Unveiling Tumorigenesis Mechanisms and Drug Therapy in Neuroblastoma by Mass Spectrometry Based Proteomics
Abstract
:1. Introduction
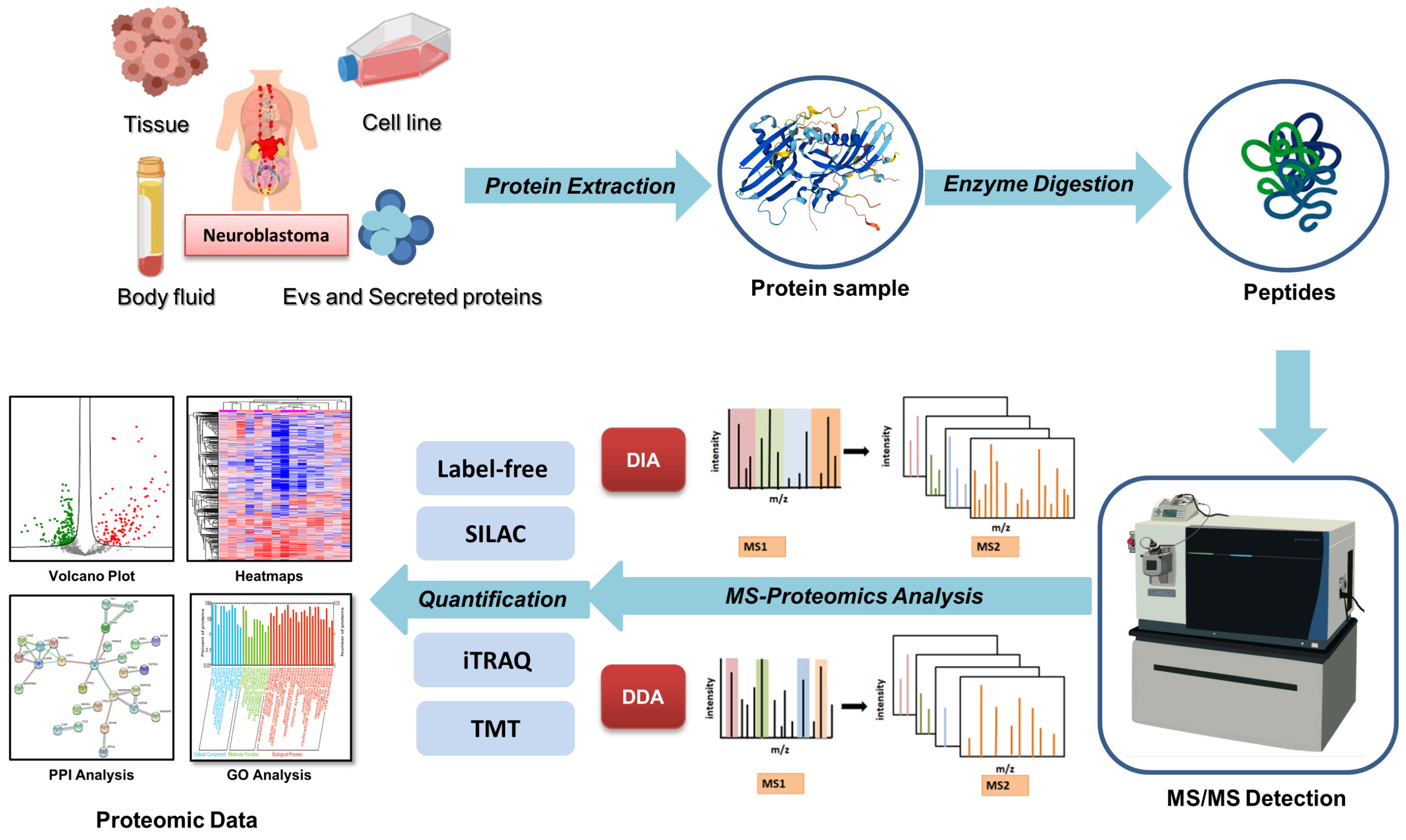
2. Advances in Mass Spectrometry-Based Proteomics Technologies
| The Classifications on MS-Based Proteomics Techniques | Characteristics of Methods | Advantages | |
|---|---|---|---|
| Data acquisition methods | DDA [29] | Selected numbers of fragmentation spectra of peptides are measured | Widely used, compatible with all quantification methods |
| DIA [31] | All the precursor ions in the given range are acquired for the fragmentation | High proteome coverage, high reproducibility | |
| Targeted MS approaches [41] | Including selected reaction monitoring, multiple reaction monitoring and parallel reaction monitoring | Monitor the biologically important proteins and peptides in a complex mixture, high sensitivity | |
| 4D-proteomics with DIA [33,34] | Ion mobility is introduced as the fourth separation dimension for peptide ions | High coverage, high reproducibility, capable of resolving PTM isoforms | |
| Quantification methods | Label-free [35] | Measurement based on the intensity of peptide signals in MS without the use of labels | Cost-effective |
| SILAC [36] | Culturing cells in isotope-containing culture media | High proteome coverage, high dynamic range and quantification accuracy | |
| iTRAQ/TMTs [37,38] | Add isobaric tags directly to enzyme-digested peptides | High proteome coverage, easy workflow with sample multiplexing | |
| MS-based proteomics on PTMs [39,40] | Affinity enrichment process in pretreatment | Capable to explore subtle alteration in PTM level | |
3. Proteomics and Neuroblastoma
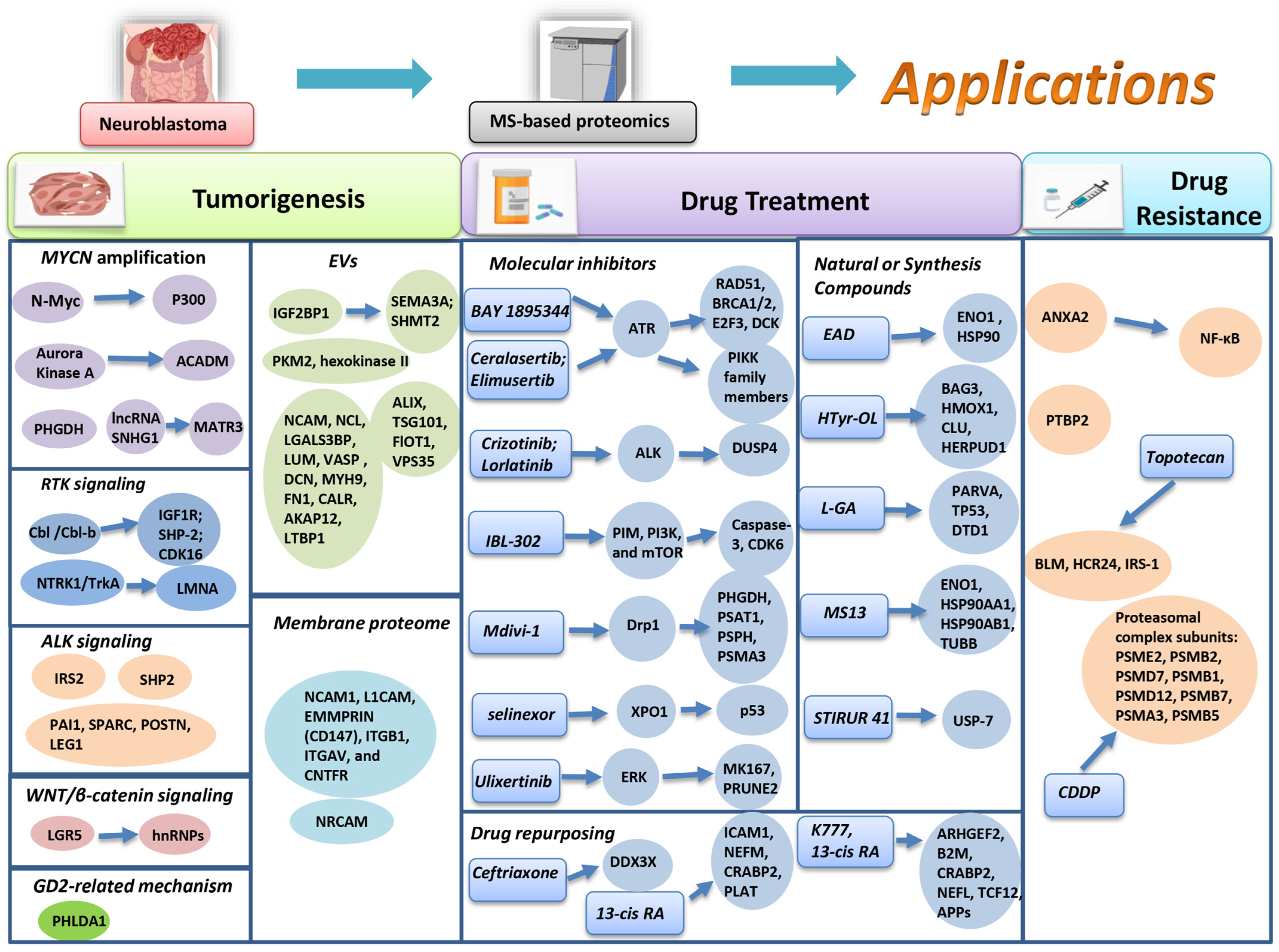
3.1. Proteomics Application in Neuroblastoma Tumorigenesis
| Authors | Samples | MS-Proteomic Methods | Proteins Surveyed by Proteomics | Critical NB-Related Proteins Identified | Molecular Functions and Mechanisms |
|---|---|---|---|---|---|
| Cheng et al. [49] | SK-N-BE(2) and 293T cells | Label-free proteomics; | N-Myc interacting proteins | P300 | Stabilizing N-Myc |
| Hsieh et al. [50] | Th-MYCN mouse model | iTRAQ-labeled proteomics | Proteins response to Aurora kinases inhibition | ACADM (+) | Inducing β-oxidation metabolism and reducing NB progression |
| Arlt et al. [51] | 49 NB biopsies and 13 NB cell lines | Label-free proteomics; | Proteins correlated to MYCN-amplified level | PHGDH (+) | Inducing serine synthesis and one-carbon metabolism and promoting NB proliferation. |
| Yang et al. [52] | SK-N-BE(2), SK-N-DZ and SK-N-AS cells | - | lncRNA SNHG1 interacting proteins | MATR3 | Inducing RNA splicing and enhancing NB progression |
| Pedersen et al. [53] | SH-SY5Y cells | SILAC-labeled proteomics, TMT-labeled phosphoproteomics | Proteins response to Cbl proteins depletion | IGF1R (+), SHP2 (+), CDK16 (+) | Inducing ERK phosphorylation and promoting neurite outgrowth |
| Funke et al. [54]. | IMR5 cell | Label-free proteomics; phosphoproteomics | Proteins response to NTRK1/TrkA activation | Lamin A/C/LMNA (+) | Inducing stability of nuclear lamina and NB differentiation |
| Emdal et al. [55] | NB1 cell | SILAC-labeled, TMT-labeled and label-free proteomics | ALK interacting proteins | IRS2 | Stimulating PI3K-Akt-FoxO3 signaling and promoting NB cell survival |
| Uckun et al. [56] | SK-N-AS and SK-N-BE(2) cells | TMT-labeled proteomics | ALK interacting proteins | SHP2 | Interacting with ALK and promoting NB proliferation |
| Li et al. [57] | Medium of SK-N-SH cell | - | Secreted proteins from S-type cells | PAI1, SPARC, POSTN and LEG1 | Activating the STAT3 signaling and protecting cells from apoptosis |
| Hwang et al. [58] | SH-SY5Y cells | - | Proteins response to LGR5 knockdown | hnRNPH3, hnRNPA2B1 (−), and more | Activation of pre-mRNA processing and cell proliferation |
