Using Vital Signs for the Early Prediction of Necrotizing Enterocolitis in Preterm Neonates with Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Preprocessing
2.3. Model Development and Analysis
3. Results
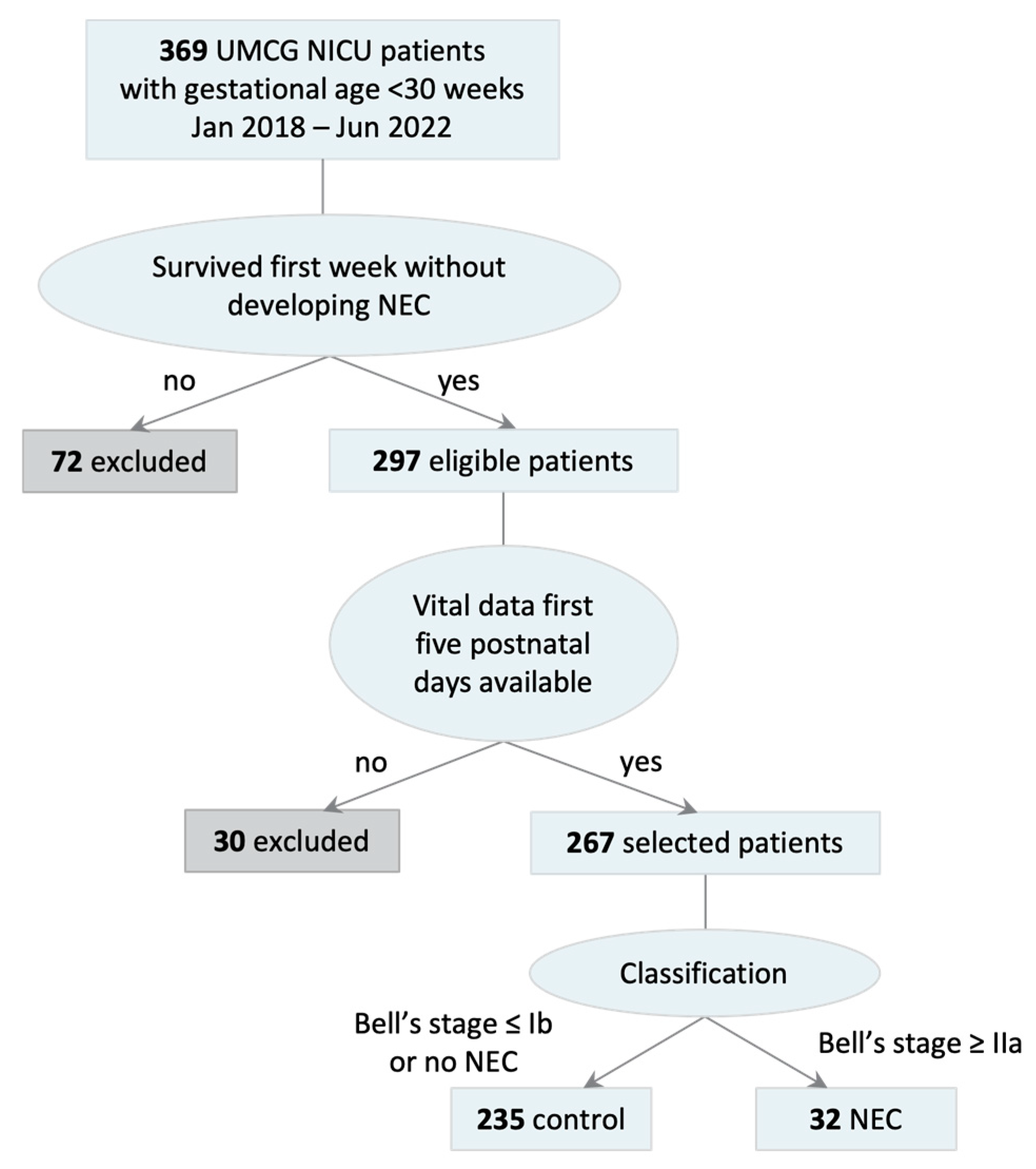
3.1. Included Patients
3.2. Prediction Models
3.3. Vital Sign Contribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, K.E. Clinical Predictors of Necrotizing Enterocolitis in Premature Infants. Nurs. Res. 2008, 57, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.L.; Doheny, K.K.; Travagli, R.A. Necrotizing Enterocolitis: It’s Not All in the Gut. Exp. Biol. Med. 2020, 245, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.M.; Berryhill, T.F.; Ellenburg, J.L.; Jilling, T.; Cleveland, D.S.; Lorenz, R.G.; Martin, C.A. Pathogenesis of Necrotizing Enterocolitis: Modeling the Innate Immune Response. Am. J. Pathol. 2015, 185, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zani, A.; Pierro, A. Necrotizing Enterocolitis: Controversies and Challenges. F1000Research 2015, 4, 1373. [Google Scholar] [CrossRef] [PubMed]
- Flahive, C.; Schlegel, A.; Mezoff, E.A. Necrotizing Enterocolitis: Updates on Morbidity and Mortality Outcomes. J. Pediatr. 2020, 220, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Nasr, T.R.; Stoll, B.J. Necrotizing Enterocolitis: Recent Scientific Advances in Pathophysiology and Prevention. Semin. Perinatol. 2008, 32, 70–82. [Google Scholar] [CrossRef]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of Neonatal Necrotising Enterocolitis in High-Income Countries: A Systematic Review. Arch. Dis. Child Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef]
- Alsaied, A.; Islam, N.; Thalib, L. Global Incidence of Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Beeby, P.J.; Jeffery, H. Risk Factors for Necrotising Enterocolitis: The Influence of Gestational Age. Arch. Dis. Child. 1992, 67, 432–435. [Google Scholar] [CrossRef]
- Heida, F.H.; Stolwijk, L.; Loos, M.L.H.J.; van den Ende, S.J.; Onland, W.; van den Dungen, F.A.M.; Kooi, E.M.W.; Bos, A.F.; Hulscher, J.B.F.; Bakx, R. Increased Incidence of Necrotizing Enterocolitis in the Netherlands After Implementation of the New Dutch Guideline for Active Treatment in Extremely Preterm Infants: Results from Three Academic Referral Centers. J. Pediatr. Surg. 2017, 52, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal Necrotizing Enterocolitis. Therapeutic Decisions Based Upon Clinical Staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining Necrotizing Enterocolitis: Current Difficulties and Future Opportunities. Pediatr. Res. 2020, 88 (Suppl. S1), 10–15. [Google Scholar] [CrossRef]
