The Rising Popularity of Growth Hormone Therapy and Ensuing Orthopedic Complications in the Pediatric Population: A Review
Abstract
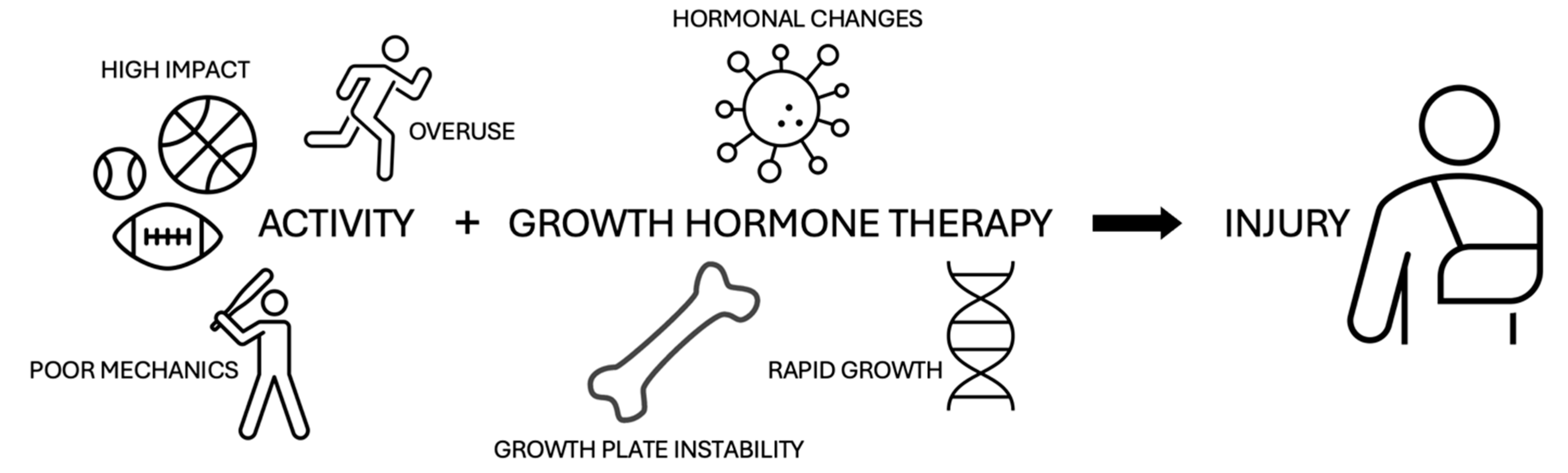
1. Introduction
2. Pathogenesis
3. Scoliosis
4. Osteochondritis Dissecans
5. Slipped Capital Femoral Epiphysis
6. Legg–Calve–Perthes Disease
7. Little League Shoulder
8. Osgood–Schlatter Disease
9. Sever’s Disease
10. Carpal Tunnel Syndrome
11. Discussion
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danowitz, M.; Grimberg, A. Clinical Indications for Growth Hormone Therapy. Adv. Pediatr. 2022, 69, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Veldhuis, J.D.; Thorner, M.O. Normal Control of Growth Hormone Secretion. Horm. Res. 1993, 40, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Kanter, G.P. US Growth Hormone Use in the Idiopathic Short Stature Era: Trends in Insurer Payments and Patient Financial Burden. J. Endocr. Soc. 2019, 3, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. Cardiovascular Disease in Former Pediatric Recipients of Growth Hormone: Another Look at Growth Hormone Safety. JAMA Pediatr. 2021, 175, e205232. [Google Scholar] [CrossRef]
- Haidar, R.K.; Nasrallah, M.P.; Der-Boghossian, A.H.; Ghanem, I.B. Orthopedic complications related to growth hormone therapy in a pediatric population. J. Pediatr. Orthop. B 2011, 20, 57–61. [Google Scholar] [CrossRef]
- Docquier, P.-L.; Mousny, M.; Jouret, M.; Bastin, C.; Rombouts, J.-J. Orthopaedic concerns in children with growth hormone therapy. Acta Orthop. Belg. 2004, 70, 299–305. [Google Scholar]
- Beber, S.A.; Gross, P.W.; Nichols, E.; Green, D.W.; Fabricant, P.D. Strong Association Between Growth Hormone Therapy and Proximal Tibial Physeal Avulsion Fractures in Children and Adolescents: A Case-Control Study. J. Bone Jt. Surg. 2023, 106, 227–231. [Google Scholar] [CrossRef]
- Baumgarten, K.M.; Oliver, H.A.; Foley, J.; Chen, D.-G.; Autenried, P.; Duan, S.; Heiser, P. Human Growth Hormone May Be Detrimental When Used to Accelerate Recovery from Acute Tendon-Bone Interface Injuries. J. Bone Jt. Surg. 2013, 95, 783–789. [Google Scholar] [CrossRef]
- Dalton, S.E. Overuse Injuries in Adolescent Athletes. Sports Med. 1992, 13, 58–70. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef]
- Isgaard, J.; Nilsson, A.; Lindahl, A.; Jansson, J.O.; Isaksson, O.G. Effects of local administration of GH and IGF-1 on longitudinal bone growth in rats. Am. J. Physiol.-Endocrinol. Metab. 1986, 250, E367–E372. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Isgaard, J.; Lindahl, A.; Peterson, L.; Isaksson, O. Effects of unilateral arterial infusion of GH and IGF-I on tibial longitudinal bone growth in hypophysectomized rats. Calcif. Tissue Int. 1987, 40, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Balkman, C.E.; Sandell, L.J.; Ratcliffe, A.; Nixon, A.J. Insulin-like growth factor-I gene expression patterns during spontaneous repair of acute articular cartilage injury. J. Orthop. Res. 2001, 19, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.L. Bone disease in patients receiving growth hormone. Kidney Int. Suppl. 1996, 53, S126–S127. [Google Scholar]
