Perspectives and Challenges of Telemedicine and Artificial Intelligence in Pediatric Dermatology
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Telemedicine Applications in Pediatric Dermatology Subsection
3.2. The Role of Artificial Intelligence in Pediatric Dermatology
4. Discussion
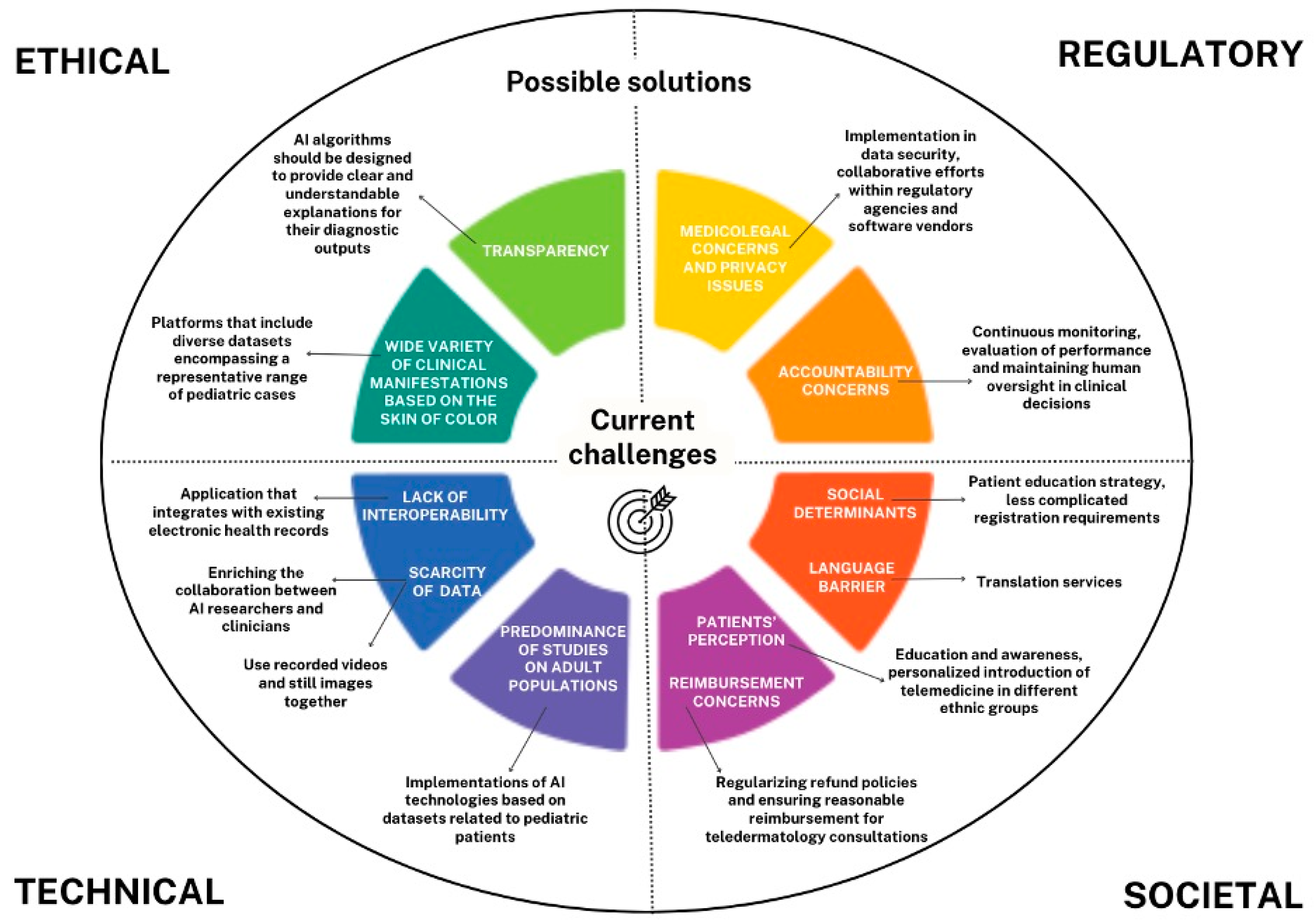
4.1. Challenges in Pediatric Dermatology Telemedicine and AI
4.1.1. Ethical and Regulatory Challenges
Lawfulness
Human Agency and Oversight
Technical Robustness and Safety
Privacy and Data Governance
Transparency
Diversity, Non-Discrimination, and Fairness
Environmental and Societal Well-Being
4.1.2. Technical Challenges
Interoperability
Data Quality and Availability (or Scarcity)
Model Flexibility and Specificity
4.1.3. Societal Challenges
Trust and Acceptance
Workforce Impact
Equity and Access
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prindaville, B.; Antaya, R.J.; Siegfried, E.C. Pediatric Dermatology: Past, Present, and Future. Pediatr. Dermatol. 2015, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Naka, F.; Makkar, H.; Lu, J. Teledermatology: Kids Are Not Just Little People. Clin. Dermatol. 2017, 35, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.K.; Hawryluk, E.B. Access to Pediatric Dermatology. Curr. Opin. Pediatr. 2022, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Burshtein, J.; Buethe, M.G.; Ghias, M.H.; Stein, A.B.; Glick, S.; Marmon, S. Efficacy, Perception, and Utilization of Pediatric Teledermatology: A Systematic Review. JAAD Int. 2023, 12, 3–11. [Google Scholar] [CrossRef]
- Duan, G.; Lambert, R.; Hight, R.; Rosenblatt, A. Comparison of Pediatric Dermatology Conditions Across Telehealth and In-Person Visits During the COVID-19 Pandemic. J. Drugs Dermatol. 2022, 21, 1260–1263. [Google Scholar] [CrossRef]
- Katzow, M.W.; Steinway, C.; Jan, S. Telemedicine and Health Disparities During COVID-19. Pediatrics 2020, 146, e20201586. [Google Scholar] [CrossRef]
- Gerber, D.E.; Barksdale, E.K.S. Approaches to Continuing COVID-19–Related Clinical Research Practices After the Pandemic—Must Cinderella Leave the Ball? JAMA Oncol. 2023, 9, 1027–1028. [Google Scholar] [CrossRef]
- Young, A.T.; Xiong, M.; Pfau, J.; Keiser, M.J.; Wei, M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020, 140, 1504–1512. [Google Scholar] [CrossRef]
- Lee, J.J.; English, J.C. Teledermatology: A Review and Update. Am. J. Clin. Dermatol. 2018, 19, 253–260. [Google Scholar] [CrossRef]
- Cartron, A.M.; Aldana, P.C.; Khachemoune, A. Pediatric Teledermatology: A Review of the Literature. Pediatr. Dermatol. 2021, 38, 39–44. [Google Scholar] [CrossRef]
- Whited, J.D. Teledermatology. Med. Clin. N. Am. 2015, 99, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Philp, J.C.; Frieden, I.J.; Cordoro, K.M. Pediatric Teledermatology Consultations: Relationship between Provided Data and Diagnosis. Pediatr. Dermatol. 2013, 30, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Batalla, A.; Suh-Oh, H.J.; Abalde, T.; Salgado-Boquete, L.; de la Torre, C. Teledermatology in Paediatrics. Observations in daily clinical practice. An. Pediatr. 2016, 84, 324–330. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.M.; Jew, O.S.; Perman, M.J.; Castelo-Soccio, L.A.; Winston, F.K.; McMahon, P.J. Diagnostic Accuracy of Pediatric Teledermatology Using Parent-Submitted Photographs: A Randomized Clinical Trial. JAMA Dermatol. 2017, 153, 1243–1248. [Google Scholar] [CrossRef]
- Pahalyants, V.; Murphy, W.S.; Gunasekera, N.S.; Das, S.; Hawryluk, E.B.; Kroshinsky, D. Evaluation of Electronic Consults for Outpatient Pediatric Patients with Dermatologic Complaints. Pediatr. Dermatol. 2021, 38, 1210–1218. [Google Scholar] [CrossRef]
- Heffner, V.A.; Lyon, V.B.; Brousseau, D.C.; Holland, K.E.; Yen, K. Store-and-Forward Teledermatology versus in-Person Visits: A Comparison in Pediatric Teledermatology Clinic. J. Am. Acad. Dermatol. 2009, 60, 956–961. [Google Scholar] [CrossRef]
