The Association Between In Utero Exposure to Painkillers and Trajectories of Hyperactivity and Emotional Problems in Children with Autism Compared with Neurotypical Peers
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Materials and Measures
2.4. Statistical Analysis
3. Results
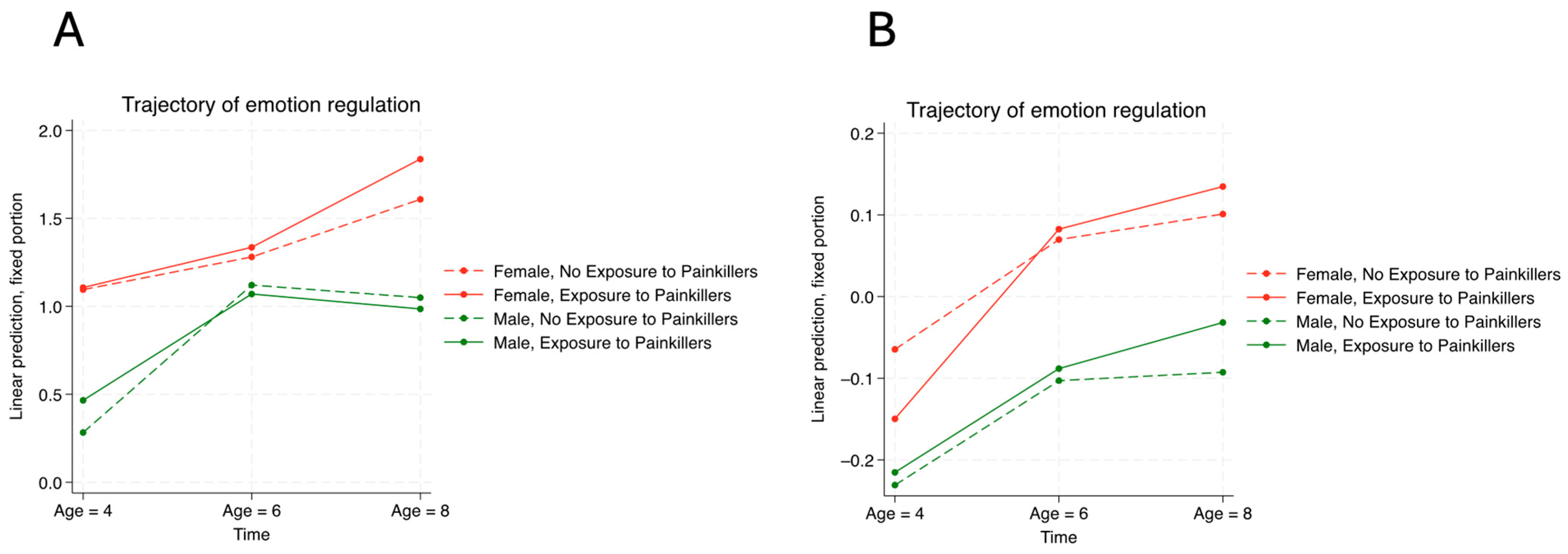
4. Discussion
4.1. Key Findings
4.2. Comparison of Current Findings and the Literature
4.3. Limitations
4.4. Conclusions and Future Research Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bérard, A.; Zhao, J.P.; Sheehy, O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: An updated analysis of the Quebec Pregnancy Cohort. BMJ Open 2017, 7, e013372. [Google Scholar] [CrossRef]
- Blotière, P.-O.; Raguideau, F.; Weill, A.; Elefant, E.; Perthus, I.; Goulet, V.; Rouget, F.; Zureik, M.; Coste, J.; Dray-Spira, R. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 2019, 93, e167–e180. [Google Scholar] [CrossRef] [PubMed]
- Damkier, P.; Brønniche, L.M.S.; Korch-Frandsen, J.F.B.; Broe, A. In utero exposure to antibiotics and risk of congenital malformations: A population-based study. Am. J. Obstet. Gynecol. 2019, 221, 648.e1–648.e15. [Google Scholar] [CrossRef] [PubMed]
- Sujan, A.C.; Rickert, M.E.; Öberg, A.S.; Quinn, P.D.; Hernández-Díaz, S.; Almqvist, C.; Lichtenstein, P.; Larsson, H.; D’onofrio, B.M. Associations of Maternal Antidepressant Use During the First Trimester of Pregnancy with Preterm Birth, Small for Gestational Age, Autism Spectrum Disorder, and Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA 2017, 317, 1553–1562. [Google Scholar] [CrossRef]
- Haas, D.M.; Marsh, D.J.; Dang, D.T.; Parker, C.B.D.; Wing, D.A.M.; Simhan, H.N.; Grobman, W.A.M.; Mercer, B.M.; Silver, R.M.; Hoffman, M.K.; et al. Prescription and Other Medication Use in Pregnancy. Obstet. Gynecol. 2018, 131, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—A call for precautionary action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Ystrom, E.; Gustavson, K.; Brandlistuen, R.E.; Knudsen, G.P.; Magnus, P.; Susser, E.; Davey Smith, G.; Stoltenberg, C.; Surén, P.; Håberg, S.E.; et al. Prenatal exposure to acetaminophen risk of ADHD. Pediatrics 2017, 140, e20163840. [Google Scholar] [CrossRef]
- Liew, Z.; Ritz, B.; Virk, J.; Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. 2016, 9, 951–958. [Google Scholar] [CrossRef]
- Stergiakouli, E.; Thapar, A.; Smith, G.D. Association of acetaminophen use during pregnancy with behavioral problems in childhood: Evidence against confounding. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Lai, M.C.; Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry 2019, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lussier, A.A.; Zhu, Y.; Smith, B.J.; Cerutti, J.; Fisher, J.; E Melton, P.; Wood, N.M.; Cohen-Woods, S.; Huang, R.-C.; Mitchell, C.; et al. Association between the timing of childhood adversity and epigenetic patterns across childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) prospective cohort. Lancet Child. Adolesc. Health 2023, 7, 532–543. [Google Scholar] [CrossRef]