- Masquijo, J.; Kothari, A. Juvenile osteochondritis dissecans (JOCD) of the knee: Current concepts review. EFORT Open Rev. 2019, 4, 201–212. [Google Scholar] [CrossRef]
- Hong, H.; Pan, X.; Song, J.; Fang, N.; Yang, R.; Xiang, L.; Wang, X.; Huang, C. Idiopathic short stature and scoliosis in children treated with growth hormone: Scoliosis and idiopathic short stature in a cross-sectional and cohort study of 6159 children. Bone Jt. J. 2023, 105-B, 439–448. [Google Scholar] [CrossRef]
- Latalski, M.; Danielewicz-Bromberek, A.; Fatyga, M.; Latalska, M.; Kröber, M.; Zwolak, P. Current insights into the aetiology of adolescent idiopathic scoliosis. Arch. Orthop. Trauma. Surg. 2017, 137, 1327–1333. [Google Scholar] [CrossRef]
- Shi, B.; Mao, S.; Liu, Z.; Sun, X.; Zhu, Z.; Zhu, F.; Cheng, J.C.Y.; Qiu, Y. Spinal growth velocity versus height velocity in predicting curve progression in peri-pubertal girls with idiopathic scoliosis. BMC Musculoskelet. Disord. 2016, 17, 368. [Google Scholar] [CrossRef]
- Moawad, D.; Bell, J.; Lippe, B. National Cooperative Growth Study; YS Medical Media Ltd.: Netanya, Israel, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Maghnie, M.; Ranke, M.B.; Geffner, M.E.; Vlachopapadopoulou, E.; Ibáñez, L.; Carlsson, M.; Cutfield, W.; Rooman, R.; Gomez, R.; Wajnrajch, M.P.; et al. Safety and Efficacy of Pediatric Growth Hormone Therapy: Results from the Full KIGS Cohort. J. Clin. Endocrinol. Metab. 2022, 107, 3287–3301. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.J.; Oh, K.E.; Kang, E.; Nam, H.-K.; Rhie, Y.-J.; Lee, K.-H. The association between idiopathic scoliosis and growth hormone treatment in short children. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 207–213. [Google Scholar] [CrossRef]
- Rappaport, E.B. Effects of Exogenous Growth Hormone on Growth Plate Cartilage in Rats. Arch. Pediatr. Adolesc. Med. 1987, 141, 497. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.M.; Hussain, H.M.; Hussain, M.S.; Ho, S.S.W. Human growth hormone and the development of osteochondritis dissecans lesions. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Akagi, R.; Sato, Y.; Enomoto, T.; Nakagawa, R.; Kimura, S.; Yamaguchi, S.; Nishikawa, S.; Sasho, T. Multiple Osteochondritis Dissecans in Multiple Joints. Case Rep. Orthop. 2021, 2021, 8828687. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Yang, A.; Kollias, C.; Bakir, C.; Carsen, S.; Lazier, J.; Innes, A.M.; Pagé, M.; Dawrant, J.; Robinson, M.-E.; et al. From “ACAN” to “I CAN”: Restoring wellness in a boy with severe osteochondritis dissecans through diagnostic precision combined with optimal medical, surgical and rehabilitation management. Bone Rep. 2023, 18, 101663. [Google Scholar] [CrossRef]