- Chen, T.S.; Goldyne, M.E.; Mathes, E.F.D.; Frieden, I.J.; Gilliam, A.E. Pediatric Teledermatology: Observations Based on 429 Consults. J. Am. Acad. Dermatol. 2010, 62, 61–66. [Google Scholar] [CrossRef]
- Lowe, A.; Dawood, S.; Al-Tayeb, A.; Hancock, P.; Pararajasingam, A.; Ali, F.; Goodwin, R.G. Evaluating Paediatric Dermatology Telephone Clinics during COVID-19 from a Dual Clinician and Patient Perspective: A Prospective Study. Clin. Exp. Dermatol. 2022, 47, 553–560. [Google Scholar] [CrossRef]
- Taslidere, N.; Kucuk, O.S. Investigation of the Effectiveness of Teledermatology in the Diagnosis of Skin Lesions in Pediatric Patients. Rev. Assoc. Med. Bras. 2023, 69, e20230253. [Google Scholar] [CrossRef]
- Tollefson, M.M.; Frieden, I.J. Early Growth of Infantile Hemangiomas: What Parents’ Photographs Tell Us. Pediatrics 2012, 130, e314–e320. [Google Scholar] [CrossRef]
- Betlloch-Mas, I.; Martínez-Miravete, M.-T.; Berbegal-DeGracia, L.; Sánchez-Vázquez, L.; Sánchez-Payá, J. Teledermatology in Paediatrics: Health-Care Impact on the Early Treatment of Infantile Haemangiomas. J. Telemed. Telecare 2021, 27, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Frieden, I.J.; Püttgen, K.B.; Drolet, B.A.; Garzon, M.C.; Chamlin, S.L.; Pope, E.; Mancini, A.J.; Lauren, C.T.; Mathes, E.F.; Siegel, D.H.; et al. Management of Infantile Hemangiomas during the COVID Pandemic. Pediatr. Dermatol. 2020, 37, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Kittler, N.W.; Frieden, I.J.; Abuabara, K.; Siegel, D.H.; Horii, K.A.; Mathes, E.F.; Blei, F.; Haggstrom, A.N.; Streicher, J.L.; Metry, D.W.; et al. Successful Use of Telemedicine for Evaluation of Infantile Hemangiomas during the Early COVID-19 Pandemic: A Cross-Sectional Study. Pediatr. Dermatol. 2022, 39, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Gehris, R.P.; Herman, E.I.X. Pediatric Teledermatology: A Review. Curr. Derm. Rep. 2020, 9, 114–122. [Google Scholar] [CrossRef]
- Marchetti, A.; Dalle, S.; Maucort-Boulch, D.; Amini-Adl, M.; Debarbieux, S.; Poulalhon, N.; Perier-Muzet, M.; Phan, A.; Thomas, L. Diagnostic Concordance in Tertiary (Dermatologists-to-Experts) Teledermoscopy: A Final Diagnosis-Based Study on 290 Cases. Dermatol. Pract. Concept. 2020, 10, e2020071. [Google Scholar] [CrossRef]
- Ying, G.; Koch, K. General Practitioner Prescription Patterns for Atopic Eczema in Children-Are They Affected by Telemedicine Advice? Australas. J. Dermatol. 2024, 65, 369–372. [Google Scholar] [CrossRef]
- McCusker, S.; King Stokes, N.; Hunjan, M.; Daly, A.; George, S.; Solman, L.; Burden-Teh, E. Paediatric Dermatology Teleconsultations: A Survey of Healthcare Professionals in the UK. Clin. Exp. Dermatol. 2023, 48, 785–789. [Google Scholar] [CrossRef]
- Snoswell, C.; Finnane, A.; Janda, M.; Soyer, H.P.; Whitty, J.A. Cost-Effectiveness of Store-and-Forward Teledermatology: A Systematic Review. JAMA Dermatol. 2016, 152, 702–708. [Google Scholar] [CrossRef]
- Seiger, K.; Hawryluk, E.B.; Kroshinsky, D.; Kvedar, J.C.; Das, S. Pediatric Dermatology eConsults: Reduced Wait Times and Dermatology Office Visits. Pediatr. Dermatol. 2020, 37, 804–810. [Google Scholar] [CrossRef]
- Calafiore, R.; Khan, A.; Anderson, D.; Wu, Z.H.; Lu, J. Impact of Dermoscopy-Aided Pediatric Teledermatology Program on the Accessibility and Efficiency of Dermatology Care at Community Health Centers. J. Telemed. Telecare 2024, 30, 519–526. [Google Scholar] [CrossRef]
- Jew, O.S.; Murthy, A.S.; Danley, K.; McMahon, P.J. Implementation of a Pediatric Provider-to-Provider Store-and-Forward Teledermatology System: Effectiveness, Feasibility, and Acceptability in a Pilot Study. Pediatr. Dermatol. 2020, 37, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Paradela-De-La-Morena, S.; Fernandez-Torres, R.; Martínez-Gómez, W.; Fonseca-Capdevila, E. Teledermatology: Diagnostic Reliability in 383 Children. Eur. J. Dermatol. 2015, 25, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Toy, J.; Gregory, A.; Rehmus, W. Barriers to Healthcare Access in Pediatric Dermatology: A Systematic Review. Pediatr. Dermatol. 2021, 38 (Suppl. S2), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Prindaville, B.; Horii, K.A.; Siegfried, E.C.; Brandling-Bennett, H. Pediatric Dermatology Workforce in the United States. Pediatr. Dermatol. 2019, 36, 166–168. [Google Scholar] [CrossRef]