| Bugara et al. [59] | IMR-32 cells | - | Proteins response to PHLDA1 activation | mitochondrion related proteins (+) | - |
| Dhamdhere et al. [60] | EVs derived from M1 and 9464D cells | TMT-labeled proteomics | Proteins correlated to IGF2BP1 level | SEMA3A (+), SHMT2 (+) | Inducing PMN formation and promoting metastasis of NB |
| Tsakaneli et al. [61] | EVs isolated from TET21-N NB cells | Label-free proteomics | Proteins correlated to MYCN-amplified level | PKM2 (+), hexokinase II/HK2 (+) | Enhancing histone H3 phosphorylation and promoting the metabolic activity in NB |
| Fonseka et al. [62]. | Exosomes from SK-N-BE2 and SH-SY5Y cells | Label-free proteomics | Proteins correlated to MYCN-amplified level | Alix (+), TSG101 (+), FlOT1 (+) and VPS35 (+), and more | Regulating cell communication and signal transduction |
| Morini et al. [63] | Plasma exosomes from HR-NB patients and LR-NB patients and healthy controls | - | Proteins correlated to NB risk level | NCAM1 (+), NCL (+), LGALS3BP (+), LUM (−), VASP (−), DCN (−), MYH9 (+), FN1 (+), CALR (−), AKAP12 (−) and LTBP1 (+),and more | - |
| Garcia et al. [64]. | SH-Y5Y cells | Label-free proteomics | Membrane proteins | NCAM1, L1CAM, EMMPRIN (CD147), ITGB1, ITGAV, CNTFR, and more | - |
| Gangras et al. [65]. | SH-SY5Y cells | - | Membrane proteins | NRCAM | - |
3.1.1. MYCN-Related Mechanism
3.1.2. RTK Signaling
3.1.3. ALK Signaling
3.1.4. WNT/β-Catenin Signaling Pathway
3.1.5. Ganglioside GD2 Related Mechanism
3.1.6. Extracellular Vesicles (EVs)
3.1.7. Membrane Proteomics
3.2. Proteomics Application in Drug Treatment
3.2.1. Inhibitors of NB Molecular Risk Factors
| Authors | Samples | MS-Proteomic Methods | Drug Name | Information of Drug | Protein Targets and the Response to Drug | Related Mechanisms of Drug |
|---|---|---|---|---|---|---|
| Szydzik et al. [101] | CLB-BAR and CLB-GE cells | TMT-labeled proteomics, phosphoproteomics | BAY 1895344 | ATR inhibitor | RAD51 (−), BRCA1/2 (−), E2F3 (−), DCK (−) and more | Inhibiting E2F transcription and DNA repair machinery pathways |
| Borenas et al. [102]. | CLB-BAR cells | Phosphoproteomics | Ceralasertib, Elimusertib | ATR inhibitor | phospho-ATM (+),phospho-DNAPK (+), and more | Decreasing ATR and mTOR signaling |
| Van et al. [105] | CLB-BAR, CLB-GE, SK-N-AS cells | Label-free phosphoproteomics | Crizotinib or lorlatinib | ALK inhibitor | DUSP4 (−) and more | Inducing feedback loop of ERK and ALK signaling |
| Mohlin et al. [110] | LU-NB-3 cells | Label-free proteomics, phosphoproteomics | IBL-302 | PIM, PI3K, mTOR inhibitor | Caspase3 (+) and CDK6 (+), and more | Inducing programmed cell death and cell cycle signaling |
| Wang et al. [113]. | SK-N-BE(2) cells | formaldehyde-H2 and formaldehyde-D2-labled proteomics and phosphoproteomics | Mdivi-1 | Mitochondrial division inhibitor | PHGDH (+), PSAT1 (+), PSPH (+), PSMA3 (−), and more | Inducing serine synthesis, curtailing proliferation, and more |
| Chiangjong et al. [119] | SK-N-BE2 and SH-SY5Y cells | - | miR-204-loaded REPs | MiR-204 mimic | - | Suppressing mRNA splicing and SLIT/ROBO pathway |
| Nguyen et al. [123] | KCNR, SH-SY5Y cell lines and PDXs | TMT-labeled proteomics, phosphoproteomics | Selinexor | XPO1 inhibitor | P53 (+), and more | Increasing p53-mediated cytotoxicity |
| Yu et al. [127] | NGP cells | Label-free proteomics | Ulixertinib | ERK inhibitor | MK167 (+), PRUNE2 (+), and more | Inhibiting Cycle-related and DNA replication/synthesis pathways |
| Chandel et al. [130] | SH-SY5Y cells | Label-free proteomics | EAD | Limonoid | ENO1 (−) and HSP90 (−),and more | Preventing proliferation and triggering apoptosis in NB |
