Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Enrollment and Participants
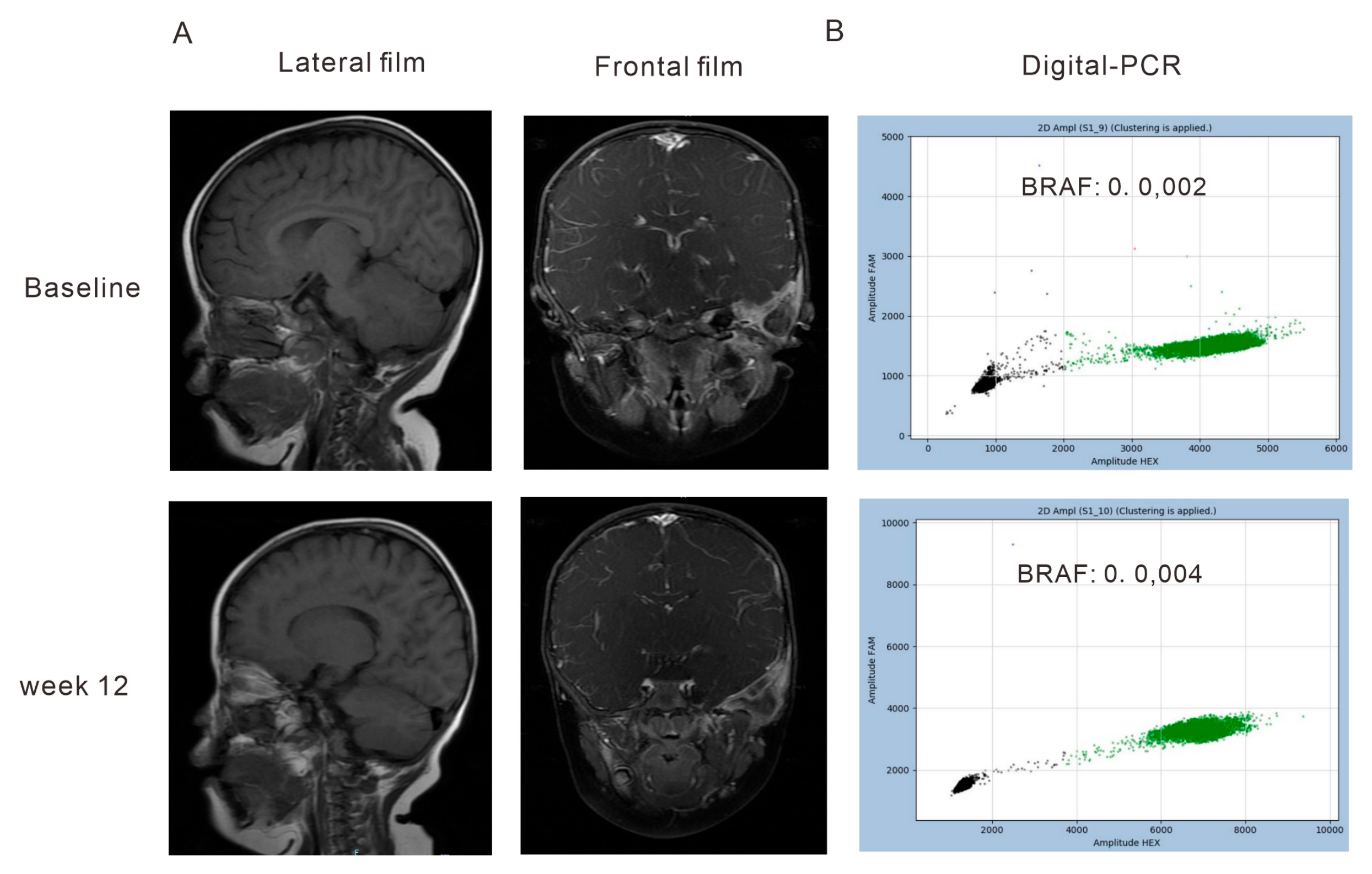
2.3. Quantification of cfDNA and ltDNA by PCR or NGS
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
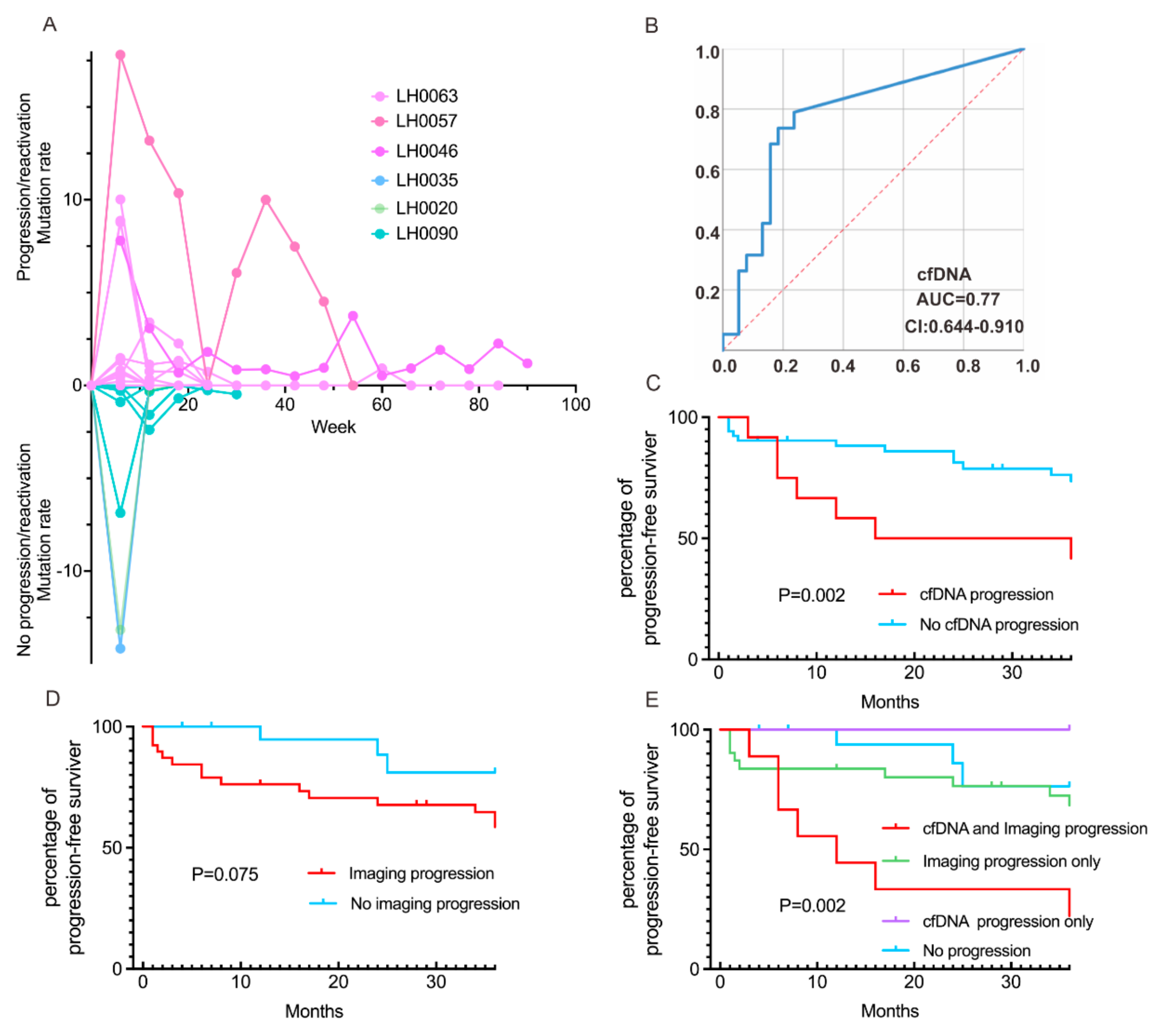
3.2. Correlation of the Dynamics of cfDNA Molecular Responses with Clinical Outcomes
3.3. Concordance of cfDNA Molecular and Radiographic Responses on Clinical Outcomes
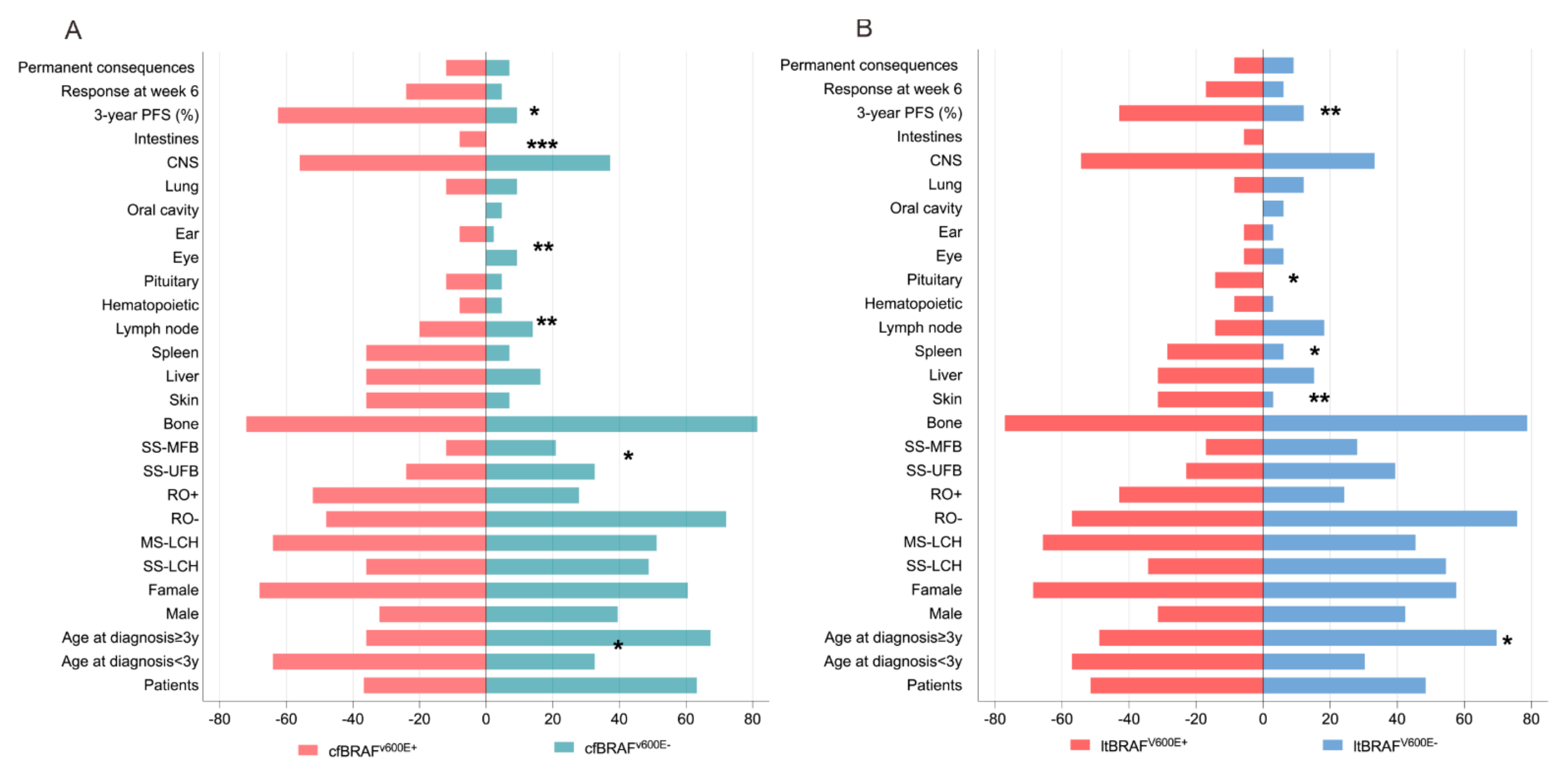
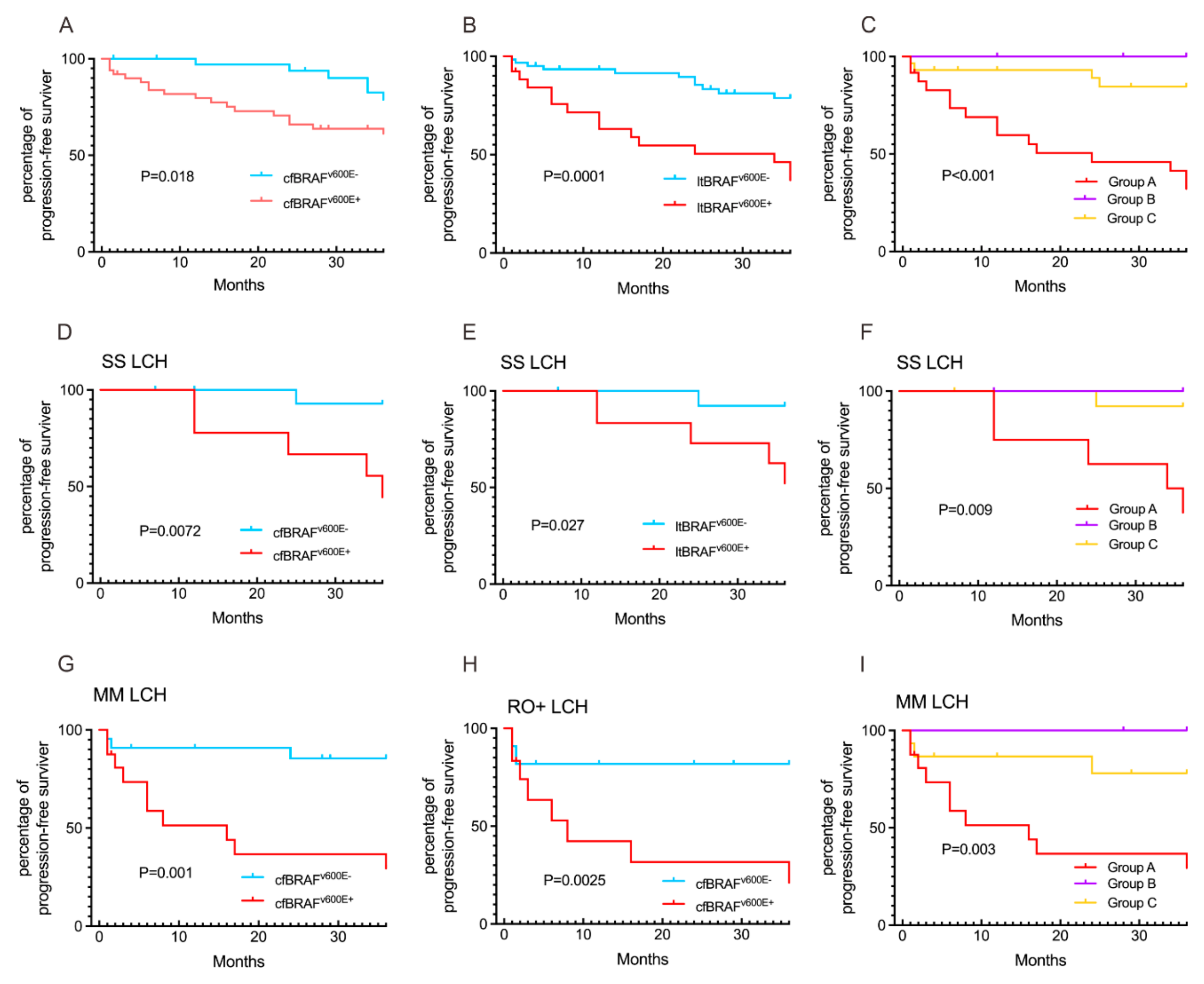
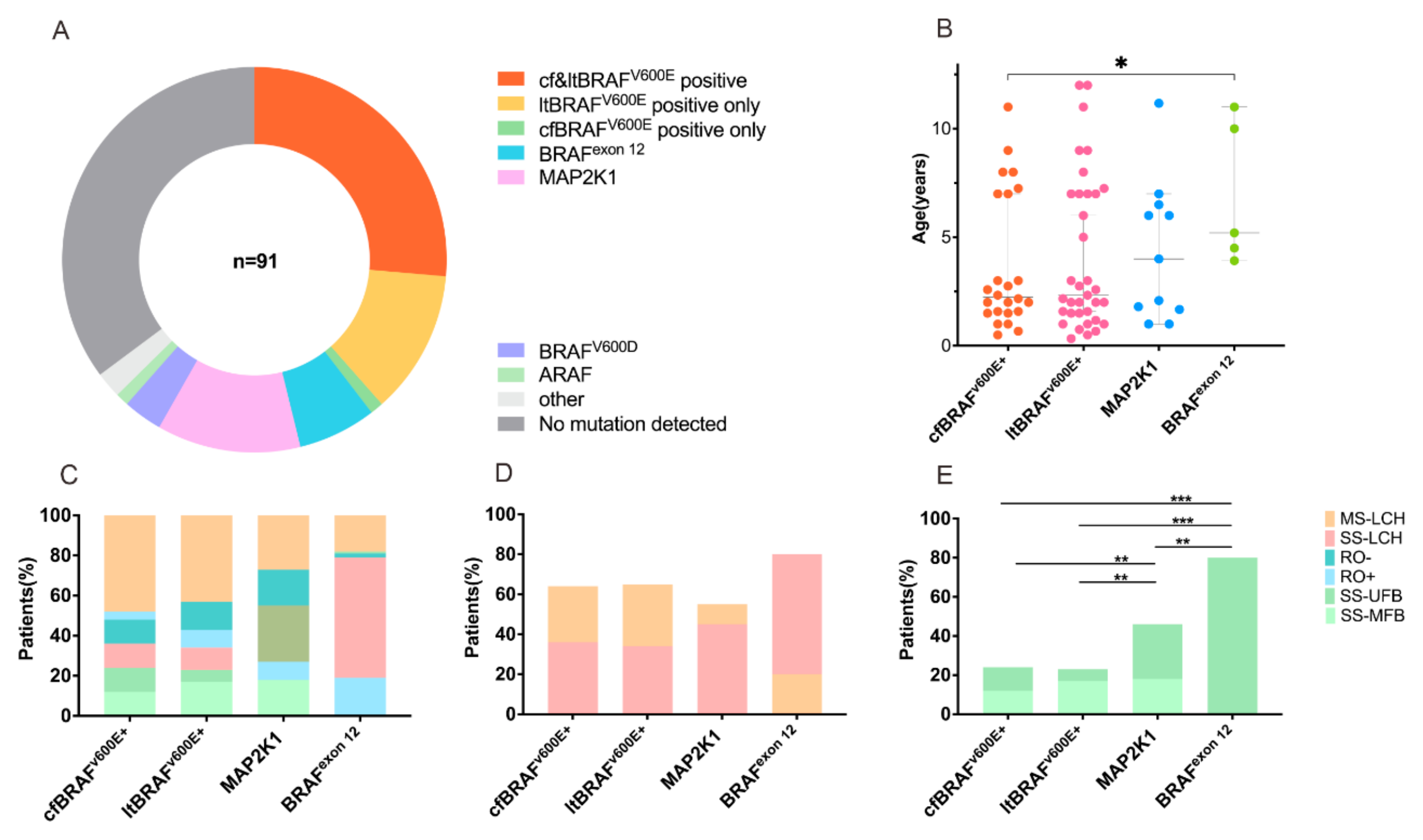
3.4. Comparison of cfBRAFV600E and ltBRAFV600E Status in Relation to LCH Clinical Presentations and Outcomes
3.5. MAP2K1 and BRAFexon12 Mutations in Relation to LCH Clinical Presentations and Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, C.E.; Longo, D.L.; Merad, M.; McClain, K.L. Langerhans-Cell Histiocytosis. N. Engl. J. Med. 2018, 379, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Hagey, D.W.; Kvedaraite, E.; Akber, M.; Görgens, A.; Javadi, J.; Von Bahr Greenwood, T.; Björklund, C.; Åkefeldt, S.O.; Hannegård-Hamrin, T.; Arnell, H.; et al. Myeloid cells from Langerhans cell histiocytosis patients exhibit increased vesicle trafficking and an altered secretome capable of activating NK cells. Haematologica 2023, 108, 2422–2434. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Y.; Han, L.; Feng, S.; Cao, J.; Sun, Y.; Cao, Z.; Cui, X. Somatic ARAF mutations in pediatric Langerhans cell histiocytosis: Clinicopathologic, genetic and functional profiling. Clin. Exp. Med. 2023, 23, 5269–5279. [Google Scholar] [CrossRef] [PubMed]
- Krooks, J.; Minkov, M.; Weatherall, A.G. Langerhans cell histiocytosis in children: History, classification, pathobiology, clinical manifestations, and prognosis. J. Am. Acad. Dermatol. 2018, 78, 1035–1044. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C. Clinical features and treatment of Langerhans cell histiocytosis. Acta Paediatr. 2021, 110, 2892–2902. [Google Scholar] [CrossRef]