- Hansen, I.; Abeck, D.; Kött, J.; Schneider, S.W.; Abeck, F. The Potential of Telemedicine for Dermatological Care of Pediatric Patients in Germany. J. Dtsch. Dermatol. Ges. 2023, 21, 141–145. [Google Scholar] [CrossRef]
- Cline, A.; Gao, J.C.; Berk-Krauss, J.; Kaplan, L.; Bienenfeld, A.; Desai, A.; Huang, A.; Bleicher, B.; Chopra, R.; Shukla, S.; et al. Sustained Reduction in No-Show Rate with the Integration of Teledermatology in a Federally Qualified Health Center. J. Am. Acad. Dermatol. 2021, 85, e299–e301. [Google Scholar] [CrossRef]
- Cline, A.; Jacobs, A.K.; Fonseca, M.; Marmon, S. The Impact of Telemedicine on No-Show Rates in Pediatric Dermatology: A Multicenter Retrospective Analysis of Safety-Net Clinics. J. Am. Acad. Dermatol. 2022, 86, e235–e237. [Google Scholar] [CrossRef]
- Kohn, L.L.; Pickett, K.; Day, J.A.; Torres-Zegarra, C.; Plost, G.; Gurnee, E.; Prok, L.; Olson, C.A.; Manson, S.M.; Bruckner, A.L. When Is Synchronous Telehealth Acceptable for Pediatric Dermatology? Pediatr. Dermatol. 2022, 39, 236–242. [Google Scholar] [CrossRef]
- Fogel, A.L.; Teng, J.M.C. Pediatric Teledermatology: A Survey of Usage, Perspectives, and Practice. Pediatr. Dermatol. 2015, 32, 363–368. [Google Scholar] [CrossRef]
- Bridges, C.; Morris, C.; McElroy, J.A.; Quinn, K.; Dyer, J.; Becevic, M. Utility of Dermatology Extension for Community Healthcare Outcomes (ECHO) Sessions in the Adult and Paediatric Population. J. Telemed. Telecare 2021, 27, 376–381. [Google Scholar] [CrossRef]
- Lewis, H.; Becevic, M.; Myers, D.; Helming, D.; Mutrux, R.; Fleming, D.; Edison, K. Dermatology ECHO—An Innovative Solution to Address Limited Access to Dermatology Expertise. Rural Remote Health 2018, 18, 4415. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, D.F.; Boscardin, C.K.; Frieden, I.J.; Mathes, E.F.D. Can You See Me Now? Video Supplementation for Pediatric Teledermatology Cases. Pediatr. Dermatol. 2017, 34, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, S.; Calacione, R.; Fagotti, S.; Finizio, L.; Scaini, M.; Schiesari, L.; Gatti, A. Teledermatology with General Practitioners and Pediatricians during COVID-19 Outbreak in Italy: Preliminary Data from a Second-Level Dermatology Department in North-Eastern Italy. Dermatol. Ther. 2020, 33, e14040. [Google Scholar] [CrossRef] [PubMed]
- Ragamin, A.; Schappin, R.; Tan Nguyen, N.; Nouwen, A.E.M.; Hoekstra, L.F.; Schuttelaar, M.L.A.; Pasmans, S.G.M.A. Remote Severity Assessment in Atopic Dermatitis: Validity and Reliability of the Remote Eczema Area and Severity Index and Self-Administered Eczema Area and Severity Index. JAAD Int. 2023, 13, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zacher, N.C.; Pickett, K.L.; Schmiege, S.J.; Olson, C.A.; Bruckner, A.L.; Kohn, L.L. Retrospective Chart Review of Patient Socioeconomic Status and Language Preference Associated with Live Video Telehealth in a Pediatric Dermatology Practice. Pediatr. Dermatol. 2023, 40, 651–654. [Google Scholar] [CrossRef]
- Ferrante, G.; Licari, A.; Fasola, S.; Marseglia, G.L.; La Grutta, S. Artificial Intelligence in the Diagnosis of Pediatric Allergic Diseases. Pediatr. Allergy Immunol. 2021, 32, 405–413. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Schuman, A. AI in Pediatrics: Past, Present, and Future. Contemp Pediatr. 2019, 36, 38–45. [Google Scholar]
- Issa, C.J.; Reimer-Taschenbrecker, A.; Paller, A.S. A Call for Implementing Augmented Intelligence in Pediatric Dermatology. Pediatr. Dermatol. 2023, 40, 584–586. [Google Scholar] [CrossRef]