- Witbreuk, M.; Van Kemenade, F.J.; Van Der Sluijs, J.A.; Jansma, E.P.; Rotteveel, J.; Van Royen, B.J. Slipped capital femoral epiphysis and its association with endocrine, metabolic and chronic diseases: A systematic review of the literature. J. Child. Orthop. 2013, 7, 213–223. [Google Scholar] [CrossRef]
- Mathew, S.E.; Larson, A.N. Natural History of Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2019, 39 (Suppl. S1), S23–S27. [Google Scholar] [CrossRef]
- Perry, D.C.; Metcalfe, D.; Lane, S.; Turner, S. Childhood Obesity and Slipped Capital Femoral Epiphysis. Pediatrics 2018, 142, e20181067. [Google Scholar] [CrossRef]
- Loder, R.T.; Skopelja, E.N. The Epidemiology and Demographics of Slipped Capital Femoral Epiphysis. ISRN Orthop. 2011, 2011, 486512. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, Y.J.; Song, M.H.; Cho, T.-J.; Choi, I.H.; Shin, C.H. Incidence and Clinical Characteristics of Slipped Capital Femoral Epiphysis in Patients with Endocrinopathy: A Population-Based Cohort Study. J. Bone Jt. Surg. 2023, 106, 381–388. [Google Scholar] [CrossRef]
- Bell, J.; Parker, K.L.; Swinford, R.D.; Hoffman, A.R.; Maneatis, T.; Lippe, B. Long-Term Safety of Recombinant Human Growth Hormone in Children. J. Clin. Endocrinol. Metab. 2010, 95, 167–177. [Google Scholar] [CrossRef]
- Darendeliler, F.; Karagiannis, G.; Wilton, P. Headache, Idiopathic Intracranial Hypertension and Slipped Capital Femoral Epiphysis during Growth Hormone Treatment: A Safety Update from the KIGS Database. Horm. Res. Paediatr. 2007, 68, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fine, R.N.; Ho, M.; Tejani, A.; Blethen, S. Adverse events with rhGH treatment of patients with chronic renal insufficiency and end-stage renal disease. J. Pediatr. 2003, 142, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.L.; Arons, R.R.; Loder, R.T.; Vitale, M.G. The Epidemiology of Slipped Capital Femoral Epiphysis: An Update. J. Pediatr. Orthop. 2006, 26, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, M.P.; Der-Boghossian, A.H.; Haidar, R.K. Slipped Capital Femoral Epiphysis In A Patient with Turner Syndrome Receiving Growth Hormone Therapy. Endocr. Pract. 2012, 18, e135–e137. [Google Scholar] [CrossRef]
- Bolar, K.; Hoffman, A.R.; Maneatis, T.; Lippe, B. Long-Term Safety of Recombinant Human Growth Hormone in Turner Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 344–351. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Isaacoff, E.J.; Spiegel, D.; Gruccio, D.; Ginsberg, J.P.; Hobbie, W.; Shults, J.; Leonard, M.B. Childhood cancer survivors exposed to total body irradiation are at significant risk for slipped capital femoral epiphysis during recombinant growth hormone therapy: SCFE Risk in Cancer Survivors on rhGH. Pediatr. Blood Cancer 2013, 60, 1766–1771. [Google Scholar] [CrossRef][Green Version]