- Goyal, G.; Tazi, A.; Go, R.S.; Rech, K.L.; Picarsic, J.L.; Vassallo, R.; Young, J.R.; Cox, C.W.; Van Laar, J.; Hermiston, M.L.; et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood 2022, 139, 2601–2621. [Google Scholar] [CrossRef]
- Bigenwald, C.; Le Berichel, J.; Wilk, C.M.; Chakraborty, R.; Chen, S.T.; Tabachnikova, A.; Mancusi, R.; Abhyankar, H.; Casanova-Acebes, M.; Laface, I.; et al. BRAF(V600E)-induced senescence drives Langerhans cell histiocytosis pathophysiology. Nat. Med. 2021, 27, 851–861. [Google Scholar] [CrossRef]
- Cohen Aubart, F.; Idbaih, A.; Emile, J.F.; Amoura, Z.; Abdel-Wahab, O.; Durham, B.H.; Haroche, J.; Diamond, E.L. Histiocytosis and the nervous system: From diagnosis to targeted therapies. Neuro Oncol. 2021, 23, 1433–1446. [Google Scholar] [CrossRef]
- Cournoyer, E.; Ferrell, J.; Sharp, S.; Ray, A.; Jordan, M.; Dandoy, C.; Grimley, M.; Roy, S.; Lorsbach, R.; Merrow, A.C.; et al. Dabrafenib and trametinib in Langerhans cell histiocytosis and other histiocytic disorders. Haematologica 2023, 109, 1137. [Google Scholar] [CrossRef]
- Kim, J.R.; Yoon, H.M.; Jung, A.Y.; Cho, Y.A.; Seo, J.J.; Lee, J.S. Comparison of whole-body MRI, bone scan, and radiographic skeletal survey for lesion detection and risk stratification of Langerhans Cell Histiocytosis. Sci. Rep. 2019, 9, 317. [Google Scholar] [CrossRef]
- Gulati, N.; Allen, C.E. Langerhans cell histiocytosis: Version 2021. Hematol. Oncol. 2021, 39 (Suppl. 1), 15–23. