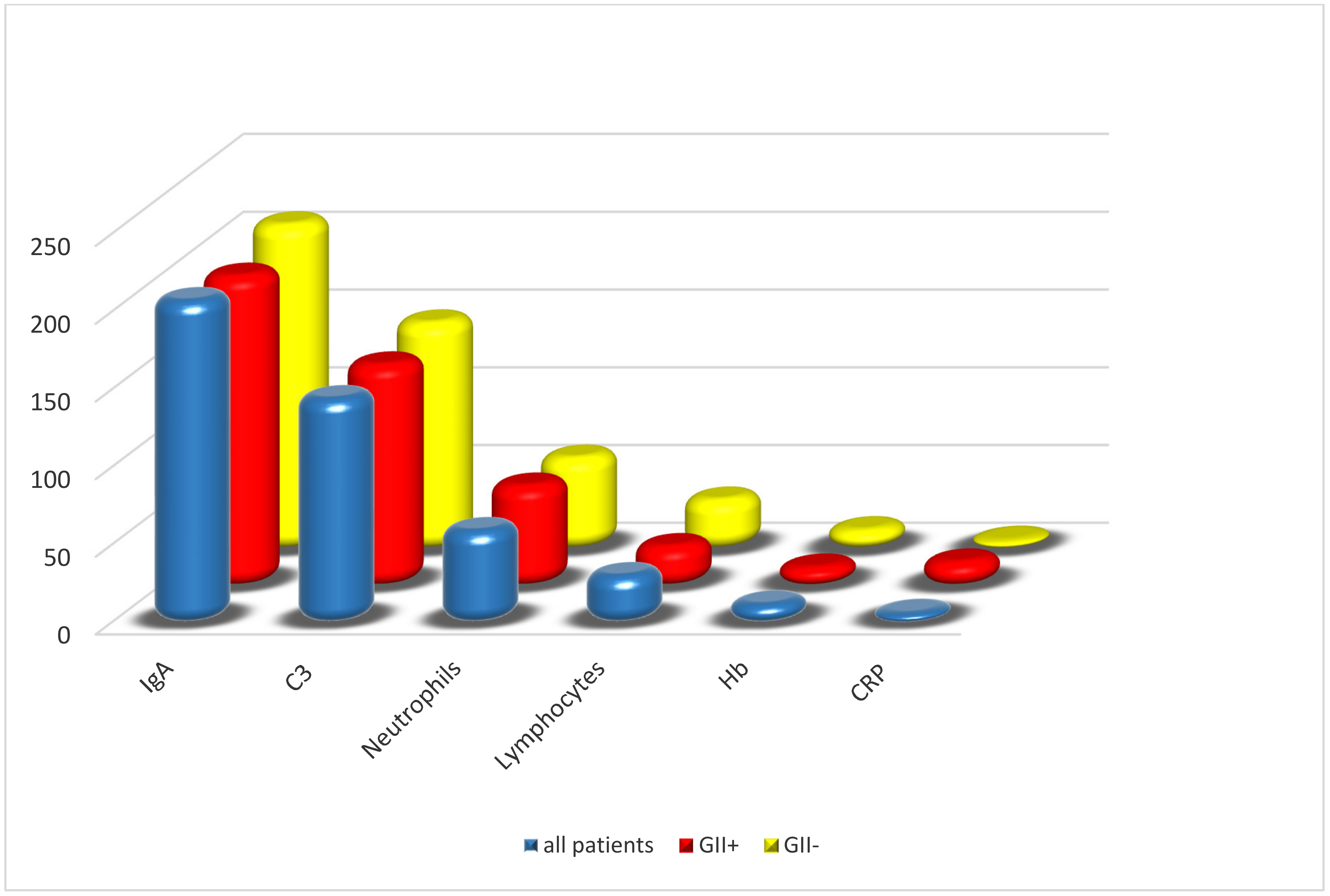
| All Patients | GII+ | GII− | p | |
|---|---|---|---|---|
| IgA (mg/dL, mean ± SD) | 208 ± 78 | 200 ± 60 | 209 ± 80 | 0.60 |
| C3 (mg/dL, mean ± SD) | 145 ± 39 | 143 ± 34 | 146 ± 39 | 0.79 |
| CRP (mg/L, median [IQR]) | 7.2 (3–17) | 16 (6–36) | 6 (3–16) | 0.24 |
| Neutrophils (%, mean ± SD) | 59.60 ± 13 | 65.17 ± 13 | 58.81 ± 12 | 0.02 |
| Lymphocytes (%, mean ± SD) | 31.40 ± 11 | 26.39 ± 11 | 32.12 ± 11 | 0.02 |
| Hb (g/dL, mean ± SD) | 12.7 ± 1 | 12.6 ± 1 | 12.7 ± 0.9 | 0.62 |
| All Patients | GII+ | GII- | p | |
|---|---|---|---|---|
| N/TN (%) | N/TN (%) | N/TN (%) | ||
| S. pyogenes+ | 35/147 (24) | 0/14 (0) | 35/98 (35) | 0.0001 |
| Vitamin D < 20 ng/mL | 21/195 (11) | 9/23 (39) | 12/172 (7) | 0.0001 |
| Vitamin D > 30 ng/mL | 20/19 (11) | 11/23 (48) | 143/172 (83) | 0.0001 |
| Rash > 50% of body | 56/195 (29) | 10/23 (43) | 46/172 (27) | 0.1 |
| Rash > 4 weeks | 38/195 (19) | 9/23 (41) | 29/172 (17) | 0.007 |
| Need for surgery | 4/195 (2) | 4/23 (17) | 0/172 (0) | - |
| Renal involvement | 29/195 (15) | 4/23 (17) | 25/172 (15) | 0.75 |
| Proteinuria | 29/195 (15) | 4/23 (17) | 25/172 (15) | 0.75 |
| Hematuria | 24/195 (12) | 3/23 (13) | 21/172 (12) | 1 |
| Berger’s disease | 3/195 (2) | 1/23 (4) | 2/172 (1) | 0.3 |
