Pediatric Cranial Vault Lesions: A Tailored Approach According to Bony Involvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics and Presurgical Evaluation
2.2. Surgical Strategy and Postoperative Assessment
2.3. Statistical Analysis
3. Results
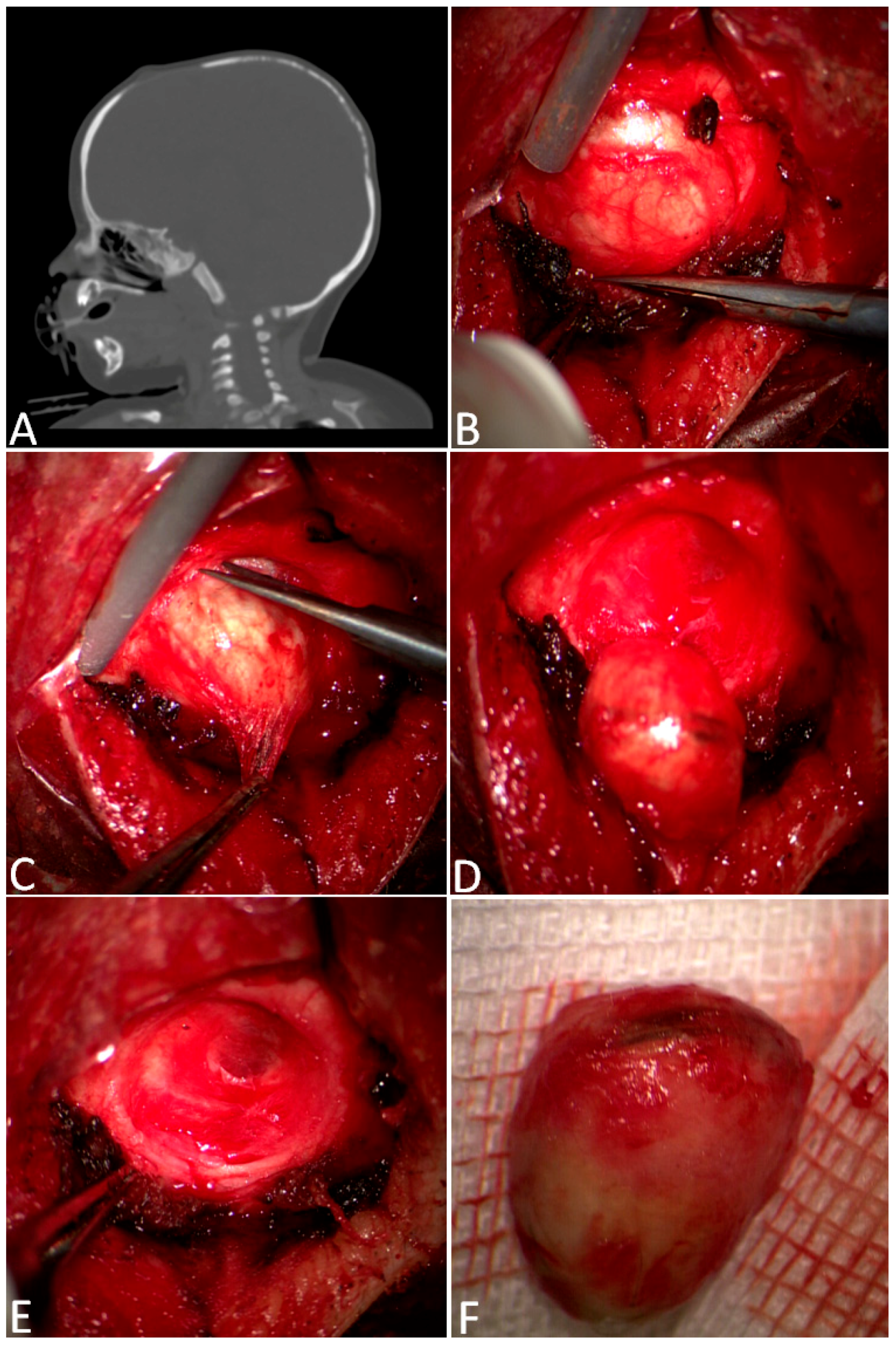
3.1. Surgical Techniques
3.2. Histopathological Examination and Follow-Up
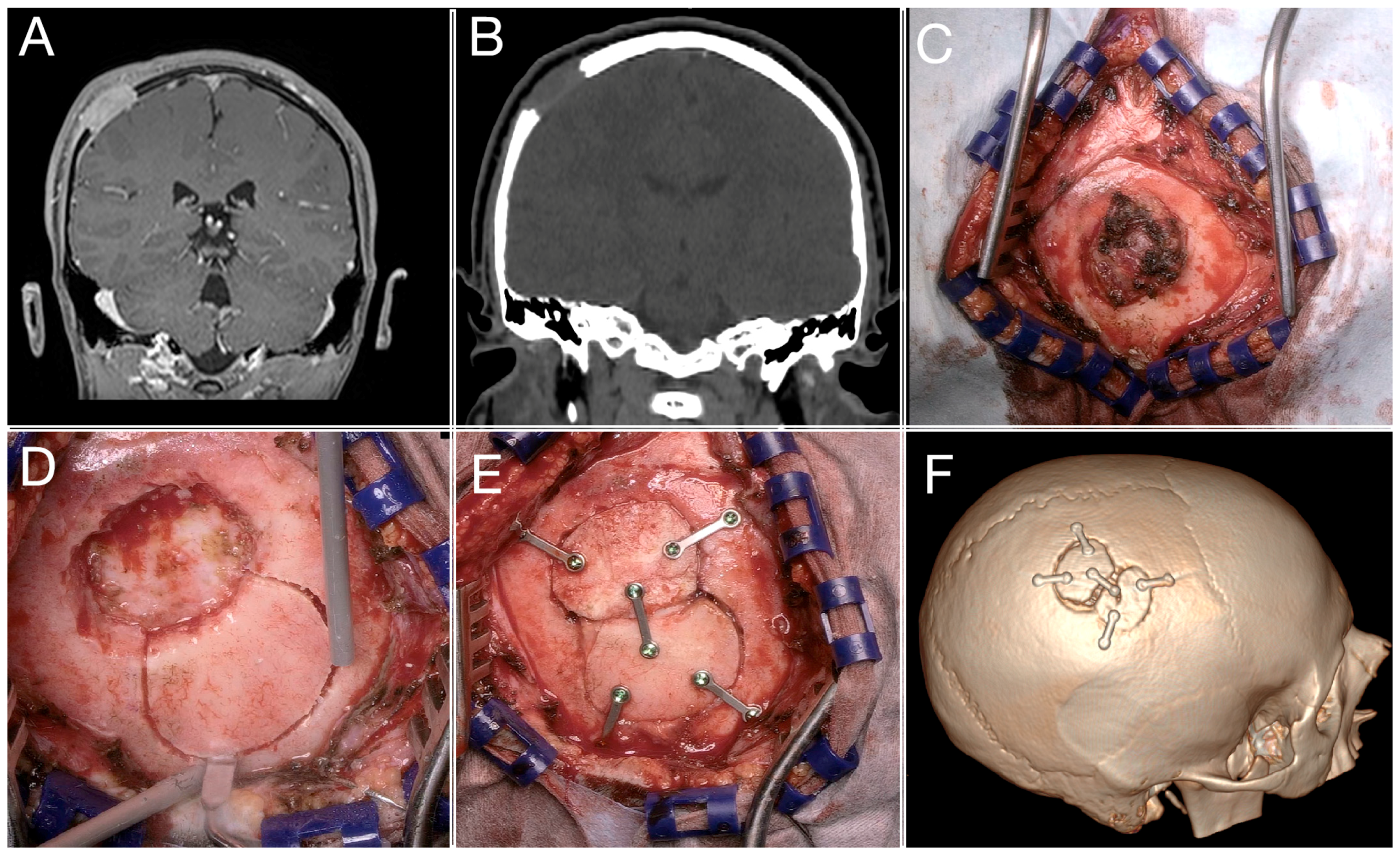
3.3. A Complex Case: Melanocytic Neuroectodermal Tumor
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruge, J.R.; Tomita, T.; Naidich, T.P.; Hahn, Y.S.; McLone, D.G. Scalp and Calvarial Masses of Infants and Children. Neurosurgery 1988, 22, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Barnett, R.R.; Piazza, M.G.; Elton, S.W. Pediatric Neurosurgery in Primary Care: Masses of the Scalp and Skull in Children. Pediatr. Clin. N. Am. 2021, 68, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Cummings, T.J.; George, T.M.; Fuchs, H.E.; McLendon, R.E. The Pathology of Extracranial Scalp and Skull Masses in Young Children. Clin. Neuropathol. 2004, 23, 33–43. [Google Scholar]
- Sorenson, E.P.; Powel, J.E.; Rozzelle, C.J.; Tubbs, R.S.; Loukas, M. Scalp Dermoids: A Review of Their Anatomy, Diagnosis, and Treatment. Child’s Nerv. Syst. 2013, 29, 375–380. [Google Scholar] [CrossRef]
- Skadorwa, T.; Ciszek, B. Clinical Characteristics of Benign Pediatric Cranial Vault Tumors: Surgical Considerations Based on 100 Cases. Pediatr. Neurosurg. 2016, 52, 13–19. [Google Scholar] [CrossRef]
- Prior, A.; Anania, P.; Pacetti, M.; Secci, F.; Ravegnani, M.; Pavanello, M.; Piatelli, G.; Cama, A.; Consales, A. Dermoid and Epidermoid Cysts of Scalp: Case Series of 234 Consecutive Patients. World Neurosurg. 2018, 120, 119–124. [Google Scholar] [CrossRef]