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, J.; Jeziorski, E.; Marec-Berard, P.; Barkaoui, M.A.; Pagnier, A.; Rohrlich, P.S.; Chevallier, A.; Carausu, L.; Aladjidi, N.; Rigaud, C.; et al. Childhood Langerhans cell histiocytosis hematological involvement: Severity associated with BRAFV600E loads. Blood 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Li, J.; Cao, X.X. Signaling pathways, microenvironment, and targeted treatments in Langerhans cell histiocytosis. Cell Commun. Signal. 2022, 20, 195. [Google Scholar] [CrossRef]
- Sconocchia, T.; Foßelteder, J.; Sconocchia, G.; Reinisch, A. Langerhans cell histiocytosis: Current advances in molecular pathogenesis. Front. Immunol. 2023, 14, 1275085. [Google Scholar] [CrossRef]
- Lang, M.; Chang, L.; Cai, H.; Lin, H.; Liu, Z.Z.; Duan, M.H.; Zhou, D.B.; Cao, X.X. BRAF deletion in adult patients with Langerhans cell histiocytosis correlates with multisystem disease and poor outcome. Clin. Cancer Res. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Jouenne, F.; Tazi, A. The MAPK Pathway in Pulmonary Langerhans Cell Histiocytosis. Arch. Bronconeumol. 2023, 59, 347–349. [Google Scholar] [CrossRef]
- Ji, H.; Long, X.; Gu, J.; Jin, J.; Mao, X.; Wang, Z.; Ma, H.; Chen, L. Longitudinal Monitoring of Plasma Circulating Tumour DNA Enables the Prediction of Early Relapse in Patients with Non-Hodgkin Lymphoma: A Case Series. Diagnostics 2021, 11, 2055. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, A.; Duan, M.; Cai, H.; Gao, X.; Liu, T.; Sun, J.; Liang, Z.; Zhou, D.; Cao, X.; et al. Diverse kinase alterations and myeloid-associated mutations in adult histiocytosis. Leukemia 2022, 36, 573–576. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Wang, D.; Cui, L.; Li, N.; Lian, H.; Ma, H.; Zhao, Y.; Zhang, L.; Liu, W.; et al. Clinical study of MAP2K1-mutated Langerhans cell histiocytosis in children. J. Cancer Res. Clin. Oncol. 2022, 148, 2517–2527. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.S. Proteomics technologies for cancer liquid biopsies. Mol. Cancer 2022, 21, 53. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, F.; Zimmermann, S.Y.; Egeler, R.M.; Nanduri, V.R.; Cammarata, B.; Donadieu, J.; Lehrnbecher, T.; Haupt, R. Langerhans cell histiocytosis and associated malignancies: A retrospective analysis of 270 patients. Eur. J. Cancer 2022, 172, 138–145. [Google Scholar] [CrossRef]
- Heritier, S.; Helias-Rodzewicz, Z.; Lapillonne, H.; Terrones, N.; Garrigou, S.; Normand, C.; Barkaoui, M.A.; Miron, J.; Plat, G.; Aladjidi, N.; et al. Circulating cell-free BRAF(V600E) as a biomarker in children with Langerhans cell histiocytosis. Br. J. Haematol. 2017, 178, 457–467. [Google Scholar] [CrossRef]
- Makras, P.; Yavropoulou, M.P.; Chatziioannou, S.N.; Anastasilakis, A.D.; Georgakopoulou, D.; Tsoli, M.; Chatzelis, E.; Kaltsas, G. Efficacy of denosumab monotherapy among adults with Langerhans cell histiocytosis: A prospective clinical trial. Am. J. Hematol. 2023, 98, E168–E171. [Google Scholar] [CrossRef]
- Cui, L.; Wang, C.J.; Lian, H.Y.; Zhang, L.; Ma, H.H.; Wang, D.; Chen, F.F.; Zhang, Q.; Yang, Y.; Wei, A.; et al. Clinical outcomes and prognostic risk factors of Langerhans cell histiocytosis in children: Results from the BCH-LCH 2014 protocol study. Am. J. Hematol. 2023, 98, 598–607. [Google Scholar] [CrossRef]
- Evseev, D.; Osipova, D.; Kalinina, I.; Raykina, E.; Ignatova, A.; Lyudovskikh, E.; Baidildina, D.; Popov, A.; Zhogov, V.; Semchenkova, A.; et al. Vemurafenib combined with cladribine and cytarabine results in durable remission of pediatric BRAF V600E-positive LCH. Blood Adv. 2023, 7, 5246–5257. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chang, L.W.; Wang, J.; Xie, M.; Chen, L.L.; Liu, W. Ursodeoxycholic acid prevention on cholestasis associated with total parenteral nutrition in preterm infants: A randomized trial. World J. Pediatr. WJP 2022, 18, 100–108. [Google Scholar] [CrossRef]