- Zhang, A.J.; Lindberg, N.; Chamlin, S.L.; Haggstrom, A.N.; Mancini, A.J.; Siegel, D.H.; Drolet, B.A. Development of an Artificial Intelligence Algorithm for the Diagnosis of Infantile Hemangiomas. Pediatr. Dermatol. 2022, 39, 934–936. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef]
- Mehta, P.P.; Sun, M.; Betz-Stablein, B.; Halpern, A.; Soyer, H.P.; Weber, J.; Kose, K.; Rotemberg, V. Improving Artificial Intelligence−Based Diagnosis on Pediatric Skin Lesions. J. Investig. Dermatol. 2023, 143, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. arXiv 2019, arXiv:1610.02391. [Google Scholar]
- Hadj-Rabia, S.; Schneider, H.; Navarro, E.; Klein, O.; Kirby, N.; Huttner, K.; Wolf, L.; Orin, M.; Wohlfart, S.; Bodemer, C.; et al. Automatic Recognition of the XLHED Phenotype from Facial Images. Am. J. Med. Genet. A 2017, 173, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Zhang, E.; Caussade, M.-C.; Brown, T.; Stockton Hogrogian, G.; Yan, A.C. Pediatric Dermatologists versus AI Bots: Evaluating the Medical Knowledge and Diagnostic Capabilities of ChatGPT. Pediatr. Dermatol. 2024, 41, 831–834. [Google Scholar] [CrossRef]
- Madiega, T. Artificial Intelligence Act. In European Parliament: European Parliamentary Research Service; EU Parliament: Brussels, Belgium, 2021. [Google Scholar]
- Floridi, L. Establishing the Rules for Building Trustworthy Ai. Nat. Mach. Intell. 2019, 1, 261–262. [Google Scholar] [CrossRef]
- Edison, K.E.; Dyer, J.A.; Whited, J.D.; Mutrux, R. Practice Gaps. The Barriers and the Promise of Teledermatology. Arch. Dermatol. 2012, 148, 650–651. [Google Scholar] [CrossRef]
- Long, V.; Chandran, N.S. A Glance at the Practice of Pediatric Teledermatology Pre- and Post–COVID-19: Narrative Review. JMIR Dermatol. 2022, 5, e34228. [Google Scholar] [CrossRef]
- Fogel, A.L.; Kvedar, J.C. Reported Cases of Medical Malpractice in Direct-to-Consumer Telemedicine. JAMA 2019, 321, 1309–1310. [Google Scholar] [CrossRef]
- General Data Protection Regulation (GDPR) Compliance Guidelines. Available online: https://gdpr.eu/ (accessed on 14 October 2024).
- Shu, L.-Q.; Sun, Y.-K.; Tan, L.-H.; Shu, Q.; Chang, A.C. Application of Artificial Intelligence in Pediatrics: Past, Present and Future. World J. Pediatr. 2019, 15, 105–108. [Google Scholar] [CrossRef]
- Holmes, A.N.; Chansky, P.B.; Simpson, C.L. Teledermatology Consultation Can Optimize Treatment of Cutaneous Disease by Nondermatologists in Under-Resourced Clinics. Telemed. J. E Health 2020, 26, 1284–1290. [Google Scholar] [CrossRef]
- Burshtein, J.; Buethe, M.G. Artificial Intelligence in Dermatology: A Review of Literature and Application to Pediatric Dermatology. SKIN J. Cutan. Med. 2024, 8, 1250–1257. [Google Scholar] [CrossRef]
- Daneshjou, R.; Vodrahalli, K.; Novoa, R.A.; Jenkins, M.; Liang, W.; Rotemberg, V.; Ko, J.; Swetter, S.M.; Bailey, E.E.; Gevaert, O.; et al. Disparities in Dermatology AI Performance on a Diverse, Curated Clinical Image Set. Sci. Adv. 2022, 8, eabq6147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Yang, Y.; Gao, Y. Artificial Intelligence-Aided Decision Support in Paediatrics Clinical Diagnosis: Development and Future Prospects. J. Int. Med. Res. 2020, 48, 300060520945141. [Google Scholar] [CrossRef] [PubMed]
- Andryani, N.A.C.; Achmad, S.; Ario, M.K.; Jerikho; Sun, S.; Daniel, L.; Constantine, N.; Mazaya, M.; Gondokaryono, S.P.; Puspitosari, D. AI-Based Paediatric Teledermatology Analysis and Proposed Framework. In Proceedings of the 2023 IEEE International Biomedical Instrumentation and Technology Conference (IBITeC), Yogyakarta, Indonesia, 9–10 November 2023; pp. 165–170. [Google Scholar]
- Ahuja, G.; Khushbakht, M.; Joe, J.; Eskinder, H.; Ekwunazu, C.; Boos, M.D. Pediatric Teledermatology: A Tool for Combating Dermatology Care Disparities. Dermatol. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Nami, N.; Giannini, E.; Burroni, M.; Fimiani, M.; Rubegni, P. Teledermatology: State-of-the-Art and Future Perspectives. Expert Rev. Dermatol. 2012, 7, 1–3. [Google Scholar] [CrossRef]