- Serrallach, B.L.; Orman, G.; Hicks, M.J.; Desai, N.; Kralik, S.; Huisman, T.A.G.M. Conventional and Advanced MR Imaging Findings in a Cohort of Pathology-Proven Dermoid Cysts of the Pediatric Scalp and Skull. Neuroradiol. J. 2022, 35, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Matsuo, M. CT and MRI Features of Scalp Lesions. Radiol. Medica 2019, 124, 1049–1061. [Google Scholar] [CrossRef]
- McDermott, T.; Amarneh, M.; Sato, Y.; Watal, P.; Charmi, V.; Fuortes, M.; Faruqui, S.; Sato, T.S. Pediatric Focal Calvarial Lesions: An Illustrated Review. Pediatr. Radiol. 2023, 53, 2699–2711. [Google Scholar] [CrossRef]
- Khalid, S.; Ruge, J. Considerations in the Management of Congenital Cranial Dermoid Cysts. J. Neurosurg. Pediatr. 2017, 20, 30–34. [Google Scholar] [CrossRef]
- Sinclair, R.D.; Darley, C.; Dawber, R.P.R. Congenital inclusion dermoid cysts of the scalp. Australas. J. Dermatol. 1992, 33. [Google Scholar] [CrossRef]
- Riebel, T.; David, S.; Thomale, U.W. Calvarial Dermoids and Epidermoids in Infants and Children: Sonographic Spectrum and Follow-Up. Child’s Nerv. Syst. 2008, 24, 1327–1332. [Google Scholar] [CrossRef]
- Aslan, Ö.; Özveren, F.; Kotil, K.; Özdemir, B.; Kuşçuoǧlu, U.; Bilge, T. Congenital Dermoid Cyst of the Anterior Fontanelle in Turkish Children - Four Case Reports. Neurol. Med. Chir. 2004, 44, 150–152. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.; Zhao, L.; Hu, X.; Cai, C. Congenital Dermoid Inclusion Cyst over the Anterior Fontanel in Chinese Children. Clin. Dysmorphol. 2020, 29, 81–85. [Google Scholar] [CrossRef]
- De Carvalho, G.T.C.; Fagundes-Pereyra, W.J.; Marques, J.A.P.; Dantas, F.L.R.; De Sousa, A.A. Congenital Inclusion Cysts of the Anterior Fontanelle. Surg. Neurol. 2001, 56, 400–405. [Google Scholar] [CrossRef]