- Lueong, S.S.; Herbst, A.; Liffers, S.-T.; Bielefeld, N.; Horn, P.A.; Tannapfel, A.; Reinacher-Schick, A.; Hinke, A.; Hegewisch-Becker, S.; Kolligs, F.T.; et al. Serial Circulating Tumor DNA Mutational Status in Patients with KRAS-Mutant Metastatic Colorectal Cancer from the Phase 3 AIO KRK0207 Trial. Clin. Chem. 2020, 66, 1510–1520. [Google Scholar] [CrossRef]
- Long, X.; Xu, Q.; Lou, Y.; Li, C.; Gu, J.; Cai, H.; Wang, D.; Xu, J.; Li, T.; Zhou, X.; et al. The utility of non-invasive liquid biopsy for mutational analysis and minimal residual disease assessment in extramedullary multiple myeloma. Br. J. Haematol. 2020, 189, e45–e48. [Google Scholar] [CrossRef]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA A Cancer J. Clin. 2020, 71, 176–190. [Google Scholar] [CrossRef]
- Tandon, S.; Weitzman, S.; Joyce, B.; McGuire, B.; Stephens, D.; Whitlock, J.; Hawkins, C.; Ngan, B.Y.; Abla, O. Expression and Clinical Correlation of PD-1/PD-L1 and VE1(BRAFp.V600E) in Pediatric Langerhans Cell Histiocytosis. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023035. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, L.; Ma, H.H.; Wang, C.J.; Wang, D.; Lian, H.Y.; Li, W.J.; Zhang, Q.; Li, N.; Wang, T.Y.; et al. Circulating cell-free BRAF V600E during chemotherapy is associated with prognosis of children with Langerhans cell histiocytosis. Haematologica 2020, 105, e444–e447. [Google Scholar] [CrossRef]


| ltDNA and cfDNA | Missing Detection | p | |
|---|---|---|---|
| Patients, n | 91 | 28 | |
| Age at diagnosis, y | 4.0 (2–7) | 5.37 (3.25–9.25) | 0.098 |
| Gender, n (%) | |||
| Male | 59 (64.8) | 20 (71.4) | 0.518 |
| Female | 32 (35.2) | 8 (28.6) | |
| Disease extent at diagnosis, n (%) | |||
| SS-LCH | 56 (61.5) | 20 (71.4) | 0.174 |
| MS-LCH-RO- | 7 (7.7) | 4 (14.3) | |
| MS-LCH-RO+ | 28 (30.8) | 4 (14.3) | |