- Yeoh, S.L.; Eastwood, J.; Wright, I.M.; Morton, R.; Melhuish, E.; Ward, M.; Oei, J.L. Cognitive and Motor Outcomes of Children with Prenatal Opioid Exposure a Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e197025. [Google Scholar] [CrossRef] [PubMed]
- Sanson, A.V.; Nicholson, J.; Ungerer, J.; Zubrick, S.; Wilson, K.; Ainley, J.; Berthelsen, D.; Bittman, M.; Broom, D.; Harrison, L.; et al. Introducing the Longitudinal Study of Australian Children. LSAC Discussion Paper. 2002. Available online: https://openresearch-repository.anu.edu.au/server/api/core/bitstreams/9f3020a9-970f-43bc-b1a4-307e4dbbf8c5/content (accessed on 19 December 2024).
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child. Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Bergström, M.; Baviskar, S. A Systematic Review of Some Reliability and Validity Issues regarding the Strengths and Difficulties Questionnaire Focusing on Its Use in Out-of-Home Care. J. Evid.-Based Soc. Work. 2020, 18, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Fowler, T.; Rice, F.; Scourfield, J.; Bree, M.v.D.; Thomas, H.; Harold, G.; Hay, D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am. J. Psychiatry 2003, 160, 1985–1989. [Google Scholar] [CrossRef]
- Jansone, K.; Eichler, A.; Fasching, P.A.; Kornhuber, J.; Kaiser, A.; Millenet, S.; Banaschewski, T.; Nees, F. Association of Maternal Smoking during Pregnancy with Neurophysiological and ADHD-Related Outcomes in School-Aged Children. Int. J. Environ. Res. Public. Health 2023, 20, 4716. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.; Warne, N.; Rice, F.; Langley, K.; Maughan, B.; Pickles, A.; Thapar, A.; Collishaw, S. Using a cross-cohort comparison design to test the role of maternal smoking in pregnancy in child mental health and learning: Evidence from two UK cohorts born four decades apart. Int. J. Epidemiol. 2021, 49, 390–399. [Google Scholar] [CrossRef]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, K.J.; Chow, C.H.T.; Dobson, K.G.; Pope, E.I.; Schmidt, L.A.; Van Lieshout, R.J. Mental health of extremely low birth weight survivors: A systematic review and meta-analysis. Psychol. Bull. 2017, 143, 347–383. [Google Scholar] [CrossRef] [PubMed]
- Galéra, C.; Côté, S.M.; Bouvard, M.P.; Pingault, J.-B.; Melchior, M.; Michel, G.; Boivin, M.; Tremblay, R.E. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch. Gen. Psychiatry 2011, 68, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.X.; Liu, C.; Schoeler, T.; Cecil, C.A.; Barker, E.D.; Viding, E.; Greven, C.U.; Pingault, J. The role of birth weight on the causal pathway to child and adolescent ADHD symptomatology: A population-based twin differences longitudinal design. J. Child. Psychol. Psychiatry 2018, 59, 1036–1043. [Google Scholar] [CrossRef]
- Hultman, C.M.; Torrång, A.; Tuvblad, C.; Cnattingius, S.; Larsson, J.O.; Lichtenstein, P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: A prospective Swedish twin study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, E.; Sjölander, A.; Almqvist, C.; Anckarsäter, H.; D’Onofrio, B.M.; Lichtenstein, P.; Larsson, H. Birth weight as an independent predictor of ADHD symptoms: A within-twin pair analysis. J. Child. Psychol. Psychiatry 2015, 56, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.P.; Bolat, G.U.; Bolat, H.; Matijasevich, A.; Santos, I.S.; Silveira, R.C.; Procianoy, R.S.; Rohde, L.A.; Moreira-Maia, C.R. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: A meta-analysis. Pediatrics 2018, 141, e20171645. [Google Scholar] [CrossRef] [PubMed]