- Rose, A.T.; Patel, R.M. A Critical Analysis of Risk Factors for Necrotizing Enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 374–379. [Google Scholar] [CrossRef]
- Van der Heide, M.; Hulscher, J.B.F.; Bos, A.F.; Kooi, E.M.W. Near-Infrared Spectroscopy as a Diagnostic Tool for Necrotizing Enterocolitis in Preterm Infants. Pediatr. Res. 2021, 90, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Schat, T.E.; Heida, F.H.; Schurink, M.; van der Laan, M.E.; Hulzebos, C.V.; Bos, A.F.; Kooi, E.M.W.; Hulscher, J.B.F. The Relation Between Splanchnic Ischaemia and Intestinal Damage in Necrotising Enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 2016, 101, F533–F539. [Google Scholar] [CrossRef]
- Palleri, E.; Wackernagel, D.; Wester, T.; Bartocci, M. Low Splanchnic Oxygenation and Risk for Necrotizing Enterocolitis in Extremely Preterm Newborns. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.; Aldairem, A.; Alrashed, M.; Saleh, K.B.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Japkowicz, N.; Stephen, S. The Class Imbalance Problem: A Systematic Study. Intell. Data Anal. 2002, 6, 429–449. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Compared to What? Finding Controls for Case-Control Studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Setia, M.S. Methodology Series Module 2: Case-Control Studies. Indian J. Dermatol. 2016, 61, 146–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christ, M.; Braun, N.; Neuffer, J.; Kempa-Liehr, A. Time Series Feature Extraction on Basis of Scalable Hypothesis Tests (tsfresh—A Python Package). Neurocomputing 2018, 307, 72–77. [Google Scholar] [CrossRef]
- Ozdemir, S.; Susarla, D. Feature Engineering Made Easy: Identify Unique Features from Your Dataset in Order to Build Powerful Machine Learning Systems; Packt Publishing: Birmingham, UK, 2018. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Hypothesis Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Forman, G.; Scholz, M. Apples-to-Apples in Cross-Validation Studies: Pitfalls in Classifier Performance Measurement. SIGKDD Explor. Newsl. 2010, 12, 49–57. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 27 November 2024).