| Laghezza et al. [131] | SH-SY5Y cells | Label-free proteomics | HTyr-OL | Polyphenol | BAG3 (+), HMOX1 (+), CLU (+), HERPUD1 (+), and more | Inducing apoptotic signaling pathway |
| Forbes et al. [132]. | SH-SY5Y, IMR-32, BE(2)-C, GI-M-EN, SK-N-AS cells | LFQ proteomics, phosphoproteomic | L-Glyceraldehyde | Monosaccharide | PARVA (+), TP53 (+), DTD1 (+), and more | Increasing oxidoreductase activity and inhibiting cell growth |
| Lee et al. [133] | SH-SY5Y cells | Label-free proteomics | MS13 | Curcumin analog | ENO1 (−), HSP90AA1 (+), HSP90AB1 (+), TUBB (−), and more | Inducing glycolysis and PTM-modification pathways |
| Morretta et al. [134] | HTLA-230 cells | Label-free proteomics | STIRUR 41 | Pyrazolyl-urea and dihydro-imidazo-pyrazolyl-urea compounds | USP-7 (−) | - |
| Chittavanich et al. [135] | RB organoids | Label-free proteomics | Ceftriaxone | Third generation cephalosporin antibiotic for MYCN-driven tumors’ inhibition | DDX3X (−) | Inhibiting MYCN translation |
| Halakos et al. [136] | SK-N-SH cells | Label-free proteomics | 13-cis RA | Vitamin A derivative used in the clinic post-chemotherapy | ICAM1 (+), NEFM (+), CRABP2 (+), PLAT (+) and more | Reducing ECM and collagen metabolic process, and promoting neurofilament formation |
| Halakos et al. [137] | SK-N-SH cells | Label-free proteomics | K777 and 13-cis RA | K777: cathepsin inhibitor | ARHGEF2 (+), B2M (+), CRABP2 (+), NEFL (+), TCF12 (+), APP protein family (+), and more | Inducing neuronal differentiation |
3.2.2. Natural or Synthesis Compounds for NB Treatment
3.2.3. Drug Repurposing
3.2.4. Proteomic Investigation of Drug Resistance
| Authors | Samples | MS Proteomic Methods | Drugs | Protein Targets and the Response to Chemoresistant Status | Related Mechanisms Associated with Drug Resistance |
|---|---|---|---|---|---|
| Wang et al. [152] | SK-N-BE(1) and SK-N-BE(2) cells | SILAC-labeled MS | - | ANXA2 (+) | Inducing NF-κB signaling and drug resistance |
| Tang et al. [154] | Tumors from NB patients | TMT-labled MS | - | PTBP2 (−) | Inducing alternative splicing pathway and repolarization of monocytes |
| Chae et al. [156] | SK-N-SH cells | TMT-labeled proteomics, phosphoproteomics | Topotecan | BLM (+), HCR24 (+), and phospho-IRS1 (+) | Activating DNA repair, cholesterol-mediated activity, and insulin/mTOR signaling |
| Merlos Rodrigo et al. [158] | UKF-NB-4 cells | Label-free proteomics | Cisplatin | Proteasomal complex subunits (+) | Activating lysosomal/proteasomal pathways |
4. Proteomics Application in Intratumor Heterogeneity of NB
4.1. Genetic and Proteomic Level Analysis in NB Intratumor Heterogeneity
4.2. The Application of Emerging Proteomic Techniques in NB Intratumor Heterogeneity
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, V.; Jurkovic Mlakar, S.; Lopez, G.; Maris, J.M.; Ansari, M.; Gumy-Pause, F. 11q deletion in neuroblastoma: A review of biological and clinical implications. Mol. Cancer 2017, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Schmidt, M.L.; Cohn, S.L.; Maris, J.M.; London, W.B.; Buxton, A.; Stram, D.; Castleberry, R.P.; Shimada, H.; Sandler, A.; et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N. Engl. J. Med. 2010, 363, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kalinovsky, D.V.; Doronin, I.I.; Deyev, S.M.; Kholodenko, R.V. Neuroblastoma Origin and Therapeutic Targets for Immunotherapy. J. Immunol. Res. 2018, 2018, 7394268. [Google Scholar] [CrossRef]
- Swift, C.C.; Eklund, M.J.; Kraveka, J.M.; Alazraki, A.L. Updates in Diagnosis, Management, and Treatment of Neuroblastoma. Radiographics 2018, 38, 566–580. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Janoueix-Lerosey, I.; Delattre, O. Recent insights into the biology of neuroblastoma. Int. J. Cancer 2014, 135, 2249–2261. [Google Scholar] [CrossRef]