| Involvement, n (%) | |||
| Bone | 75 (82.4) | 25 (89.3) | 0.386 |
| Skin | 12 (13.2) | 2 (7.1) | 0.385 |
| Liver | 20 (22.0) | 2 (7.1) | 0.077 |
| Spleen | 14 (15.4) | 1 (3.6) | 0.100 |
| Lymph node | 16(17.6) | 2 (7.1) | 0.178 |
| Hematopoietic | 6 (6.6) | 0 (0.0) | 0.163 |
| Pituitary | 6 (6.6) | 1 (3.6) | 0.552 |
| Eye | 4 (4.4) | 3 (10.7) | 0.214 |
| Ear | 6 (6.6) | 1 (3.6) | 0.552 |
| Oral cavity | 3 (3.3) | 0 (0.0) | 0.330 |
| Lung | 9 (9.9) | 2 (7.1) | 0.661 |
| CNS | 40 (44.0) | 7 (25.0) | 0.073 |
| Intestines | 2 (2.2) | 1 (3.6) | 0.685 |
| 3-year PFS rate, % | 71.4 | 89.3 | 0.054 |
| Progression/Reactivation | CR/PR | p | |
|---|---|---|---|
| Molecular progression, n (%) | 7 (58.3) | 5 (41.7) | 0.011 * |
| No molecular progression, n (%) | 12 (21.8) | 43 (78.2) | |
| Radiologic progression, n (%) | 16 (41.0) | 23 (59.0) | 0.045 * |
| No radiologic progression, n (%) | 3 (15.0) | 17 (85.0) | |
| Molecular and radiologic progression, n (%) | 6 (75.0) | 2 (25.0) | 0.004 * |
| No molecular and radiologic progression, n (%) | 3 (16.7) | 15 (83.3) |
| cfBRAFV600E+ | cfBRAFV600E- | p | ltBRAFV600E+ | ltBRAFV600E- | p | Group A | Group B | Group C | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n. | 25 | 43 | 35 | 33 | 24 | 12 | 32 | |||
| Age at diagnosis, y | 2.17 (1.5–7) | 5.08 (2–9) | 0.036 * | 2.33 (1.5–7) | 5.08 (2–9) | 0.068 | 2.083 (1.5–6) | 6.5 (1.38–8.75) | 5.04 (2–9) | 0.062 |
| <3y | 16 (64.0) | 14 (32.6) | 0.012 * | 20 (57.1) | 10 (30.3) | 0.026 * | 16 (66.7) | 4 (33.3) | 10 (31.3) | 0.021 *,b |
| ≥3y | 9 (36.0) | 29 (67.4) | 15 (42.9) | 23 (69.7) | 8 (33.3) | 8 (66.7) | 22 (68.8) | |||
| Gender, n (%) | ||||||||||
| Male | 8 (32.0) | 17 (39.5) | 0.534 | 11 (31.4) | 14 (42.4) | 0.347 | 8 (33.3) | 3 (25.0) | 14 (43.8) | 0.471 |
| Female | 17 (68.0) | 26 (60.5) | 24 (68.6) | 19 (57.6) | 16 (66.7) | 9 (75.0) | 18 (56.3) | |||