- Pedregosa, F.; Varoguaux, G.; Gramfort, A.; Michel, V.; Thirion, B. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Alpaydin, E. Introduction to Machine Learning, 3rd ed.; MIT Press: Cambridge, UK, 2014. [Google Scholar]
- Chen, X.; Cheng, G.; Wang, F.L.; Tao, X.; Xie, H.; Xu, L. Machine and Cognitive Intelligence for Human Health: Systematic Review. Brain Inform. 2022, 9, 5. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Rusconi, B.; Jiang, X.; Sidhu, R.; Ory, D.S.; Warner, B.B.; Tarr, P.I. Gut Sphingolipid Composition as a Prelude to Necrotizing Enterocolitis. Sci. Rep. 2018, 8, 10984. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Bhattacharya, N.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Song, Y.S.; Morowitz, M.J.; Banfield, J.F. Necrotizing Enterocolitis is Preceded by Increased Gut Bacterial Replication, Klebsiella, and Fimbriae-Encoding Bacteria. Sci. Adv. 2019, 5, eaax5727. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, J.M.; Liu, S.; Olaloye, O.O.; Prochaska, E.C.; Yanowitz, T.; Riley, M.M.; Buland, J.R.; Brozanski, B.S.; Good, M.; Konnikova, L. Gestational Age-Specific Complete Blood Count Signatures in Necrotizing Enterocolitis. Front. Pediatr. 2021, 9, 604899. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Underwood, M.A. Probiotics and Necrotizing Enterocolitis. Semin. Pediatr. Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Beirnaert, C.; Mahieu, L.; Laukens, K.; Meysman, P.; Mulder, A.; Van Laere, D. Clinical Decision Support for Improved Neonatal Care: The Development of a Machine Learning Model for the Prediction of Late-Onset Sepsis and Necrotizing Enterocolitis. J. Pediatr. 2024, 266, 113869. [Google Scholar] [CrossRef]
- Gephart, S.M.; Spitzer, A.R.; Effken, J.A.; Dodd, E.; Halpern, M.; McGrath, J.M. Discrimination of GutCheckNEC: A Clinical Risk Index for Necrotizing Enterocolitis. J. Perinatol. 2014, 34, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Hooven, T.; Lin, Y.C.; Salleb-Aouissi, A. Multiple Instance Learning for Predicting Necrotizing Enterocolitis in Premature Infants Using Microbiome Data. In Proceedings of the ACM Conference on Health, Inference, and Learning, Toronto, ON, Canada, 2–4 April 2020; pp. 99–109. [Google Scholar]
- Osotsi, A.; Oravecz, Z.; Li, Q.; Smyth, J.; Brick, T.R. Individualized Modeling to Distinguish Between High and Low Arousal States Using Physiological Data. J. Healthc. Inform. Res. 2020, 4, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Valbusa, G.; Buscema, M. Detection of an Autism EEG Signature from Only Two EEG Channels Through Features Extraction and Advanced Machine Learning Analysis. Clin. EEG Neurosci. 2021, 52, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.M.; Yeung, T.; Choisne, J. A Comparison of Machine Learning Models’ Accuracy in Predicting Lower-Limb Joints’ Kinematics, Kinetics, and Muscle Forces from Wearable Sensors. Sci. Rep. 2023, 13, 5046. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, K.; Qayyum, A.; Ghaly, M.; Al-Fuqaha, A.; Razi, A.; Qadir, J. Explainable, Trustworthy, and Ethical Machine Learning for Healthcare: A Survey. Comput. Biol. Med. 2022, 149, 106043. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, K.; Aschner, J. HeRO Monitoring to Reduce Mortality in NICU Patients. Res. Rep. Neonatol. 2012, 2, 65–76. [Google Scholar] [CrossRef]
- Schat, T.E.; van Zoonen, A.G.J.F.; van der Laan, M.E.; Mebius, M.J.; Bos, A.F.; Hulzebos, C.V.; Boezen, H.M.; Hulscher, J.B.; Kooi, E.M. Early Cerebral and Intestinal Oxygenation in the Risk Assessment of Necrotizing Enterocolitis in Preterm Infants. Early Hum. Dev. 2019, 131, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Palleri, E.; van der Heide, M.; Hulscher, J.B.F.; Bartocci, M.; Wester, T.; Kooi, E.M.W. Clinical Usefulness of Splanchnic Oxygenation in Predicting Necrotizing Enterocolitis in Extremely Preterm Infants: A Cohort Study. BMC Pediatr. 2023, 23, 336. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Samek, W.; Montavon, G.; Vedaldi, A.; Hansen, L.; Müller, K.R. (Eds.) Explainable AI: Interpreting, Explaining and Visualizing Deep Learning; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Mishra, P. Explainability for Time Series Models. In Practical Explainable AI Using Python; Apress: Berkeley, CA, USA, 2022. [Google Scholar] [CrossRef]
