Outbreak of Acute Fulminant Myocarditis in Children in Campania Region, Italy: A Case Series
Abstract
1. Introduction
2. Methods
Diagnosis of Fulminant Myocarditis
3. Results
3.1. Outbreak of Fulminant Myocarditis
3.2. Case Series
4. Discussion
4.1. Etiology
4.2. Diagnosis
4.3. Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klugman, D.; Berger, J.T.; Sable, C.A.; He, J.; Khandelwal, S.G.; Slonim, A.D. Pediatric patients hospitalized with myocarditis: A multi-institutional analysis. Pediatr. Cardiol. 2010, 31, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Butts, R.J.; Boyle, G.J.; Deshpande, S.R.; Gambetta, K.; Knecht, K.R.; Prada-Ruiz, C.A.; Richmond, M.E.; West, S.C.; Lal, A.K. Characteristics of Clinically Diagnosed Pediatric Myocarditis in a Contemporary Multi-Center Cohort. Pediatr. Cardiol. 2017, 38, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Skajaa, N.; Horváth-Puhó, E.; Adelborg, K.; Bøtker, H.E.; Rothman, K.R.; Sørensen, H.T. Lack of seasonality in occurrence of pericarditis, myocarditis, and endocarditis. Ann. Epidemiol. 2019, 37, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Lal, A.K.; Chen, S.; Čiháková, D.; Cooper, L.T., Jr.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A. Diagnosis and Management of Myocarditis in Children: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e123–e135. [Google Scholar] [CrossRef]
- Canter, C.E.; Simpson, K.E. Diagnosis and treatment of myocarditis in children in the current era. Circulation 2014, 129, 115–128. [Google Scholar] [CrossRef]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis—Diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef]
- Veronese, G.; Ammirati, E.; Cipriani, M.; Frigerio, M. Fulminant myocarditis: Characteristics, treatment, and outcomes. Anatol. J. Cardiol. 2018, 19, 279–286. [Google Scholar] [CrossRef]
- Carthy, R.E., 3rd; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef]
- Nagai, T.; Inomata, T.; Kohno, T.; Sato, T.; Tada, A.; Kubo, T.; Nakamura, K.; Oyama-Manabe, N.; Ikeda, Y.; Fujino, T.; et al. Guideline on the Diagnosis and Treatment of Myocarditis. Circ. J. 2023, 87, 674–754. [Google Scholar] [CrossRef]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O. Recognition and initial management of fulminant myocarditis: A scientific statement from the American heart association. Circulation 2020, 141, e69–e92. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017, 136, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Veronese, G.; Cipriani, M.; Petrella, D.; Pedrotti, P.; Giannattasio, C.; Garascia, A.; Oliva, F.; Klingel, K.; Frigerio, M.; Ammirati, E. Not every fulminant lymphocytic myocarditis fully recovers. J. Cardiovasc. Med. 2018, 19, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jerome, K.R.; Reichenbach, D.D. Fatal parvovirus myocarditis in a 5-year-old girl. Hum. Pathol. 2001, 32, 342–345. [Google Scholar] [CrossRef]