- Crockett, J.L.; Cordoro, K.M. Pediatric Dermatology eConsultation: Insights to Reduce Barriers to Utilization and Increase Access to Care. Pediatr. Dermatol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Du-Harpur, X.; Watt, F.M.; Luscombe, N.M.; Lynch, M.D. What Is AI? Applications of Artificial Intelligence to Dermatology. Br. J. Dermatol. 2020, 183, 423–430. [Google Scholar] [CrossRef]
- Ukoha, E.P.; Davis, K.; Yinger, M.; Butler, B.; Ross, T.; Crear-Perry, J.; Perron-Burdick, M.; Nijagal, M.A. Ensuring Equitable Implementation of Telemedicine in Perinatal Care. Obstet. Gynecol. 2021, 137, 487–492. [Google Scholar] [CrossRef]
- George, S.M.; Hamilton, A.; Baker, R. Pre-Experience Perceptions about Telemedicine among African Americans and Latinos in South Central Los Angeles. Telemed. J. E Health 2009, 15, 525–530. [Google Scholar] [CrossRef]
- Pineider, J.L.; Rangu, S.A.; Shaw, K.S.; Cipriano, S.D.; Oza, V.S. Pediatric Consultative Dermatology: A Survey of the Society for Pediatric Dermatology Workforce Reveals Shortcomings in Existing Practice Models of Pediatric Dermatology Consult Services in the United States. Pediatr. Dermatol. 2024, 41, 270–274. [Google Scholar] [CrossRef]
- Kennedy, J.; Arey, S.; Hopkins, Z.; Tejasvi, T.; Farah, R.; Secrest, A.M.; Lipoff, J.B. Dermatologist Perceptions of Teledermatology Implementation and Future Use After COVID-19: Demographics, Barriers, and Insights. JAMA Dermatol. 2021, 157, 595–597. [Google Scholar] [CrossRef]
- Duan, G.Y.; Ruiz De Luzuriaga, A.M.; Schroedl, L.M.; Rosenblatt, A.E. Disparities in Telemedicine Use during the COVID-19 Pandemic among Pediatric Dermatology Patients. Pediatr. Dermatol. 2022, 39, 520–527. [Google Scholar] [CrossRef]

| Author Year | Type of Study | Sample Size (Pediatric Population) | Age Range (Years) | Country | Primary Outcome | Secondary Outcomes | Significant Findings |
|---|---|---|---|---|---|---|---|
| Heffner et al., 2009 [16] | Cross-sectional study | 135 | 3 months–18 years, 6 months | USA | To assess the ability of a pediatric dermatologist to accurately diagnose rashes based on history and digital images. | To determine interrater agreement on SAF images. | Concordance between in-person and photographic diagnosis by the primary dermatologist was 82%. Concordance between the two dermatologists on images was 73%, whereas interrater agreement between the two dermatologists, one viewing the patient in person and the other viewing photographs alone, was 69%. |
| Chen et al., 2009 [17] | Retrospective cohort study | 429 | 0–12 | USA | To identify the major problems emerging in the use of teledermatology in the pediatric population. | In total, 42% of diagnoses were discordant, especially for tinea versicolor, seborrheic dermatitis, pityriasis rosea, xerosis, and lichen striatus. Agreement in management between the PCP and teledermatologist was found in only 28% of cases. Topical steroids are underused by PCPs. | |
| Tollefson et al., 2012 [20] | Cross-sectional study | 30 | USA | To examine early IH growth using parental photographs. | To assess the prevalence of hemangioma precursors evident at birth. | The majority of this growth occurs between 5.5 and 7.5 weeks of age. In total, 65% of patients had a hemangioma precursor on the first day of life. | |
| Philp et al., 2013 [12] | Retrospective cohort study | 395 | 0–18 | USA | To assess whether historical data and the quality of photographs influence the ability to render a diagnosis. | A diagnosis was made in 75% of cases, regardless of the historical data provided. Previous treatments are the only data related to a more likely diagnosis. Poor image quality was not a significant barrier to providing a diagnosis. | |