- Wood, J.; Couture, D.; David, L.R. Midline Dermoid Cyst Resulting in Frontal Bone Erosion. J. Craniofacial Surg. 2012, 23, 131–134. [Google Scholar] [CrossRef]
- Yan, C.; Low, D.W. A Rare Presentation of a Dermoid Cyst with Draining Sinus in a Child: Case Report and Literature Review. Pediatr. Dermatol. 2016, 33, e244–e248. [Google Scholar] [CrossRef]
- Tateshima, S.; Numoto, R.T.; Abe, S.; Yasue, M.; Abe, T. Rapidly Enlarging Dermoid Cyst over the Anterior Fontanel: A Case Report and Review of the Literature. Child’s Nerv. Syst. 2000, 16, 875–878. [Google Scholar] [CrossRef]
- Akhaddar, A.; Jiddane, M.; Chakir, N.; El Hassani, R.; Moustarchid, B.; Bellakhdar, F. Cerebellar Abscesses Secondary to Occipital Dermoid Cyst with Dermal Sinus: Case Report. Surg. Neurol. 2002, 58, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Park, S.H. A Study of 77 Cases of Surgically Excised Scalp and Skull Masses in Pediatric Patients. Child’s Nerv. Syst. 2008, 24, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.; Bassani, L.; Ma, T.; Tanweer, O.; Elliott, R.E.; Harter, D.H.; Wisoff, J.H. Open and Endoscopic Excision of Calvarial Dermoid and Epidermoid Cysts: A Single Center Experience on 128 Consecutive Cases. Child’s Nerv. Syst. 2016, 32, 2351–2356. [Google Scholar] [CrossRef]
- Orozco-Covarrubias, L.; Lara-Carpio, R.; Saez-De-Ocariz, M.; Duran-Mckinster, C.; Palacios-Lopez, C.; Ruiz-Maldonado, R. Dermoid Cysts: A Report of 75 Pediatric Patients. Pediatr. Dermatol. 2013, 30, 706–711. [Google Scholar] [CrossRef]
- Kantorova, V.I. The Role of the Dura Mater in Induced Regeneration of Cranial Vault Bones. Sov. J. Dev. Biol. 1972, 3, 371–376. [Google Scholar]