- Mittal, M.; Momtaz, D.; Gonuguntla, R.; Singh, A.; Dave, D.; Mohseni, M.; Torres-izquierdo, B.; Schaibley, C.; Hosseinzadeh, P. The Effect of Human Growth Hormone Treatment on the Development of Slipped Capital Femoral Epiphysis: A Cohort Analysis With 6 Years of Follow-up. J. Pediatr. Orthop. 2024, 44, e344–e350. [Google Scholar] [CrossRef]
- Perry, D.C.; Hall, A.J. The Epidemiology and Etiology of Perthes Disease. Orthop. Clin. North. Am. 2011, 42, 279–283. [Google Scholar] [CrossRef]
- Laine, J.C.; Novotny, S.A.; Tis, J.E.; Sankar, W.N.; Martin, B.D.; Kelly, D.M.; Gilbert, S.R.; Shah, H.; Joseph, B.; Kim, H.K.W.; et al. Demographics and Clinical Presentation of Early-Stage Legg-Calvé-Perthes Disease: A Prospective, Multicenter, International Study. J. Am. Acad. Orthop. Surg. 2021, 29, e85–e91. [Google Scholar] [CrossRef]
- Lamback, E.B.; Chiarini, S.; Roposch, A.; Dattani, M.T. Congenital growth hormone deficiency associated with hip dysplasia and Legg-Calve-Perthes disease. Clin. Endocrinol. 2021, 94, 590–597. [Google Scholar] [CrossRef]
- Shi, Y.; Ying, Y.; Luo, X.; Hou, L. Growth Hormone Treatment in Children with Perthes Disease and GrowthHormone Deficiency: A Case Report and Literature Review. Endocr. Metab. Immune. Disord. Drug Targets 2023, 23, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Germain-Lee, E.L.; Dunbar, N.S. Legg-Calve-Perthes disease in an 8-year old girl with Acrodysostosis type 1 on growth hormone therapy: Case report. Int. J. Pediatr. Endocrinol. 2020, 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Baş, V.N.; Uytun, S.; Vurdem, Ü.E.; Torun, Y.A. Hypopituitarism and Legg-Calve-Perthes disease related to difficult delivery. Korean J. Pediatr. 2015, 58, 270. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Wolf, M.; Herman, M. Acute and Chronic Growth Plate Injuries. Pediatr. Rev. 2017, 38, 129–138. [Google Scholar] [CrossRef]
- Brown, T.; Moran, M. Pediatric Sports-Related Injuries. Clin. Pediatr. 2019, 58, 199–212. [Google Scholar] [CrossRef]
- Heyworth, B.E.; Kramer, D.E.; Martin, D.J.; Micheli, L.J.; Kocher, M.S.; Bae, D.S. Trends in the Presentation, Management, and Outcomes of Little League Shoulder. Am. J. Sports Med. 2016, 44, 1431–1438. [Google Scholar] [CrossRef]
- Kosko, B.; Richey, B.; Cardin, S.; White, K.; Youmans, D.H.; Service, B.; Osbahr, D.C. Little League Shoulder and Subsequent Proximal Humeral Fracture in the Setting of Human Growth Hormone Use: A Case Report. JBJS Case Connect. 2023, 13, e22. [Google Scholar] [CrossRef]
- Circi, E.; Atalay, Y.; Beyzadeoglu, T. Treatment of Osgood–Schlatter disease: Review of the literature. Musculoskelet. Surg. 2017, 101, 195–200. [Google Scholar] [CrossRef]
- Corbi, F.; Matas, S.; Álvarez-Herms, J.; Sitko, S.; Baiget, E.; Reverter-Masia, J.; López-Laval, I. Osgood-Schlatter Disease: Appearance, Diagnosis and Treatment: A Narrative Review. Healthcare 2022, 10, 1011. [Google Scholar] [CrossRef]