| Disease extent at diagnosis, n (%) | ||||||||||
| SS-LCH | 9 (36.0) | 21 (48.8) | 0.304 | 12 (34.3) | 18 (54.5) | 0.093 | 8 (33.3) | 5 (41.7) | 17 (53.1) | 0.330 |
| MS-LCH | 16 (64.0) | 22 (51.2) | 23 (65.7) | 15 (45.5) | 16 (66.7) | 7 (58.3) | 15 (46.9) | |||
| Detailed subtype, n (%) | ||||||||||
| RO- | 12 (48.0) | 31 (72.1) | 0.047 * | 20 (57.1) | 25 (75.8) | 0.105 | 12 (50.0) | 9 (75.0) | 24 (75.0) | 0.114 |
| RO+ | 13 (52.0) | 12 (27.9) | 15 (42.9) | 8 (24.2) | 12 (50.0) | 3 (25.0) | 8 (25.0) | |||
| Detailed subtype, n (%) | ||||||||||
| SS-UFB | 6 (24.0) | 14 (32.6) | 0.455 | 8 (22.9) | 13 (39.4) | 0.140 | 5 (20.8) | 3 (25.0) | 12 (37.5) | 0.373 |
| SS-MFB | 3 (12.0) | 9 (20.9) | 0.352 | 6 (17.1) | 9 (28.1) | 0.281 | 3 (12.5) | 2 (16.7) | 7 (21.9) | 0.707 |
| Involvement | ||||||||||
| Bone | 18 (72.0) | 35 (81.4) | 0.368 | 27 (77.1) | 26 (78.8) | 0.870 | 17 (70.8) | 11 (91.7) | 25 (78.1) | 0.364 |
| Skin | 9 (36.0) | 3 (7.0) | 0.002 * | 11 (31.4) | 1 (3.0) | 0.002 * | 9 (37.5) | 2 (16.7) | 1 (3.1) | 0.003 * b |
| Liver | 9 (36.0) | 7 (16.3) | 0.065 | 11 (31.4) | 5 (15.2) | 0.114 | 9 (37.5) | 2 (16.7) | 5 (15.6) | 0.133 |
| Spleen | 9 (36.0) | 3 (7.0) | 0.002 * | 10 (28.6) | 2 (6.1) | 0.015 * | 9 (37.5) | 1 (8.3) | 2 (6.3) | 0.005 *b |
| Lymph node | 5 (20.0) | 6 (14.0) | 0.514 | 5 (14.3) | 6 (18.2) | 0.663 | 5 (20.8) | 0 (0.0) | 6 (18.8) | 0.281 |
| Hematopoietic | 2 (8.0) | 2 (4.7) | 0.571 | 3 (8.6) | 1 (3.0) | 0.332 | 2 (8.3) | 1 (8.3) | 1 (3.1) | 0.510 |
| Pituitary | 3 (12.0) | 2 (4.7) | 0.263 | 5 (14.3) | 0 (0.0) | 0.024 * | 3 (12.5) | 2 (16.7) | 0 (0.0) | 0.048 |
| Eye | 0 (0.0) | 4 (9.3) | 0.116 | 2 (5.7) | 2 (6.1) | 0.952 | 0 (0.0) | 2 (16.7) | 2 (6.3) | 0.121 |
| Ear | 2 (8.0) | 1 (2.3) | 0.272 | 2 (5.7) | 1 (3.0) | 0.590 | 2 (8.3) | 0 (0.0) | 1 (3.1) | 0.579 |