| Batalla et al., 2015 [13] | Retrospective and observational study | 183 | 0–15 | Spain | To describe the distribution of diseases consulted through teledermatology, avoiding FTF consultations, and the agreement between virtual and FTF diagnoses. | The most frequent diagnoses were inflammatory diseases (39%), benign pigmented lesions (23%), and infectious diseases (20%). In total, 48% of consultations were referred for a face-to-face diagnosis. The diagnostic agreement between the dermatologist who evaluated the virtual consultation and the one evaluating the FTF consultation was 89%. | |
| Paradela et al., 2015 [32] | Retrospective and observational study | 383 | 0–15 | Spain | To evaluate the trustworthiness of SAF teledermatology and its potential to decrease FTF visits. | A total of 55.9% of diagnoses were concordant between pediatricians and teledermatologists. A lower accuracy was associated with incomplete clinical data or bad-quality photographic images. In total, 58.4% of FTF visits were avoided. | |
| Feigenbaum et al., 2017 [42] | Controlled trial | 31 | USA | To assess whether recorded videos added to stationary images can enhance diagnosis and management in pediatric teledermatology, rather than relying solely on images. | Supplemental videos helped significantly with management accuracy. In less common conditions, the use of videos and still images outperformed the sole use of images in terms of management. | ||
| O’Connor et al., 2017 [14] | Prospective cohort study | 40 | 0–18 | USA | To evaluate concordance between diagnoses based on in-person examination and those based on parental photographs. | To assess the effect of photography instructions on in-person photograph-based vs. examination-based diagnoses. | Overall concordance between photograph-based and in-person diagnoses was 83%. The provision of photographic instruction did not statistically influence diagnostic concordance. |
| Bridges et al., 2019 [40] | Retrospective cross-sectional study | 44 | USA | To assess the effect of Dermatology ECHO, a telemonitoring program connecting PCPs and specialists, over 2 years. | In total, 45% of patients had correct diagnoses. Among them, 77% profited from expert treatment recommendations. | ||
| Bergamo et al., 2020 [43] | Monocentric prospective study | 32 | Italy | During the COVID-19 pandemic, a direct line for teledermatology was provided, where a dermatologist replied to all GP and pediatrician requests. | In 86% of teledermatology consultations, diagnosis and management were provided without the need for a visit. Teledermatology does not allow for second-level investigations during the visit. This can cause a delay in diagnosis. | ||
| Marchetti et al., 2020 [25] | Retrospective cohort study | 290 (44 children) | 0–15 | France | To assess diagnostic concordance in tertiary (dermatologist-to-expert) teledermoscopy and its efficiency. | Diagnostic concordance was found in 77% of cases. Final concordance on the benign or malignant nature of the lesion was observed in 77.3% of cases. | |
| Betlloch-Mas et al., 2020 [21] | Retrospective descriptive study | 432 | 0–14 | Spain | To evaluate the role of teledermatology as a tool in pediatric settings. | To assess whether teledermatology was effective in reducing the age of propranolol initiation in presumed IH. | In total, 48.12% of cases consulted via teledermatology were resolved remotely. After the implementation of telemedicine (2015–2018), children with IHs began treatment at a mean age of 4.5 months, before which treatment began at 7.1 months (2008–2014). |
| Seiger et al., 2020 [29] | Retrospective cohort study | 188 | 0–18 | USA | To assess the duration of waiting times and the avoidance of FTF dermatology visits via a pediatric dermatology eConsult program. | To assess recommendations for FTF dermatology visits and potential cost savings. | In total, 31.9% of cases were referred to FTF evaluation. After the eConsult, the mean wait time for an initial FTF evaluation was reduced by 31%. The program acted as a triage to avoid FTF visits and offered cost savings. |
