Subjective and Objective Assessment of the Preferred Rotational Cervical Spine Position in Infants with an Upper Cervical Spine Dysfunction: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Outcome
2.2. Secondary Outcome
2.3. Study Procedure
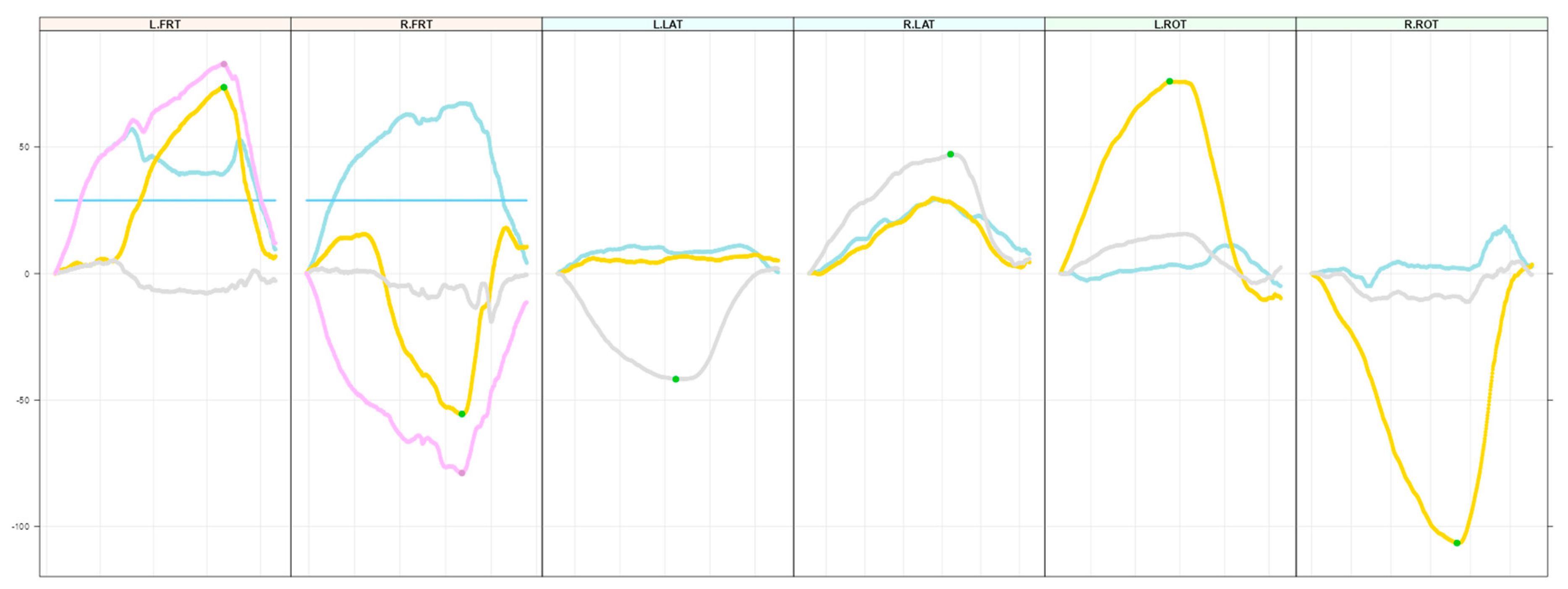
2.4. Data Preparation
2.5. Statistical Analysis
3. Results
3.1. Demographics and Descriptive Statistics
3.2. Subjective and Objective Assessments
3.3. Test–Retest Reliability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomczak, K.K.; Rosman, N.P. Torticollis. J. Child Neurol. 2013, 28, 365–378. [Google Scholar] [CrossRef] [PubMed]
- van Vlimmeren, L.A.; Helders, P.J.; van Adrichem, L.N.; Engelbert, R.H. Diagnostic strategies for the evaluation of asymmetry in infancy—A review. Eur. J. Pediatr. 2004, 163, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sacher, R. Geburtstrauma und (Hals-)Wirbelsäule. Manuelle Medizin 2003, 41, 9–14. [Google Scholar] [CrossRef]
- Sacher, R. Geburtstrauma und (Hals-)Wirbelsäule. Manuelle Medizin 2003, 41, 15–21. [Google Scholar] [CrossRef]
- Driehuis, F.; Keijsers, N.L.W.; Nijhuis-van der Sanden, M.W.G.; De Bie, R.A.; Staal, J.B.; Hoogeboom, T.J. Measurement of range-of-motion in infants with indications of upper cervical dysfunction using the Flexion-Rotation-Test and Lateral-Flexion-Test: A blinded inter-rater reliability study in a clinical practice setting. J. Man. Manip. Ther. 2020, 29, 40–50. [Google Scholar] [CrossRef]
- Hautopp, L.; Wester, S.; Bang, B.; Buus, L.; Grindsted, J.; Christensen, K.; Knudsen, B.; Vinther, A. Benefit of physiotherapeutic treatment in children with torticollis. Dan. Med. J. 2014, 61, A4970. [Google Scholar]
- Kind, H.P.; Kränzlin, P. Neugeborenen-Tortikollis; Zürcherische Arbeitsgemeinschaft Praktizierender Pädiater (ZAPP): Zurich, Switzerland, 1994. [Google Scholar]
- Rogers, G.F.; Oh, A.K.; Mulliken, J.B. The role of congenital muscular torticollis in the development of deformational plagiocephaly. Plast. Reconstr. Surg. 2009, 123, 643–652. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Coulter, C.; Sargent, B. Physical Therapy Management of Congenital Muscular Torticollis: A 2018 Evidence-Based Clinical Practice Guideline from the APTA Academy of Pediatric Physical Therapy. Pediatr. Phys. Ther. 2018, 30, 240–290. [Google Scholar] [CrossRef]
- Sweeney, J.K.; Blackburn, S. Neonatal physiological and behavioral stress during neurological assessment. J. Perinat. Neonatal Nurs. 2013, 27, 242–252; quiz 253–254. [Google Scholar] [CrossRef]