| Oral cavity | 0 (0.0) | 2 (4.7) | 0.274 | 0 (0.0) | 2 (6.1) | 0.139 | 0 (0.0) | 0 (0.0) | 2 (6.3) | 0.663 |
| Lung | 3 (12.0) | 4 (9.3) | 0.724 | 3 (8.6) | 4 (12.1) | 0.630 | 3 (12.5) | 0 (0.0) | 4 (12.5) | 0.660 |
| CNS | 14 (56.0) | 16 (37.2) | 0.132 | 19 (54.3) | 11 (33.3) | 0.083 | 13 (54.2) | 7 (58.3) | 10 (31.3) | 0.128 |
| Intestines | 2 (8.0) | 0 (0.0) | 0.060 | 2 (5.7) | 0 (0.0) | 0.163 | 2 (8.3) | 0 (0.0) | 0 (0.0) | 0.150 |
| 3-year PFS rate, n (%) | 37.5 | 90.7 | <0.001 * | 57.1 | 87.9 | 0.005 * | 34.8 | 100 | 87.5 | <0.001 *b |
| Response at the 6th week | 6 (24.0) | 2 (4.7) | 0.042 * | 6 (17.1) | 2 (6.1) | 0.296 | 6 (25.0) | 0 (0.0) | 2 (6.3) | 0.110 |
| Permanent consequences developed during follow-up, n (%) | 3 (12.0) | 3 (7.0) | 0.481 | 3 (8.6) | 3 (9.1) | 0.940 | 2 (8.3) | 2 (16.7) | 2 (6.3) | 0.463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhu, Y.; Chen, Y.; Wang, Y.; Zhang, D.; Zhang, J.; Wang, Y.; Zhang, A.; Hu, Q.; Liu, A. Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children. Children 2024, 11, 1449. https://doi.org/10.3390/children11121449
Liu S, Zhu Y, Chen Y, Wang Y, Zhang D, Zhang J, Wang Y, Zhang A, Hu Q, Liu A. Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children. Children. 2024; 11(12):1449. https://doi.org/10.3390/children11121449
Chicago/Turabian StyleLiu, Siying, Yongbing Zhu, Yu Chen, Yaqin Wang, Dedong Zhang, Jiasi Zhang, Yao Wang, Ai Zhang, Qun Hu, and Aiguo Liu. 2024. "Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children" Children 11, no. 12: 1449. https://doi.org/10.3390/children11121449
APA StyleLiu, S., Zhu, Y., Chen, Y., Wang, Y., Zhang, D., Zhang, J., Wang, Y., Zhang, A., Hu, Q., & Liu, A. (2024). Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children. Children, 11(12), 1449. https://doi.org/10.3390/children11121449