- Bültmann, B.D.; Klingel, K.; Sotlar, K.; Bock, C.T.; Baba, H.A.; Sauter, M.; Kandolf, R. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: An endothelial cell-mediated disease. Hum. Pathol. 2003, 34, 92–95. [Google Scholar] [CrossRef]
- Pelzl, L.; Mantino, S.; Sauter, M.; Manuylova, T.; Vogel, U.; Klingel, K. Lymphocytic Myocarditis in Children with Parvovirus B19 Infection: Pathological and Molecular Insights. Biomedicines 2024, 12, 1909. [Google Scholar] [CrossRef]
- Kim, I.C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef]
- Maglione, M.; Di Nardo, G.; Di Marco, G.M.; D’Anna, C.; Muzzica, S.; Savoia, F.; Calì, C.; Grieco, M.; Cardaropoli, D.; Cosimi, R.; et al. Echocardiographic Findings and Conduction Abnormalities in Children with Multisystem Inflammatory Syndrome. Indian J. Pediatr. 2023, 90, 316. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Pönkä, A. Carditis associated with mycoplasma pneumoniae infection. Acta Med. Scand. 1979, 206, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Higaki, T.; Satoh, H.; Nakano, T. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Gu, L.; Yin, S.J.; Yang, R.; Xie, Y.; Guo, X.Z.; Fu, Y.X.; Cheng, D. Age-specific Mycoplasma pneumoniae pneumonia-associated myocardial damage in children. J. Int. Med. Res. 2013, 41, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Padget, R.L.; Zeitz, M.J.; Blair, G.A.; Wu, X.; North, M.D.; Tanenbaum, M.T.; Stanley, K.E.; Phillips, C.M.; King, D.R.; Lamouille, S.; et al. Acute Adenoviral Infection Elicits an Arrhythmogenic Substrate Prior to Myocarditis. Circ. Res. 2024, 134, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Da, M.; Yang, X.; Xu, Y.; Qi, J. A retrospective analysis of clinical characteristics and outcomes of pediatric fulminant myocarditis. BMC Pediatr. 2024, 24, 553. [Google Scholar] [CrossRef]
- Giordani, A.S.; Baritussio, A.; Vicenzetto, C.; Peloso-Cattini, M.G.; Pontara, E.; Bison, E.; Fraccaro, C.; Basso, C.; Iliceto, S.; Marcolongo, R.; et al. Fulminant Myocarditis: When One Size Does Not Fit All—A Critical Review of the Literature. Eur. Cardiol. 2023, 18, e15. [Google Scholar] [CrossRef]