- Oppenheimer, A.J.; Mesa, J.; Buchman, S.R. Current and Emerging Basic Science Concepts in Bone Biology: Implications in Craniofacial Surgery. J. Craniofacial Surg. 2012, 23, 30–36. [Google Scholar] [CrossRef]


| Tumor Type | N° | Rapid Growth and Pain | Adjuvant Therapies |
|---|---|---|---|
| Dermoid cysts | 62 | 0 | 0 |
| Myofibroma | 9 | 0 | 0 |
| Langerhans cell histiocytosis | 9 | 8 | 1 |
| Cavernomas | 3 | 2 | 0 |
| Eosinophilic granuloma | 2 | 2 | 1 |
| Osteoid osteoma | 1 | 0 | 0 |
| Rhabdomyosarcoma | 1 | 1 | 1 |
| Melanocytic neuroectodermal tumor | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbotti, A.; Vetrano, I.G.; Casali, C.; Galbiati, T.F.; Mariani, S.; Porto, E.; Erbetta, A.; Chiaravalli, S.; Valentini, L.G. Pediatric Cranial Vault Lesions: A Tailored Approach According to Bony Involvement. Children 2024, 11, 1377. https://doi.org/10.3390/children11111377
Barbotti A, Vetrano IG, Casali C, Galbiati TF, Mariani S, Porto E, Erbetta A, Chiaravalli S, Valentini LG. Pediatric Cranial Vault Lesions: A Tailored Approach According to Bony Involvement. Children. 2024; 11(11):1377. https://doi.org/10.3390/children11111377
Chicago/Turabian StyleBarbotti, Arianna, Ignazio G. Vetrano, Cecilia Casali, Tommaso F. Galbiati, Sabrina Mariani, Edoardo Porto, Alessandra Erbetta, Stefano Chiaravalli, and Laura G. Valentini. 2024. "Pediatric Cranial Vault Lesions: A Tailored Approach According to Bony Involvement" Children 11, no. 11: 1377. https://doi.org/10.3390/children11111377
APA StyleBarbotti, A., Vetrano, I. G., Casali, C., Galbiati, T. F., Mariani, S., Porto, E., Erbetta, A., Chiaravalli, S., & Valentini, L. G. (2024). Pediatric Cranial Vault Lesions: A Tailored Approach According to Bony Involvement. Children, 11(11), 1377. https://doi.org/10.3390/children11111377