- Shawraba, F.; Hammoud, H.; Mrad, Y.; Saker, Z.; Fares, Y.; Harati, H.; Bahmad, H.F.; Nabha, S. Biomarkers in Neuroblastoma: An Insight into Their Potential Diagnostic and Prognostic Utilities. Curr. Treat. Options Oncol. 2021, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Cheung, N.V. Targets and Antibody Formats for Immunotherapy of Neuroblastoma. J. Clin. Oncol. 2020, 38, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.H.Y.; Juan, H.F. Quantitative proteomics in lung cancer. J. Biomed. Sci. 2017, 24, 37. [Google Scholar] [CrossRef]
- Pandey, A.; Mann, M. Proteomics to study genes and genomes. Nature 2000, 405, 837–846. [Google Scholar] [CrossRef]
- Smiles, W.J.; Catalano, L.; Stefan, V.E.; Weber, D.D.; Kofler, B. Metabolic protein kinase signalling in neuroblastoma. Mol. Metab. 2023, 75, 101771. [Google Scholar] [CrossRef]
- Kumar, P.; Koach, J.; Nekritz, E.; Mukherjee, S.; Braun, B.S.; DuBois, S.G.; Nasholm, N.; Haas-Kogan, D.; Matthay, K.K.; Weiss, W.A.; et al. Aurora Kinase A inhibition enhances DNA damage and tumor cell death with (131)I-MIBG therapy in high-risk neuroblastoma. EJNMMI Res. 2024, 14, 54. [Google Scholar] [CrossRef]
- Paccosi, E.; Costantino, M.; Balzerano, A.; Filippi, S.; Brancorsini, S.; Proietti-De-Santis, L. Neuroblastoma Cells Depend on CSB for Faithful Execution of Cytokinesis and Survival. Int. J. Mol. Sci. 2021, 22, 10070. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, F.; Limones-Gonzalez, J.E.; Mendoza-Almanza, B.; Esparza-Ibarra, E.L.; Gallegos-Flores, P.I.; Ayala-Lujan, J.L.; Godina-Gonzalez, S.; Salinas, E.; Mendoza-Almanza, G. Understanding Cervical Cancer through Proteomics. Cells 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Pascovici, D.; Wu, J.X.; McKay, M.J.; Joseph, C.; Noor, Z.; Kamath, K.; Wu, Y.; Ranganathan, S.; Gupta, V.; Mirzaei, M. Clinically Relevant Post-Translational Modification Analyses-Maturing Workflows and Bioinformatics Tools. Int. J. Mol. Sci. 2018, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.H.; Goyette, J.; Thomas, P.S. Proteomics as a method for early detection of cancer: A review of proteomics, exhaled breath condensate, and lung cancer screening. J. Gen. Intern. Med. 2008, 23 (Suppl. 1), 78–84. [Google Scholar] [CrossRef] [PubMed]
- Alessandro, R.; Fontana, S.; Kohn, E.; De Leo, G. Proteomic strategies and their application in cancer research. Tumori 2005, 91, 447–455. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, S.; Stenoien, D.L.; Pasa-Tolic, L. High-throughput proteomics. Annu. Rev. Anal. Chem. 2014, 7, 427–454. [Google Scholar] [CrossRef]
- Duong, V.A.; Lee, H. Bottom-Up Proteomics: Advancements in Sample Preparation. Int. J. Mol. Sci. 2023, 24, 5350. [Google Scholar] [CrossRef]
- Miller, R.M.; Smith, L.M. Overview and considerations in bottom-up proteomics. Analyst 2023, 148, 475–486. [Google Scholar] [CrossRef]
- Ryu, J.; Thomas, S.N. Quantitative Mass Spectrometry-Based Proteomics for Biomarker Development in Ovarian Cancer. Molecules 2021, 26, 2674. [Google Scholar] [CrossRef]
- Li, K.W.; Gonzalez-Lozano, M.A.; Koopmans, F.; Smit, A.B. Recent Developments in Data Independent Acquisition (DIA) Mass Spectrometry: Application of Quantitative Analysis of the Brain Proteome. Front. Mol. Neurosci. 2020, 13, 564446. [Google Scholar] [CrossRef]