- Shi, Y.; Payeur, P.; Frize, M.; Bariciak, E. Thermal and RGB-D Imaging for Necrotizing Enterocolitis Detection. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 1 June–1 July 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Gao, W.; Pei, Y.; Liang, H.; Lv, J.; Chen, J.; Zhong, W. Multimodal AI System for the Rapid Diagnosis and Surgical Prediction of Necrotizing Enterocolitis. IEEE Access 2021, 9, 51050–51064. [Google Scholar] [CrossRef]
- Lin, Y.C.; Salleb-Aouissi, A.; Hooven, T.A. Interpretable Prediction of Necrotizing Enterocolitis from Machine Learning Analysis of Premature Infant Stool Microbiota. BMC Bioinform. 2022, 23, 104. [Google Scholar] [CrossRef] [PubMed]
| Variable | Variable Type | Missing Data (%) | Imputed Data (%) |
|---|---|---|---|
| Gestational age | Static | NA | NA |
| Birth weight | Static | NA | NA |
| Sex | Static | NA | NA |
| Heart rate | Time series | 9.07 | 8.94 |
| Respiratory rate | Time series | 18.69 | 15.14 |
| Arterial oxygenation | Time series | 13.50 | 11.84 |
| Cerebral oxygenation | Time series | 30.25 | 27.16 |
| Splanchnic oxygenation | Time series | 61.81 | 50.91 |
| Group | All (N = 267) | Control (N = 235) | NEC (N = 32) | Difference NEC/Control |
|---|---|---|---|---|
| Male, N (%) | 145 (54.31) | 124 (52.77) | 21 (65.63) | p = 0.171 |
| GA (weeks + days), median (IQR) | 27 + 6 (26 + 3 − 29 + 1) | 27 + 6 (26 + 4 − 29 + 1) | 27 + 2 (26 + 0 − 28 + 1) | p = 0.045 |
| BW (grams), median (IQR) | 1000 (830–1260) | 1010 (840–1280) | 878 (749–1038) | p < 0.001 |
| NEC onset (days), median (IQR) | - | - | 14.50 (10.00–22.25) | |
| NEC laparotomy, N (%) | - | - | 9 (28.13) | |
| Died, N (%) | 18 (6.74) | 11 (4.68) | 7 (21.88) | p =< 0.001 |
| A/N steroids | ||||
| N known | 228 | 207 | 21 | |
| complete (%) | 143 (62.28) | 130 (62.80) | 12 (57.14) | p = 0.242 |
| incomplete (%) | 57 (25.00) | 49 (23.67) | 8 (38.10) | |
| none (%) | 29 (12.72) | 28 (13.53) | 1 (4.76) | |
| Antibiotics < 72 h P/N | ||||
| N known | 236 | 213 | 23 | |
| yes (%) | 169 (71.61) | 153 (71.83) | 16 (69.57) | p = 0.819 |
| Algorithm/Measure | F1-Score | AUC-PR |
|---|---|---|
| SVM | 0.82 ± 0.04 | 0.82 ± 0.04 |
| LR | 0.82 ± 0.05 | 0.83 ± 0.04 |
| XGBoost | 0.76 ± 0.06 | 0.77 ± 0.04 |
| Variable | Contribution (%) |
|---|---|
| Splanchnic oxygenation | 40.1 ± 8.2 |
| Cerebral oxygenation | 24.8 ± 7.4 |
| Arterial oxygenation | 14.5 ± 3.2 |
| Heart rate | 12.9 ± 2.4 |
| Respiratory rate | 7.6 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeven, R.; Kupers, T.; Brunsch, C.L.; Hulscher, J.B.F.; Kooi, E.M.W. Using Vital Signs for the Early Prediction of Necrotizing Enterocolitis in Preterm Neonates with Machine Learning. Children 2024, 11, 1452. https://doi.org/10.3390/children11121452
Verhoeven R, Kupers T, Brunsch CL, Hulscher JBF, Kooi EMW. Using Vital Signs for the Early Prediction of Necrotizing Enterocolitis in Preterm Neonates with Machine Learning. Children. 2024; 11(12):1452. https://doi.org/10.3390/children11121452
Chicago/Turabian StyleVerhoeven, Rosa, Thijmen Kupers, Celina L. Brunsch, Jan B. F. Hulscher, and Elisabeth M. W. Kooi. 2024. "Using Vital Signs for the Early Prediction of Necrotizing Enterocolitis in Preterm Neonates with Machine Learning" Children 11, no. 12: 1452. https://doi.org/10.3390/children11121452
APA StyleVerhoeven, R., Kupers, T., Brunsch, C. L., Hulscher, J. B. F., & Kooi, E. M. W. (2024). Using Vital Signs for the Early Prediction of Necrotizing Enterocolitis in Preterm Neonates with Machine Learning. Children, 11(12), 1452. https://doi.org/10.3390/children11121452