| Edema of extremities | 76/195 (39) | 9/23 (39) | 67/172 (39) | 0.99 |
| Joint involvement | 123/195 (11) | 16/23 (70) | 107/172 (62) | 0.49 |
| Headache | 9/195 (5) | 1/23 (4) | 8/172 (5) | 0.35 |
| Genital involvement | 21/195 (11) | 9/23 (39) | 12/172 (7) | 0.001 |
| Fever | 37/195 (19) | 6/23 (26) | 31/172 (18) | 0.35 |
| Use of corticosteroids | 53/195 (56) | 18/23 (78) | 35/172 (20) | 0.001 |
| Use of NSAIDs | 49/195 (25) | 6/23 (26) | 43/172 (25) | 1 |
| Positive prognosis | 192/195 (98) | 22/23 (96) | 169/171 (99) | 0.89 |
| GII+ | GII− | p | OR (95% CI) | |
|---|---|---|---|---|
| N/TN (%) | N/TN (%) | |||
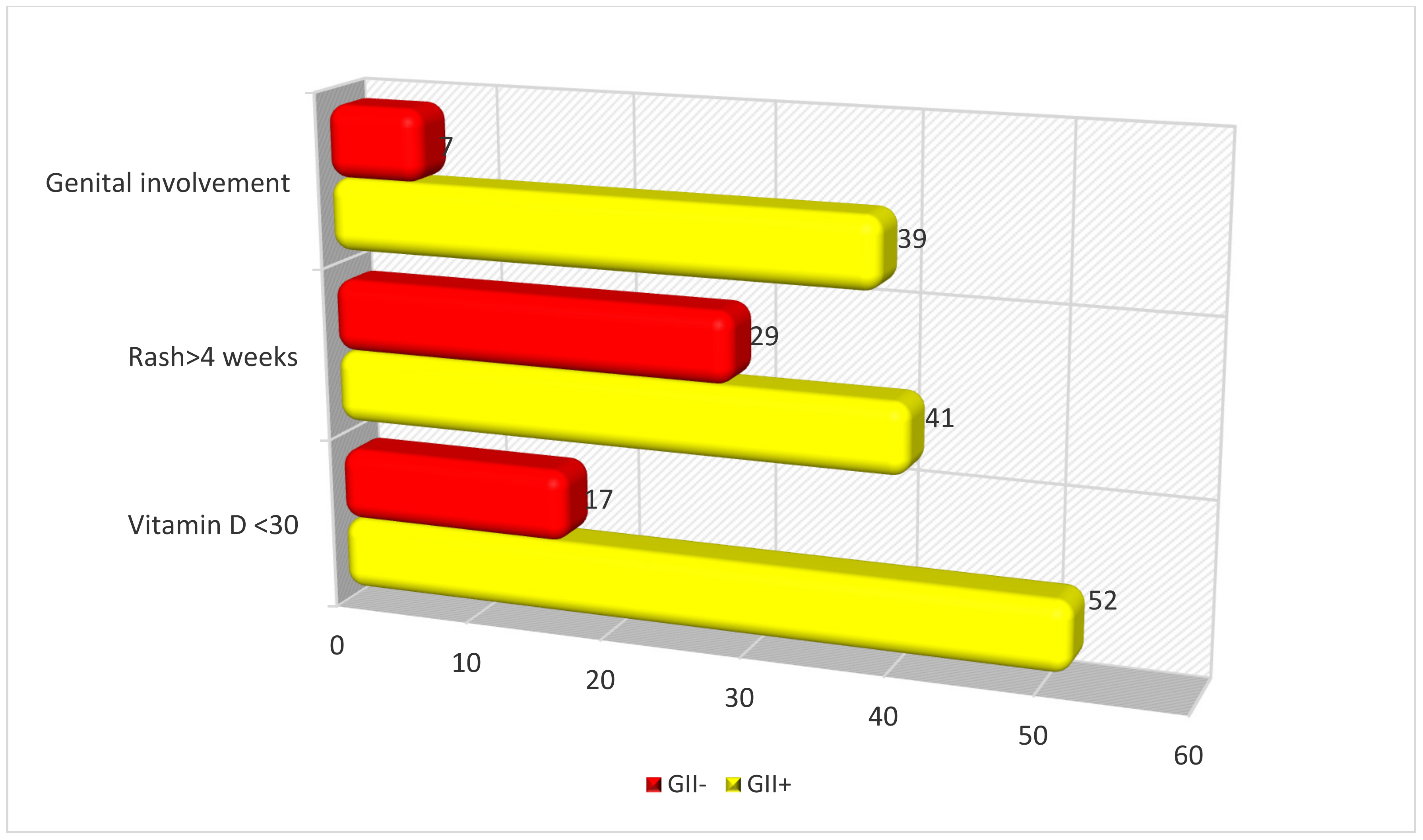
| Vitamin D < 30 ng/mL | 12/23 (52) | 29/172 (17) | 0.003 | 5.6 (1.8–17.6) |
| Rash > 4 weeks | 9/23 (41) | 29/172 (17) | 0.034 | 3.2 (1.1–9.2) |
| Genital involvement | 9/23 (39) | 12/172 (7) | 0.05 | 7.1 (1.8–28.0) |
| All Patients | Vitamin D < 30 | Vitamin D > 30 | p | |
|---|---|---|---|---|
| IgA (mg/dL, mean ± SD) | 208 ± 78 | 223 ± 81 | 204 ± 77 | 0.17 |
| C3 (mg/dL, mean ± SD) | 145 ± 39 | 143 ± 47 | 146 ± 36 | 0.74 |
| CRP (mg/L, median [IQR]) | 7.2 (3–17) | 12 (3–28) | 6 (3–15) | 0.24 |
| Neutrophils (%, mean ± SD) | 59.60 ± 13 | 61.80 ± 11 | 58.97 ± 13 | 0.18 |
| Lymphocytes (%, mean ± SD) | 31.40 ± 11 | 29.71 ± 11 | 31.91 ± 11 | 0.27 |