- Elias, J.E.; Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 2007, 4, 207–214. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Rost, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Q.; Yu, B.; Han, B.; Yang, B. 4D-quantitative proteomics signature of asthenozoospermia and identification of extracellular matrix protein 1 as a novel biomarker for sperm motility. Mol. Omics 2022, 18, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, S.; Zhang, Y.; Olga, V.; Li, Y.; Diao, X.; Zhou, H. Coral and it’s symbionts responses to the typical global marine pollutant BaP by 4D-Proteomics approach. Environ. Pollut. 2022, 307, 119440. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.D.; Frank, M.; Ha, A.; Bludau, I.; Voytik, E.; Kaspar-Schoenefeld, S.; Lubeck, M.; Raether, O.; Bache, N.; et al. diaPASEF: Parallel accumulation-serial fragmentation combined with data-independent acquisition. Nat. Methods 2020, 17, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; VerBerkmoes, N.C.; Langston, M.A.; Uberbacher, E.; Hettich, R.L.; Samatova, N.F. Detecting differential and correlated protein expression in label-free shotgun proteomics. J. Proteome Res. 2006, 5, 2909–2918. [Google Scholar] [CrossRef]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Swiatly, A.; Horala, A.; Matysiak, J.; Hajduk, J.; Nowak-Markwitz, E.; Kokot, Z.J. Understanding Ovarian Cancer: iTRAQ-Based Proteomics for Biomarker Discovery. Int. J. Mol. Sci. 2018, 19, 2240. [Google Scholar] [CrossRef]
- Zhang, L.; Elias, J.E. Relative Protein Quantification Using Tandem Mass Tag Mass Spectrometry. Methods Mol. Biol. 2017, 1550, 185–198. [Google Scholar]
- Srinivasan, A.; Sing, J.C.; Gingras, A.C.; Rost, H.L. Improving Phosphoproteomics Profiling Using Data-Independent Mass Spectrometry. J. Proteome Res. 2022, 21, 1789–1799. [Google Scholar] [CrossRef]
- Sahu, I.; Zhu, H.; Buhrlage, S.J.; Marto, J.A. Proteomic approaches to study ubiquitinomics. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194940. [Google Scholar] [CrossRef]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell Proteom. 2012, 11, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Kumar, C.; Zeng, W.F.; Strauss, M.T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Zeng, W.F.; Chi, H.; Luo, C.; Liu, C.; Zhan, J.; He, S.M.; Zhang, Z. pDeep: Predicting MS/MS Spectra of Peptides with Deep Learning. Anal. Chem. 2017, 89, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Voytik, E.; Treit, P.V.; Doll, S.; Kleinhempel, A.; Niu, L.; Muller, J.B.; Buchholtz, M.L.; Bader, J.M.; Teupser, D.; et al. Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol. Med. 2019, 11, e10427. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Ge, J.; Tang, G.; Xiong, D.; Zhu, D.; Ding, X.; Zhou, X.; Sang, M. Machine learning-based identification of biomarkers and drugs in immunologically cold and hot pancreatic adenocarcinomas. J. Transl. Med. 2024, 22, 775. [Google Scholar] [CrossRef]
- Lee, T.; Natalwala, J.; Chapple, V.; Liu, Y. A brief history of artificial intelligence embryo selection: From black-box to glass-box. Hum. Reprod. 2024, 39, 285–292. [Google Scholar] [CrossRef]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Oresic, M. Deep learning meets metabolomics: A methodological perspective. Brief. Bioinform. 2021, 22, 1531–1542. [Google Scholar] [CrossRef]
- Cifani, P.; Kentsis, A. Towards comprehensive and quantitative proteomics for diagnosis and therapy of human disease. Proteomics 2017, 17, 1600079. [Google Scholar] [CrossRef]
- Cheng, C.; He, T.; Chen, K.; Cai, Y.; Gu, Y.; Pan, L.; Duan, P.; Wu, Y.; Wu, Z. P300 Interacted with N-Myc and Regulated Its Protein Stability via Altering Its Post-Translational Modifications in Neuroblastoma. Mol. Cell Proteom. 2023, 22, 100504. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Cheung, C.H.Y.; Liu, Y.L.; Hou, C.L.; Hsu, C.L.; Huang, C.T.; Yang, T.S.; Chen, S.F.; Chen, C.N.; Hsu, W.M.; et al. Quantitative Proteomics of Th-MYCN Transgenic Mice Reveals Aurora Kinase Inhibitor Altered Metabolic Pathways and Enhanced ACADM To Suppress Neuroblastoma Progression. J. Proteome Res. 2019, 18, 3850–3866. [Google Scholar] [CrossRef]