- Lucenti, L.; Sapienza, M.; Caldaci, A.; Cristo, C.; Testa, G.; Pavone, V. The Etiology and Risk Factors of Osgood–Schlatter Disease: A Systematic Review. Children 2022, 9, 826. [Google Scholar] [CrossRef]
- Gigante, A.; Bevilacqua, C.; Bonetti, M.; Greco, F. Increased external tibial torsion in Osgood-Schlatter disease. Acta Orthop. Scand. 2003, 74, 431–436. [Google Scholar] [CrossRef] [PubMed]
- De Lucena, G.L.; Dos Santos Gomes, C.; Guerra, R.O. Prevalence and Associated Factors of Osgood-Schlatter Syndrome in a Population-Based Sample of Brazilian Adolescents. Am. J. Sports Med. 2011, 39, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D.; Albright, J.A.; Callanan, T.C.; Hall, R.P.; Zhang, H.; Daniels, A.H.; Cruz, A.I. Incidence of Tibial Tubercle Fractures in Patients With and Without Osgood-Schlatter Disease. J. Pediatr. Orthop. 2024, 44, e763–e766. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, P.; Fan, M.; Zhuang, H.; Guo, R.; Zheng, P.; Tang, K. Risk factor analysis for tibial tubercle avulsion fractures in children. Int. Orthop. 2023, 47, 2347–2356. [Google Scholar] [CrossRef]
- James, A.M.; Williams, C.M.; Haines, T.P. Health related quality of life of children with calcaneal apophysitis: Child & parent perceptions. Health Qual. Life Outcomes 2016, 14, 95. [Google Scholar] [CrossRef]
- Hart, E.; Meehan, W.P.; Bae, D.S.; d’Hemecourt, P.; Stracciolini, A. The Young Injured Gymnast: A Literature Review and Discussion. Curr. Sports Med. Rep. 2018, 17, 366–375. [Google Scholar] [CrossRef]
- Launay, F. Sports-related overuse injuries in children. Orthop. Traumatol. Surg. Res. 2015, 101, S139–S147. [Google Scholar] [CrossRef]
- Ayuk, J.; Sheppard, M.C. Growth hormone and its disorders. Postgrad. Med. J. 2006, 82, 24–30. [Google Scholar] [CrossRef]
- Kurnaz, E.; Savas-Erdeve, S.; Aycan, Z.; Çetinkaya, S. Sever’s Disease in a Patient Receiving Growth Hormone with no Causative Relation. J. Clin. Diagn. Res. 2018, 12, SD04–SD05. [Google Scholar] [CrossRef]
- Aboonq, M.S. Pathophysiology of carpal tunnel syndrome. Neurosciences 2015, 20, 4–9. [Google Scholar]
- Joshi, A.; Patel, K.; Mohamed, A.; Oak, S.; Zhang, M.H.; Hsiung, H.; Zhang, A.; Patel, U.K. Carpal Tunnel Syndrome: Pathophysiology and Comprehensive Guidelines for Clinical Evaluation and Treatment. Cureus 2022, 14, e27053. [Google Scholar] [CrossRef] [PubMed]
- Maneatis, T.; Baptista, J.; Connelly, K.; Blethen, S. Growth hormone safety update from the National Cooperative Growth Study. J. Pediatr. Endocrinol. Metab. 2000, 13 (Suppl. S2), 1035–1044. [Google Scholar] [PubMed]
- Tang, H.-C.; Cheng, Y.-Y.; Guo, H.-R. Association between hormone replacement therapy and carpal tunnel syndrome: A nationwide population-based study. BMJ Open 2022, 12, e055139. [Google Scholar] [CrossRef] [PubMed]
- Vouzouneraki, K.; Esposito, D.; Mukka, S.; Granfeldt, D.; Ragnarsson, O.; Dahlqvist, P.; Olsson, D.S. Carpal tunnel syndrome in acromegaly: A nationwide study. Eur. J. Endocrinol. 2021, 184, 209–216. [Google Scholar] [CrossRef]