| Hb (g/dL, mean ± SD) | 12.7 ± 1 | 12.6 ± 1 | 12.7 ± 1 | 0.86 |
| All Patients | Vitamin D < 30 | Vitamin D > 30 | p | |
|---|---|---|---|---|
| N/TN (%) | N/TN (%) | N/TN (%) | ||
| S. pyogenes + | 35/147 (24) | 4/32 (12) | 31/115 (27) | 0.1 |
| GI involvement | 33/195 (17) | 12/41 (29) | 11/154 (7) | 0.0001 |
| Rash > 50% of body | 56/195 (29) | 15/41 (37) | 41/154 (27) | 0.21 |
| Rash > 4 weeks | 38/195 (19) | 14/41 (34) | 25/154 (16) | 0.01 |
| Need for surgery | 4/195 (2) | 4/41 (10) | 0/154 (0) | 0.001 |
| Renal involvement | 29/195 (15) | 12/41 (29) | 17/154 (11) | 0.004 |
| Proteinuria | 29/195 (15) | 10/41 (24) | 10/154 (6) | 0.001 |
| Hematuria | 24/195 (12) | 10/41 (24) | 14/154 (9) | 0.01 |
| Berger’s disease | 3/195 (2) | 3/41 (7) | 0/154 (0) | 0.01 |
| Edema of extremities | 76/195 (39) | 18/41 (44) | 58/154 (38) | 0.46 |
| Joint involvement | 123/195 (11) | 30/41 (73) | 93/154 (60) | 0.49 |
| Headache | 9/195 (5) | 2/41 (5) | 7/154 (5) | 0.9 |
| Genital involvement | 7/154 (5) | 7/154 (5) | 11/154 (7) | 0.01 |
| Fever | 37/195 (19) | 11/41 (27) | 26/154 (17) | 0.1 |
| Use of corticosteroids | 53/195 (56) | 29/41 (71) | 24/154 (16) | 0.0001 |
| Use of NSAIDs | 49/195 (25) | 9/41 (22) | 40/154 (26) | 0.59 |
| Positive prognosis | 192/195 (99) | 38/41 (93) | 153/153 (100) | 0.03 |