- Arlt, B.; Zasada, C.; Baum, K.; Wuenschel, J.; Mastrobuoni, G.; Lodrini, M.; Astrahantseff, K.; Winkler, A.; Schulte, J.H.; Finkler, S.; et al. Inhibiting phosphoglycerate dehydrogenase counteracts chemotherapeutic efficacy against MYCN-amplified neuroblastoma. Int. J. Cancer 2021, 148, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.W.; Sahu, D.; Chang, Y.W.; Hsu, C.L.; Hsieh, C.H.; Huang, H.C.; Juan, H.F. RNA-Binding Proteomics Reveals MATR3 Interacting with lncRNA SNHG1 To Enhance Neuroblastoma Progression. J. Proteome Res. 2019, 18, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.K.; Pfeiffer, A.; Karemore, G.; Akimov, V.; Bekker-Jensen, D.B.; Blagoev, B.; Francavilla, C.; Olsen, J.V. Proteomic investigation of Cbl and Cbl-b in neuroblastoma cell differentiation highlights roles for SHP-2 and CDK16. iScience 2021, 24, 102321. [Google Scholar] [CrossRef] [PubMed]
- Funke, L.; Bracht, T.; Oeck, S.; Schork, K.; Stepath, M.; Dreesmann, S.; Eisenacher, M.; Sitek, B.; Schramm, A. NTRK1/TrkA Signaling in Neuroblastoma Cells Induces Nuclear Reorganization and Intra-Nuclear Aggregation of Lamin A/C. Cancers 2021, 13, 5293. [Google Scholar] [CrossRef]
- Emdal, K.B.; Pedersen, A.K.; Bekker-Jensen, D.B.; Lundby, A.; Claeys, S.; De Preter, K.; Speleman, F.; Francavilla, C.; Olsen, J.V. Integrated proximal proteomics reveals IRS2 as a determinant of cell survival in ALK-driven neuroblastoma. Sci. Signal 2018, 11, aap9752. [Google Scholar] [CrossRef]
- Uckun, E.; Siaw, J.T.; Guan, J.; Anthonydhason, V.; Fuchs, J.; Wolfstetter, G.; Hallberg, B.; Palmer, R.H. BioID-Screening Identifies PEAK1 and SHP2 as Components of the ALK Proximitome in Neuroblastoma Cells. J. Mol. Biol. 2021, 433, 167158. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Li, L.; Or, P.M.; Wai Wong, C.; Liu, T.; Ho, W.L.H.; Chan, A.M. Tumour-derived substrate-adherent cells promote neuroblastoma survival through secreted trophic factors. Mol. Oncol. 2021, 15, 2011–2025. [Google Scholar] [CrossRef]
- Hwang, M.; Han, M.H.; Park, H.H.; Choi, H.; Lee, K.Y.; Lee, Y.J.; Kim, J.M.; Cheong, J.H.; Ryu, J.I.; Min, K.W.; et al. LGR5 and Downstream Intracellular Signaling Proteins Play Critical Roles in the Cell Proliferation of Neuroblastoma, Meningioma and Pituitary Adenoma. Exp. Neurobiol. 2019, 28, 628–641. [Google Scholar] [CrossRef]
- Bugara, B.; Durbas, M.; Kudrycka, M.; Malinowska, A.; Horwacik, I.; Rokita, H. Silencing of the PHLDA1 leads to global proteome changes and differentiation pathways of human neuroblastoma cells. Front. Pharmacol. 2024, 15, 1351536. [Google Scholar] [CrossRef]
- Dhamdhere, M.R.; Gowda, C.P.; Singh, V.; Liu, Z.; Carruthers, N.; Grant, C.N.; Sharma, A.; Dovat, S.; Sundstrom, J.M.; Wang, H.G.; et al. IGF2BP1 regulates the cargo of extracellular vesicles and promotes neuroblastoma metastasis. Oncogene 2023, 42, 1558–1571. [Google Scholar] [CrossRef]
- Tsakaneli, A.; Carregari, V.C.; Morini, M.; Eva, A.; Cangemi, G.; Chayka, O.; Makarov, E.; Bibbo, S.; Capone, E.; Sala, G.; et al. MYC regulates metabolism through vesicular transfer of glycolytic kinases. Open Biol. 2021, 11, 210276. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, P.; Liem, M.; Ozcitti, C.; Adda, C.G.; Ang, C.S.; Mathivanan, S. Exosomes from N-Myc amplified neuroblastoma cells induce migration and confer chemoresistance to non-N-Myc amplified cells: Implications of intra-tumour heterogeneity. J. Extracell. Vesicles 2019, 8, 1597614. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Raggi, F.; Bartolucci, M.; Petretto, A.; Ardito, M.; Rossi, C.; Segalerba, D.; Garaventa, A.; Eva, A.; Cangelosi, D.; et al. Plasma-Derived Exosome Proteins as Novel Diagnostic and Prognostic Biomarkers in Neuroblastoma Patients. Cells 2023, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Faca, V.; Jarzembowski, J.; Zhang, Q.; Park, J.; Hanash, S. Comprehensive profiling of the cell surface proteome of Sy5Y neuroblastoma cells yields a subset of proteins associated with tumor differentiation. J. Proteome Res. 2009, 8, 3791–3796. [Google Scholar] [CrossRef]