- Ghelani, S.J.; Spaeder, M.C.; Pastor, W.; Spurney, C.F.; Klugman, D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 622–627. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648, 2648a–2648d. [Google Scholar] [CrossRef]
- Schubert, S.; Opgen-Rhein, B.; Boehne, M.; Weigelt, A.; Wagner, R.; Müller, G.; Rentzsch, A.; Zu Knyphausen, E.; Fischer, M.; Papakostas, K.; et al. Severe heart failure and the need for mechanical circulatory support and heart transplantation in pediatric patients with myocarditis: Results from the prospective multicenter registry “MYKKE”. Pediatr. Transplant 2019, 23, e13548. [Google Scholar] [CrossRef]
- Matsuura, H.; Ichida, F.; Saji, T.; Ogawa, S.; Waki, K.; Kaneko, M.; Tahara, M.; Soga, T.; Ono, Y.; Yasukochi, S. Clinical Features of Acute and Fulminant Myocarditis in Children—2nd Nationwide Survey by Japanese Society of Pediatric Cardiology and Cardiac Surgery. Circ. J. 2016, 80, 2362–2368. [Google Scholar] [CrossRef]
- Robinson, J.; Hartling, L.; Vandermeer, B.; Klassen, T.P. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst. Rev. 2015, 8, CD004370. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Lazea, C.; Agoston-Coldea, L. Novel insights on acute myocarditis in pediatric patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11479–11495. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Wang, W.; Wu, S.N.; Liu, J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst. Rev. 2013, 18, CD004471. [Google Scholar] [CrossRef]
- One Health Basic and Translational Actions Addressing Unmet Needs on Emerging Infectious Diseases (INF-ACT). Available online: https://www.inf-act.it/ (accessed on 5 November 2024).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age | 4 months | 4.6 years | 1 year | 1.5 years | 2 years | 1.4 years |
| Gender | F | M | F | M | M | M |
| Chronic conditions | Moderate interatrial defect | No | No | No | No | Ureterectasia and pyelectasis |
| Symptoms before hospitalization | Fever, diarrhea | Fever, vomiting, abdominal pain | Vomiting | Fever, vomiting | Dyspnea | Vomiting |
| Duration of symptoms before hospitalization | Few hours | One week | Few hours | Three days | One week | Few hours |
| C-reactive protein (mg/L; normal value < 5) | Negative | Negative | Negative | 11.2 | Negative | 26.97 |
| Procalcitonin | Negative | Negative | Negative | Negative | Negative | Negative |
| Aspartate aminotransferase (IU/L) | 73 | Not available | Non-available | 424 | 30 | 611 |
| Lactate dehydrogenase (U/L) | 416 | Not available | Not available | 1042 | 358 | 1344 |