- Melchior, M.; Hersi, R.; Van Der Waerden, J.; Larroque, B.; Saurel-Cubizolles, M.J.; Chollet, A.; Galéra, C. Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: The EDEN mother-child birth cohort study. Eur. Psychiatry 2015, 30, 562–568. [Google Scholar] [CrossRef]
- Healy, E. Cognitive, psycho-social, and medical outcomes of very low birth weight infants at age 10–14 years in Ireland. Eur. Child. Adolesc. Psychiatry 2013, 22, S142. [Google Scholar]
- Litt, J.S.; Minich, N.; Taylor, H.G.; Tiemeier, H. The Inter-Relationships of Extremely Low Birth Weight, Asthma, and Behavior: A Study of Common Cause, Mediation, and Moderation. Acad. Pediatr. 2020, 20, 975–982. [Google Scholar] [CrossRef]
- Solomon, S.R.; Sawilowsky, S.S. Impact of rank-based normalizing transformations on the accuracy of test scores. J. Mod. Appl. Stat. Methods 2009, 8, 448–462. [Google Scholar] [CrossRef]
- Balalian, A.A.; Graeve, R.; Richter, M.; Fink, A.; Kielstein, H.; Martins, S.S.; Philbin, M.M.; Factor-Litvak, P. Prenatal exposure to opioids and neurodevelopment in infancy and childhood: A systematic review. Front. Pediatr. 2023, 11, 1071889. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lawal, O.D.; Belviso, N.; Matson, K.L.; Wang, S.; Quilliam, B.J.; Meador, K.J. Association Between Prenatal Opioid Exposure and Neurodevelopmental Outcomes in Early Childhood: A Retrospective Cohort Study. Drug Saf. 2021, 44, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Saraiya, N.; Kuzniewicz, M.W. Prenatal Opioid Exposure and Neurodevelopmental Outcomes. J. Neurosurg. Anesthesiol. 2023, 35, 142–146. [Google Scholar] [CrossRef]
- Conradt, E.; Flannery, T.; Aschner, J.L.; Annett, R.D.; Croen, L.A.; Duarte, C.S.; Friedman, A.M.; Guille, C.; Hedderson, M.M.; Hofheimer, J.A.; et al. Prenatal opioid exposure: Neurodevelopmental consequences and future research priorities. Pediatrics 2019, 144, e20190128. [Google Scholar] [CrossRef]
- Trønnes, J.N.; Lupattelli, A.; Ystrom, E.; Nordeng, H. Analysis of Prenatal Exposure to Opioid Analgesics and Scholastic Skills in Children in Fifth Grade in Norway. JAMA Netw. Open 2022, 5, e2222425. [Google Scholar] [CrossRef]
- Kwok, J.; Luedecke, E.; Hall, H.A.; Murray, A.L.; Auyeung, B. Analgesic drug use in pregnancy and neurodevelopment outcomes: An umbrella review. Neurosci. Biobehav. Rev. 2022, 136, 104607. [Google Scholar] [CrossRef]
- Trønnes, J.N.; Lupattelli, A.; Handal, M.; Skurtveit, S.; Ystrom, E.; Nordeng, H. Association of Timing and Duration of Prenatal Analgesic Opioid Exposure with Attention-Deficit/Hyperactivity Disorder in Children. JAMA Netw. Open 2021, 4, e2124324. [Google Scholar] [CrossRef] [PubMed]