| Jew et al., 2020 [31] | Prospective non-blinded cohort study | 43 | 0–18 | USA | To evaluate the efficiency of a provider-to-provider SAF teledermatology consultation process. | To assess the acceptance of SAF teledermatology among patients/parents, PCPs, and dermatologists. | The median time for PCPs to communicate teledermatology recommendations to families was 3 days. In-person follow-up visits after telemedicine were required for 23%. A total of 83% of parents, as well as all PCPs and dermatologists, were satisfied with the service. |
| Calafiore et al., 2021 [30] | Retrospective cohort study | 876 | USA | To assess the effect of a SAF teledermatology program augmented by the incorporation of dermoscopy in pediatric patient health centers. | All 536 telemedicine referrals received dermatological care within 24 h, whereas with the traditional system, the mean time to be visited by a dermatologist was 75 days. Only 12% of referrals were recommended for follow-up visits. | ||
| Pahalyants et al., 2021 [15] | Retrospective study | 310 | 0–22 | USA | To identify patients and variables associated with an efficient diagnosis and management in the SAF service. | To assess pediatrician and parental openness to teleconsultations. | Follow-up visits were recommended in 28% of cases. There was full concordance in the diagnoses of 70.1% of patients seen subsequently. A survey revealed that teleconsultations were received positively by pediatricians and parents. |
| Cline et al., 2022 [37] | Multicenter retrospective analysis | 3659 | USA | To evaluate the effect of telemedicine on no-show rates in pediatric dermatology from 3 safety-net clinics. | Telemedicine was linked to a significantly lower non-attendance rate at each site. | ||
| Kohn et al., 2022 [38] | Prospective single-center study | 519 | USA | To assess the pertinency of synchronous (live video) teledermatology in a pediatric setting. | Patient satisfaction (84.3%) with telehealth consultations was more likely to be higher compared to dermatologists (68.4%). A photo to support dermatologists in their examination was reported in 10.7% of cases. | ||
| Lowe et al., 2022 [18] | Prospective single-center cohort study | 116 | 1 month–17 years | Wales | To measure the potential of a virtual pediatric dermatology telephone clinic, both from the clinician and patient/parental perspective. | From the clinician’s viewpoint, most consultations (91%) were successfully concluded over the telephone. In total, 52% of parents felt unsatisfied as the majority (65%) preferred FTF follow-ups in the future. | |
| Kittler et al., 2022 [23] | Multicenter cross-sectional study | 281 | USA | To appraise the experiences of hemangioma specialists in managing IH using telemedicine. | The median time from referral to evaluation was 17 days. Median physician confidence in performing telemedicine evaluations was 95.0. Hybrid telemedicine and the review of photographs were favored as modalities. | ||
| Duan et al., 2022 [5] | Single-center cross-sectional study | 1444 | 0–18 | USA | To identify factors associated with disparities in telemedicine use among the pediatric dermatology population during the COVID-19 pandemic. | Being Black or African American, having a preferred non-English language, and not having public insurance represented factors of reduced use of telemedicine. | |
| Hansen et al., 2023 [35] | Retrospective cross-sectional study | 504 | 1 month–17.8 years | Germany | To describe the dermatological requests submitted by pediatricians to a dermatologist using SAF technology. | A definite diagnosis was made in 88.3% of cases. In total, 90% of the requests were processed on the same or following day. | |