| Vitamin D < 30 | Vitamin D > 30 | p | OR (95% CI) | |
|---|---|---|---|---|
| N/TN (%) | N/TN (%) | |||
| GI involvement | 12/41 (29) | 11/154 (7) | 0.02 | 1.7 (1.24–8.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigante, D.; Guerriero, C.; Silvaroli, S.; Paradiso, F.V.; Sodero, G.; Laferrera, F.; Franceschi, F.; Candelli, M. Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study. Children 2024, 11, 215. https://doi.org/10.3390/children11020215
Rigante D, Guerriero C, Silvaroli S, Paradiso FV, Sodero G, Laferrera F, Franceschi F, Candelli M. Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study. Children. 2024; 11(2):215. https://doi.org/10.3390/children11020215
Chicago/Turabian StyleRigante, Donato, Cristina Guerriero, Sara Silvaroli, Filomena Valentina Paradiso, Giorgio Sodero, Francesco Laferrera, Francesco Franceschi, and Marcello Candelli. 2024. "Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study" Children 11, no. 2: 215. https://doi.org/10.3390/children11020215
APA StyleRigante, D., Guerriero, C., Silvaroli, S., Paradiso, F. V., Sodero, G., Laferrera, F., Franceschi, F., & Candelli, M. (2024). Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study. Children, 11(2), 215. https://doi.org/10.3390/children11020215