- Reuter, I.; Knaup, S.; Romanos, M.; Lesch, K.P.; Drepper, C.; Lillesaar, C. Developmental exposure to acetaminophen does not induce hyperactivity in zebrafish larvae. J. Neural Transm. 2016, 123, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, R.; Levine, H.; Gorelik, E.; Reif, S.; Perlman, A.; Matok, I. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: A systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am. J. Epidemiol. 2018, 187, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jones, J.P.; Anderson, L.G.; Konsoula, Z.; Nevison, C.D.; Reissner, K.J.; Parker, W. Acetaminophen causes neurodevelopmental injury in susceptible babies and children: No valid rationale for controversy. Clin. Exp. Pediatr. 2023, 67, 126–139. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Kriebel, D. Prenatal and perinatal analgesic exposure and autism: An ecological link. Environ. Health 2013, 12, 41. [Google Scholar] [CrossRef]
- Chang, H.; Shaw, D.S.; Cheong, J. The Development of Emotional and Behavioral Control in Early Childhood:Heterotypic Continuity and Relations to Early School Adjustment. J. Child. Adolesc. Behav. 2015, 3, 204. [Google Scholar] [CrossRef]
- Ogundele, M.O. Behavioural and emotional disorders in childhood: A brief overview for paediatricians. World J. Clin. Pediatr. 2018, 7, 9–26. [Google Scholar] [CrossRef]
- Ozerova, E.; Martinsone, B.; Cefai, C.; Conte, E. Social-Emotional Skills, Behavioural Problems and Learning Outcomes of Elementary School Children. In Proceedings of the ATEE 2022 Annual Conference, Riga, Latvia, 29–31 August 2022. [Google Scholar] [CrossRef]
- Maguire, L.K.; Niens, U.; McCann, M.; Connolly, P. Emotional development among early school-age children: Gender differences in the role of problem behaviours. Educ. Psychol. 2016, 36, 1408–1428. [Google Scholar] [CrossRef] [PubMed]
- Mkhitaryan, M.; Avetisyan, T.; Mkhoyan, A.; Avetisyan, L.; Yenkoyan, K. A case–control study on pre-, peri-, and neonatal risk factors associated with autism spectrum disorder among Armenian children. Sci. Rep. 2024, 14, 12308. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Ames, J.L.; Qian, Y.; Alexeeff, S.; Ashwood, P.; Gunderson, E.P.; Wu, Y.W.; Boghossian, A.S.; Yolken, R.; Van de Water, J.; et al. Inflammatory Conditions During Pregnancy and Risk of Autism and Other Neurodevelopmental Disorders. Biol. Psychiatry Glob. Open Sci. 2024, 4, 39–50. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 1–16. [Google Scholar] [CrossRef]

| Exposure (+) | Exposure (−) | ||||
|---|---|---|---|---|---|
| Percentage | SD | Percentage | SD | p-Value | |
| ASD Diagnosis | 3.99% | 0.30% | 3.74% | 0.25% | 0.492 |
| Gender (Male) | 51.09% | 0.60% | 51.03% | 0.55% | 0.939 |
| Maternal Smoking During Pregnancy | 15.73% | 0.44% | 12.76% | 0.37% | <0.001 |
| Mean | SD | Mean | SD | p-Value | |
| Birth Weight (gm) | 3424.79 | 555.92 | 3397.89 | 579.2 | 0.0036 |
| Hyperactivity (Mean) * | 3.45 | 2.35 | 3.37 | 2.3 | 0.0843 |
| Emotional Problems * | 1.69 | 1.75 | 1.63 | 1.74 | 0.0955 |
| Children with ASD | ||||
|---|---|---|---|---|
| Predictor | Hyperactivity | Emotional Problems | ||
| Coefficient | p-Value | Coefficient | p-Value | |
| Time | ||||