- LoBue, V.; Reider, L.B.; Kim, E.; Burris, J.L.; Oleas, D.S.; Buss, K.A.; Pérez-Edgar, K.; Field, A.P. The importance of using multiple outcome measures in infant research. Infancy 2020, 25, 420–437. [Google Scholar] [CrossRef]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.-S. Validity and Reliability of Wearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.W.M.; Palsson, T.S.; Djurtoft, C.; Simonsen, M.B. Agreement between a 3D camera system and an inertial measurement unit for assessing the range of motion, head repositioning accuracy and quality of movement during neck and head movements. Eur. J. Physiother. 2024, 26, 103–110. [Google Scholar] [CrossRef]
- Papi, E.; Koh, W.S.; McGregor, A.H. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bellosta-Lopez, P.; Simonsen, M.B.; Palsson, T.S.; Djurtoft, C.; Hirata, R.P.; Christensen, S.W.M. Validity of an inertial measurement unit for the assessment of range and quality of movement during head and thoracic spine movements. Musculoskelet. Sci. Pract. 2023, 66, 102826. [Google Scholar] [CrossRef]
- Raya, R.; Garcia-Carmona, R.; Sanchez, C.; Urendes, E.; Ramirez, O.; Martin, A.; Otero, A. An Inexpensive and Easy to Use Cervical Range of Motion Measurement Solution Using Inertial Sensors. Sensors 2018, 18, 2582. [Google Scholar] [CrossRef]
- Carmona-Perez, C.; Garrido-Castro, J.L.; Torres Vidal, F.; Alcaraz-Clariana, S.; Garcia-Luque, L.; Alburquerque-Sendin, F.; Rodrigues-de-Souza, D.P. Concurrent Validity and Reliability of an Inertial Measurement Unit for the Assessment of Craniocervical Range of Motion in Subjects with Cerebral Palsy. Diagnostics 2020, 10, 80. [Google Scholar] [CrossRef]
- Barrois, R.; Tervil, B.; Cacioppo, M.; Barnerias, C.; Deladriere, E.; Leloup-Germa, V.; Hervé, A.; Oudre, L.; Ricard, D.; Vidal, P.P.; et al. Acceptability, validity and responsiveness of inertial measurement units for assessing motor recovery after gene therapy in infants with early onset spinal muscular atrophy: A prospective cohort study. J. Neuroeng. Rehabil. 2024, 21, 183. [Google Scholar] [CrossRef]
- Blok, J.; Poggensee, K.L.; Lemus, D.; Kok, M.; Pangalila, R.F.; Vallery, H.; Deferme, J.; Toussaint-Duyster, L.C.; Horemans, H. Quantification of the development of trunk control in healthy infants using inertial measurement units. IEEE Int. Conf. Rehabil. Robot. 2022, 2022, 1–6. [Google Scholar]
- World Health Organization. 2-Month Head Circumference Increments Girls/Boys 2024. Available online: https://www.who.int/tools/child-growth-standards/standards/head-circumference-velocity (accessed on 12 June 2024).