- Gangras, P.; Gelfanova, V.; Williams, G.D.; Handelman, S.K.; Smith, R.M.; Debets, M.F. Investigating SH-SY5Y Neuroblastoma Cell Surfaceome as a Model for Neuronal-Targeted Novel Therapeutic Modalities. Int. J. Mol. Sci. 2022, 23, 15062. [Google Scholar] [CrossRef]
- Misawa, A.; Hosoi, H.; Arimoto, A.; Shikata, T.; Akioka, S.; Matsumura, T.; Houghton, P.J.; Sawada, T. N-Myc induction stimulated by insulin-like growth factor I through mitogen-activated protein kinase signaling pathway in human neuroblastoma cells. Cancer Res. 2000, 60, 64–69. [Google Scholar]
- Marshall, G.M.; Liu, P.Y.; Gherardi, S.; Scarlett, C.J.; Bedalov, A.; Xu, N.; Iraci, N.; Valli, E.; Ling, D.; Thomas, W.; et al. SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability. PLoS Genet. 2011, 7, e1002135. [Google Scholar] [CrossRef]
- Otto, T.; Horn, S.; Brockmann, M.; Eilers, U.; Schuttrumpf, L.; Popov, N.; Kenney, A.M.; Schulte, J.H.; Beijersbergen, R.; Christiansen, H.; et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 2009, 15, 67–78. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Sahu, D.; Hsu, C.L.; Lin, C.C.; Yang, T.W.; Hsu, W.M.; Ho, S.Y.; Juan, H.F.; Huang, H.C. Co-expression analysis identifies long noncoding RNA SNHG1 as a novel predictor for event-free survival in neuroblastoma. Oncotarget 2016, 7, 58022–58037. [Google Scholar] [CrossRef] [PubMed]
- Edsjo, A.; Holmquist, L.; Pahlman, S. Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin. Cancer Biol. 2007, 17, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Rozen, E.J.; Shohet, J.M. Systematic review of the receptor tyrosine kinase superfamily in neuroblastoma pathophysiology. Cancer Metastasis Rev. 2022, 41, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Emdal, K.B.; Pedersen, A.K.; Bekker-Jensen, D.B.; Tsafou, K.P.; Horn, H.; Lindner, S.; Schulte, J.H.; Eggert, A.; Jensen, L.J.; Francavilla, C.; et al. Temporal proteomics of NGF-TrkA signaling identifies an inhibitory role for the E3 ligase Cbl-b in neuroblastoma cell differentiation. Sci. Signal 2015, 8, ra40. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hammonds-Odie, L.; Perron, J.; Masters, B.A.; Bixby, J.L. SHP-2 mediates target-regulated axonal termination and NGF-dependent neurite growth in sympathetic neurons. Dev. Biol. 2002, 252, 170–187. [Google Scholar] [CrossRef]
- Dohmen, M.; Krieg, S.; Agalaridis, G.; Zhu, X.; Shehata, S.N.; Pfeiffenberger, E.; Amelang, J.; Butepage, M.; Buerova, E.; Pfaff, C.M.; et al. AMPK-dependent activation of the Cyclin Y/CDK16 complex controls autophagy. Nat. Commun. 2020, 11, 1032. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mahlow, E.; Odersky, A.; Lindner, S.; Stephan, H.; Bendix, I.; Eggert, A.; Schramm, A.; Schulte, J.H. Neuroblastoma in dialog with its stroma: NTRK1 is a regulator of cellular cross-talk with Schwann cells. Oncotarget 2014, 5, 11180–11192. [Google Scholar] [CrossRef]
- Pacenta, H.L.; Macy, M.E. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug Des. Dev. Ther. 2018, 12, 3549–3561. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Guan, J.; Tucker, E.R.; Wan, H.; Chand, D.; Danielson, L.S.; Ruuth, K.; El Wakil, A.; Witek, B.; Jamin, Y.; Umapathy, G.; et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Dis. Model. Mech. 2016, 9, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Biedler, J.L.; Spengler, B.A. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003, 197, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.C.; Chockalingam, S.; Melegh, Z.; Greenhough, A.; Malik, S.; Szemes, M.; Park, J.H.; Kaidi, A.; Zhou, L.; Catchpoole, D.; et al. LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/beta-catenin signalling in neuroblastoma. Oncotarget 2015, 6, 40053–40067. [Google Scholar] [CrossRef]