| Age 6 | 0.4 | 0.165 | 0.19 | 0.568 |
| Age 8 | 0.59 | 0.068 | 0.51 | 0.309 |
| Painkiller Exposure | 0.11 | 0.831 | 0.03 | 0.960 |
| Painkiller Exposure × Time Interaction | ||||
| Painkiller × Time (Age 6) | −0.2 | 0.594 | 0.04 | 0.957 |
| Painkiller × Time (Age 8) | −0.06 | 0.887 | 0.22 | 0.746 |
| Sex (Male versus Female) | 0.01 | 0.983 | −0.8 | 0.118 |
| Painkiller × Sex | 0.14 | 0.819 | 0.17 | 0.802 |
| Maternal Smoking | −0.07 | 0.785 | 0.56 | 0.004 |
| Birth Weight | 0 | 0.896 | 0 | 0.711 |
| Income Group | −0.04 | 0.673 | 0.01 | 0.943 |
| Neurotypical Children | ||||
| Predictor | Hyperactivity | Emotional Problems | ||
| Coefficient | p-Value | Coefficient | p-Value | |
| Time | ||||
| Age 6 | 0.03 | 0.301 | 0.13 | <0.001 |
| Age 8 | −0.01 | 0.569 | 0.16 | <0.001 |
| Painkiller Exposure | −0.06 | 0.081 | −0.08 | 0.116 |
| Painkiller Exposure × Time Interaction | ||||
| Painkiller × Time (Age 6) | −0.02 | 0.761 | 0.1 | 0.063 |
| Painkiller × Time (Age 8) | 0.01 | 0.738 | 0.12 | 0.013 |
| Sex (Male versus Female) | 0.18 | 0.0001 | −0.16 | <0.001 |
| Painkiller × Sex | 0.14 | 0.008 | 0.1 | 0.115 |
| Maternal Smoking | 0.25 | 0.0001 | 0.03 | 0.62 |
| Birth Weight | 0 | 0.17 | 0 | 0.135 |
| Income Group | −0.14 | 0.0001 | −0.09 | <0.001 |
| Factors Influencing Overall Hyperactivity Symptoms | Factors Influencing Overall Emotional Problems | Does Painkiller Exposure Influence the Trajectory of Hyperactivity Symptoms? | Does Painkiller Exposure Influence the Trajectory of Emotional Problems? | |
|---|---|---|---|---|
| Children with ASD | None | None | No | No |
| Neurotypical Children | Gender; Maternal Smoking; Family Income | Gender; Gender–Painkiller Exposure Interaction; Family Income | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-I.; Khin, K.S.; John, J.R.; Walker, A.K.; Chen, Y.-C.; Nayeem, N.; Messias, E. The Association Between In Utero Exposure to Painkillers and Trajectories of Hyperactivity and Emotional Problems in Children with Autism Compared with Neurotypical Peers. Children 2024, 11, 1558. https://doi.org/10.3390/children11121558
Lin P-I, Khin KS, John JR, Walker AK, Chen Y-C, Nayeem N, Messias E. The Association Between In Utero Exposure to Painkillers and Trajectories of Hyperactivity and Emotional Problems in Children with Autism Compared with Neurotypical Peers. Children. 2024; 11(12):1558. https://doi.org/10.3390/children11121558
Chicago/Turabian StyleLin, Ping-I., Kyi Shinn Khin, James R. John, Adam K. Walker, Yi-Chia Chen, Nawar Nayeem, and Erick Messias. 2024. "The Association Between In Utero Exposure to Painkillers and Trajectories of Hyperactivity and Emotional Problems in Children with Autism Compared with Neurotypical Peers" Children 11, no. 12: 1558. https://doi.org/10.3390/children11121558
APA StyleLin, P.-I., Khin, K. S., John, J. R., Walker, A. K., Chen, Y.-C., Nayeem, N., & Messias, E. (2024). The Association Between In Utero Exposure to Painkillers and Trajectories of Hyperactivity and Emotional Problems in Children with Autism Compared with Neurotypical Peers. Children, 11(12), 1558. https://doi.org/10.3390/children11121558