- Challis, J.H. Quaternions as a solution to determining the angular kinematics of human movement. BMC Biomed. Eng. 2020, 2, 5. [Google Scholar] [CrossRef][Green Version]
- Team, R.C. R: A Language and Enviroment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Dodge, Y. Spearman Rank Correlation Coefficient. The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; pp. 502–505. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.K.; Hao, Q.; Kuspinar, A.; D’Amore, C.; Scime, G.; Ma, J.; Mayhew, A.; Bassim, C.; Wolfson, C.; Kirkland, S.; et al. Reliability and Minimal Detectable Change Values for Performance-Based Measures of Physical Functioning in the Canadian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- De Vet, H.; Terwee, C.; Mokkink, L.; Knol, D. Measurement in Medicine; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Hayton, T.; Gross, A.; Basson, A.; Olson, K.; Ang, O.; Milne, N.; Pool, J. Psychometric properties of clinician-reported and performance-based outcomes cited in a scoping review on spinal manipulation and mobilization for pediatric populations with diverse medical conditions: A systematic review. J. Man. Manip. Ther. 2024, 32, 255–283. [Google Scholar] [CrossRef]
- Waters, S.F.; West, T.V.; Mendes, W.B. Stress contagion: Physiological covariation between mothers and infants. Psychol. Sci. 2014, 25, 934–942. [Google Scholar] [CrossRef]
- Slocombe, K.E.; Seed, A.M. Cooperation in children. Curr. Biol. 2019, 29, R470–R473. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langenfeld, A.; Paravicini, I.; Hobaek Siegenthaler, M.; Wehrli, M.; Häusler, M.; Bergander, T.; Schweinhardt, P. Subjective and Objective Assessment of the Preferred Rotational Cervical Spine Position in Infants with an Upper Cervical Spine Dysfunction: A Cross-Sectional Study. Children 2024, 11, 1515. https://doi.org/10.3390/children11121515
Langenfeld A, Paravicini I, Hobaek Siegenthaler M, Wehrli M, Häusler M, Bergander T, Schweinhardt P. Subjective and Objective Assessment of the Preferred Rotational Cervical Spine Position in Infants with an Upper Cervical Spine Dysfunction: A Cross-Sectional Study. Children. 2024; 11(12):1515. https://doi.org/10.3390/children11121515
Chicago/Turabian StyleLangenfeld, Anke, Inga Paravicini, Mette Hobaek Siegenthaler, Martina Wehrli, Melanie Häusler, Torsten Bergander, and Petra Schweinhardt. 2024. "Subjective and Objective Assessment of the Preferred Rotational Cervical Spine Position in Infants with an Upper Cervical Spine Dysfunction: A Cross-Sectional Study" Children 11, no. 12: 1515. https://doi.org/10.3390/children11121515
APA StyleLangenfeld, A., Paravicini, I., Hobaek Siegenthaler, M., Wehrli, M., Häusler, M., Bergander, T., & Schweinhardt, P. (2024). Subjective and Objective Assessment of the Preferred Rotational Cervical Spine Position in Infants with an Upper Cervical Spine Dysfunction: A Cross-Sectional Study. Children, 11(12), 1515. https://doi.org/10.3390/children11121515