| Ragamin et al., 2023 [44] | Prospective observational study | 87 | 4–12 | The Netherlands | To assess the validity of the Eczema Area and Severity Index (EASI) based on images and patient-assessed severity based on the Self-Administered EASI (SA-EASI). | Excellent validity (0.90), good inter- (0.77) and intrarater reliability (0.91), and standard error of measurement (4.31) were found for the EASI based on clinical images. The quality of images influences the assessment. A moderate correlation was found (0.60) between SA-EASI and EASI. | |
| Zacher et al., 2023 [45] | Cross-sectional retrospective study | 3027 | <18 | USA | To examine whether variables such as geographic residence, ADI, ethnicity, race, or insurance type influence the use of teledermatology in pediatric settings. | Patients with a primary language other than English were less likely to access pediatric dermatology care. No significant differences were found in those variables between patients seen only in person and those seen only through telehealth. | |
| Taslidere et al., 2023 [19] | Prospective observational study | 93 | 0–16 | To determine the group of pediatric patients in which teledermatology has the highest success rate in diagnosing dermatological lesions. | Diagnostic concordance between face-to-face and teledermatology diagnoses was 74%. The agreement rate was variable between different lesions: 100% for acne and scabies, and 25% for contact dermatitis. | ||
| Ying et al., 2024 [26] | Retrospective review | 162 | New Zealand | To analyze how specialist advice influences GPs’ prescribing practices and the impact of teledermatology in the management of pediatric eczema patients. | Following a dermatology specialist’s advice, an important change in the prescribing patterns of medications was observed. Even if it is effective, teledermatology is insufficient to guarantee the correct adoption of pediatric eczema guidelines by GPs. |
| Author Year | Type of Study | Sample Size (Pediatric Population) | Age Range (Years) | Country | Primary Outcome | Secondary Outcomes | Significant Findings |
|---|---|---|---|---|---|---|---|
| Zhang et al., 2022 [50] | Training and validation study | 5834 | 0–1 | USA | An AI algorithm was trained to recognize infantile hemangiomas based on clinical images. | The algorithm reached 91.7% accuracy in the diagnosis of facial IH. | |
| Mehta et al., 2023 [52] | Training and validation study | 1536 | 0–18 | Australia | To compare the rendering of an AI model trained on a standard adult-predominant dermoscopic dataset before and after the addition of pediatric images. | Pediatric images enhanced the algorithm’s efficiency, maintaining high performance on adult images. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zama, D.; Borghesi, A.; Ranieri, A.; Manieri, E.; Pierantoni, L.; Andreozzi, L.; Dondi, A.; Neri, I.; Lanari, M.; Calegari, R. Perspectives and Challenges of Telemedicine and Artificial Intelligence in Pediatric Dermatology. Children 2024, 11, 1401. https://doi.org/10.3390/children11111401
Zama D, Borghesi A, Ranieri A, Manieri E, Pierantoni L, Andreozzi L, Dondi A, Neri I, Lanari M, Calegari R. Perspectives and Challenges of Telemedicine and Artificial Intelligence in Pediatric Dermatology. Children. 2024; 11(11):1401. https://doi.org/10.3390/children11111401
Chicago/Turabian StyleZama, Daniele, Andrea Borghesi, Alice Ranieri, Elisa Manieri, Luca Pierantoni, Laura Andreozzi, Arianna Dondi, Iria Neri, Marcello Lanari, and Roberta Calegari. 2024. "Perspectives and Challenges of Telemedicine and Artificial Intelligence in Pediatric Dermatology" Children 11, no. 11: 1401. https://doi.org/10.3390/children11111401
APA StyleZama, D., Borghesi, A., Ranieri, A., Manieri, E., Pierantoni, L., Andreozzi, L., Dondi, A., Neri, I., Lanari, M., & Calegari, R. (2024). Perspectives and Challenges of Telemedicine and Artificial Intelligence in Pediatric Dermatology. Children, 11(11), 1401. https://doi.org/10.3390/children11111401