- Davis, L.; Vedanarayanan, V.V. Carpal Tunnel Syndrome in Children. Pediatr. Neurol. 2014, 50, 57–59. [Google Scholar] [CrossRef]
- Buluc, L.; Selek, O.; Aranay, Y. Bilateral Carpal Tunnel Syndrome in a 9-year-old Boy with Acromicric Dysplasia. Orthopedics 2012, 35, e1553–e1555. [Google Scholar] [CrossRef]
- Blethen, S.L.; Allen, D.B.; Graves, D.; August, G.; Moshang, T.; Rosenfeld, R. Safety of recombinant deoxyribonucleic acid-derived growth hormone: The National Cooperative Growth Study experience. J. Clin. Endocrinol. Metab. 1996, 81, 1704–1710. [Google Scholar] [CrossRef]
- Richmond, E.; Rogol, A.D. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best. Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 749–755. [Google Scholar] [CrossRef]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H.; on behalf of the Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef]
- Oktayoglu, P. Assessment of the Presence of Carpal Tunnel Syndrome in Patients with Diabetes Mellitus, Hypothyroidism and Acromegaly. J. Clin. Diagn. Res. 2015, 9, OC14–OC18. [Google Scholar] [CrossRef]
- Galloway, K.M.; Greathouse, D.G. Carpal Tunnel Syndrome in an Adolescent with Obesity. Pediatr. Phys. Ther. 2016, 28, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Aversa, T.; Li Pomi, A.; Pepe, G.; Corica, D.; Messina, M.F.; Coco, R.; Sippelli, F.; Ferraloro, C.; Luppino, G.; Valenzise, M.; et al. Growth Hormone Treatment to Final Height in Turner Syndrome: Systematic Review. Clin. Ther. 2023, 46, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Sodero, G.; Cipolla, C.; Pane, L.C.; Sessa, L.; Malavolta, E.; Arzilli, F.; Leoni, C.; Zampino, G.; Rigante, D. Efficacy and safety of growth hormone therapy in children with Noonan syndrome. Growth Horm. IGF Res. 2023, 69–70, 101532. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zverev, S.; Tenner, Z.M.; Coladonato, C.; Lazar-Antman, M. The Rising Popularity of Growth Hormone Therapy and Ensuing Orthopedic Complications in the Pediatric Population: A Review. Children 2024, 11, 1354. https://doi.org/10.3390/children11111354
Zverev S, Tenner ZM, Coladonato C, Lazar-Antman M. The Rising Popularity of Growth Hormone Therapy and Ensuing Orthopedic Complications in the Pediatric Population: A Review. Children. 2024; 11(11):1354. https://doi.org/10.3390/children11111354
Chicago/Turabian StyleZverev, Samuel, Zachary M. Tenner, Carlo Coladonato, and Meredith Lazar-Antman. 2024. "The Rising Popularity of Growth Hormone Therapy and Ensuing Orthopedic Complications in the Pediatric Population: A Review" Children 11, no. 11: 1354. https://doi.org/10.3390/children11111354
APA StyleZverev, S., Tenner, Z. M., Coladonato, C., & Lazar-Antman, M. (2024). The Rising Popularity of Growth Hormone Therapy and Ensuing Orthopedic Complications in the Pediatric Population: A Review. Children, 11(11), 1354. https://doi.org/10.3390/children11111354