| Lactate (mmol/L) | 14.9 | 3.6 | 13.9 | 16 | 4.2 | 5.1 |
| Troponin (ng/L) | 202 | 51 | 573 | 130 | 115 | 468 |
| B-type natriuretic peptide (pg/mL) | Not available | 2820 | 3730 | 4340 | 3270 | >5000 |
| CK-MB (ng/mL; normal value < 6.2) | 2.7 | 7.36 | Not available | 25.64 | 4.2 | Not available |
| Myoglobin (ng/mL; normal value 20–72) | 147 | Not available | Not available | 4167 | 34.08 | Not available |
| Chest X-ray | Reticular-nodular pattern, cardiomegaly | Reticular-nodular pattern, bilateral pleural effusion | Reticular-nodular pattern | Not performed | Cardiomegaly | Not performed |
| Blood pressure (mmHg) | Undetectable | 87/60 | Undetectable | 110/60 | 93/69 | |
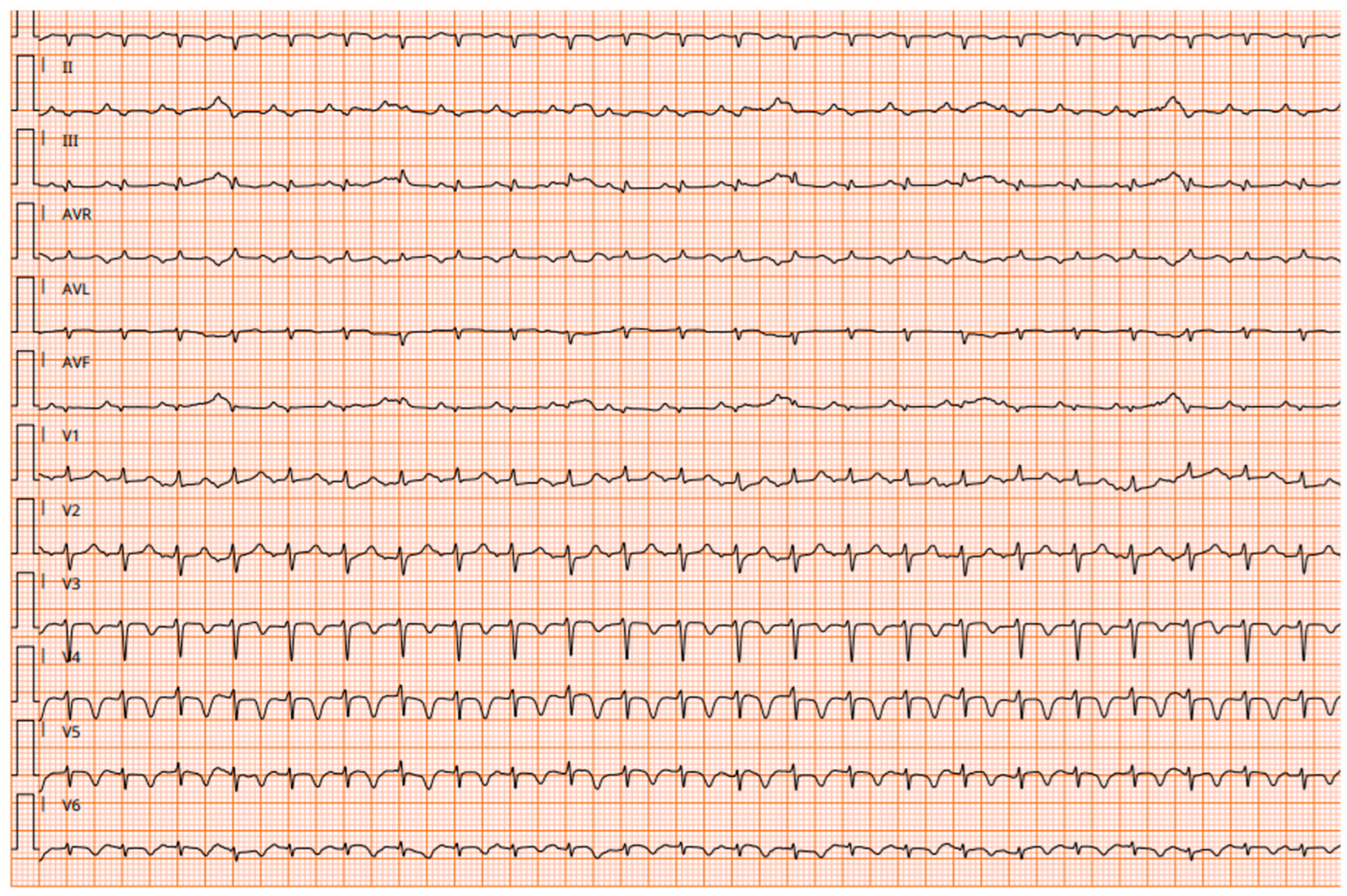
| Electrocardiography | Sinus tachycardia | Atrioventricular conduction delays | Supraventricular tachycardia and fatal ventricular tachycardia | Atrioventricular conduction delays | Sinus tachycardia, low voltage QRS complex | Non-specific ventricular repolarization abnormalities |
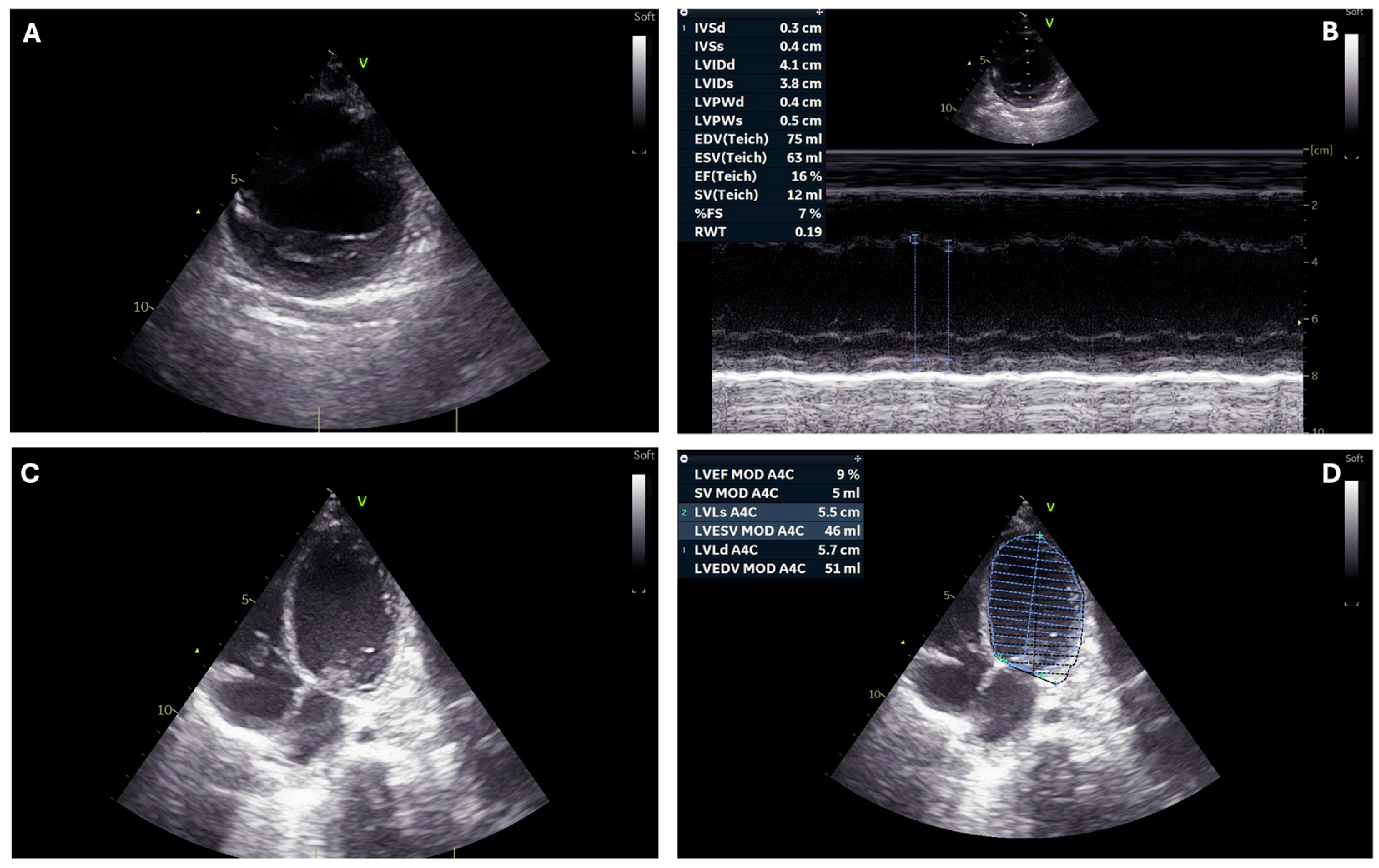
| Ventricular ejection fraction (%) | <10 | 25 | 10–15 | 10–15 | 33–35 | 10–15 |
| Outcome | Death | Discharged | Death | Death | Still hospitalized (Cardiologic Intensive Care Unit) | Still hospitalized (Cardiologic Intensive Care Unit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannattasio, A.; Maglione, M.; Di Nardo, G.; Di Marco, G.M.; Lauretta, D.; Carrella, M.C.; Furlan, D.; Savoia, F.; Tipo, V. Outbreak of Acute Fulminant Myocarditis in Children in Campania Region, Italy: A Case Series. Children 2024, 11, 1414. https://doi.org/10.3390/children11121414
Giannattasio A, Maglione M, Di Nardo G, Di Marco GM, Lauretta D, Carrella MC, Furlan D, Savoia F, Tipo V. Outbreak of Acute Fulminant Myocarditis in Children in Campania Region, Italy: A Case Series. Children. 2024; 11(12):1414. https://doi.org/10.3390/children11121414
Chicago/Turabian StyleGiannattasio, Antonietta, Marco Maglione, Giangiacomo Di Nardo, Giovanni Maria Di Marco, Daria Lauretta, Maria Chiara Carrella, Daniela Furlan, Fabio Savoia, and Vincenzo Tipo. 2024. "Outbreak of Acute Fulminant Myocarditis in Children in Campania Region, Italy: A Case Series" Children 11, no. 12: 1414. https://doi.org/10.3390/children11121414
APA StyleGiannattasio, A., Maglione, M., Di Nardo, G., Di Marco, G. M., Lauretta, D., Carrella, M. C., Furlan, D., Savoia, F., & Tipo, V. (2024). Outbreak of Acute Fulminant Myocarditis in Children in Campania Region, Italy: A Case Series. Children, 11(12), 1414. https://doi.org/10.3390/children11121414