- Larrosa, C.; Mora, J.; Cheung, N.K. Global Impact of Monoclonal Antibodies (mAbs) in Children: A Focus on Anti-GD2. Cancers 2023, 15, 3729. [Google Scholar] [CrossRef]
- Ladenstein, R.; Potschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Philippova, J.; Shevchenko, J.; Sennikov, S. GD2-targeting therapy: A comparative analysis of approaches and promising directions. Front. Immunol. 2024, 15, 1371345. [Google Scholar] [CrossRef]
- Kaczanowska, S.; Murty, T.; Alimadadi, A.; Contreras, C.F.; Duault, C.; Subrahmanyam, P.B.; Reynolds, W.; Gutierrez, N.A.; Baskar, R.; Wu, C.J.; et al. Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell 2024, 42, 35–51. [Google Scholar] [CrossRef]
- Horwacik, I.; Durbas, M.; Boratyn, E.; Sawicka, A.; Wegrzyn, P.; Krzanik, S.; Gorka, A.; Drozniak, J.; Augustyniak, E.; Kowalczyk, A.; et al. Analysis of genes involved in response to doxorubicin and a GD2 ganglioside-specific 14G2a monoclonal antibody in IMR-32 human neuroblastoma cells. Acta Biochim. Pol. 2015, 62, 423–433. [Google Scholar] [CrossRef]
- Dhamdhere, M.R.; Spiegelman, V.S. Extracellular vesicles in neuroblastoma: Role in progression, resistance to therapy and diagnostics. Front. Immunol. 2024, 15, 1385875. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Biegel, J.M.; Dhamdhere, M.; Gao, S.; Gowda, C.P.; Kawasawa, Y.I.; Spiegelman, V.S. Inhibition of the mRNA-Binding Protein IGF2BP1 Suppresses Proliferation and Sensitizes Neuroblastoma Cells to Chemotherapeutic Agents. Front. Oncol. 2021, 11, 608816. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Baylot, V.; Felsher, D.W. The MYC oncogene is a global regulator of the immune response. Blood 2018, 131, 2007–2015. [Google Scholar] [CrossRef]
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271. [Google Scholar] [CrossRef]
- Li, N.; Spetz, M.R.; Li, D.; Ho, M. Advances in immunotherapeutic targets for childhood cancers: A focus on glypican-2 and B7-H3. Pharmacol. Ther. 2021, 223, 107892. [Google Scholar] [CrossRef]
- Waas, M.; Snarrenberg, S.T.; Littrell, J.; Jones Lipinski, R.A.; Hansen, P.A.; Corbett, J.A.; Gundry, R.L. SurfaceGenie: A web-based application for prioritizing cell-type-specific marker candidates. Bioinformatics 2020, 36, 3447–3456. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. Publisher correction: The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 783. [Google Scholar] [CrossRef]
- Gilad, O.; Nabet, B.Y.; Ragland, R.L.; Schoppy, D.W.; Smith, K.D.; Durham, A.C.; Brown, E.J. Combining ATR suppression with oncogenic Ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Cancer Res. 2010, 70, 9693–9702. [Google Scholar] [CrossRef]
- Szydzik, J.; Lind, D.E.; Arefin, B.; Kurhe, Y.; Umapathy, G.; Siaw, J.T.; Claeys, A.; Gabre, J.L.; Van den Eynden, J.; Hallberg, B.; et al. ATR inhibition enables complete tumour regression in ALK-driven NB mouse models. Nat. Commun. 2021, 12, 6813. [Google Scholar] [CrossRef] [PubMed]
- Borenas, M.; Umapathy, G.; Lind, D.E.; Lai, W.Y.; Guan, J.; Johansson, J.; Jennische, E.; Schmidt, A.; Kurhe, Y.; Gabre, J.L.; et al. ALK signaling primes the DNA damage response sensitizing ALK-driven neuroblastoma to therapeutic ATR inhibition. Proc. Natl. Acad. Sci. USA 2024, 121, e2315242121. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P. Anaplastic Lymphoma Kinase as a Cancer Target in Pediatric Malignancies. Clin. Cancer Res. 2016, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef]
- Van den Eynden, J.; Umapathy, G.; Ashouri, A.; Cervantes-Madrid, D.; Szydzik, J.; Ruuth, K.; Koster, J.; Larsson, E.; Guan, J.; Palmer, R.H.; et al. Phosphoproteome and gene expression profiling of ALK inhibition in neuroblastoma cell lines reveals conserved oncogenic pathways. Sci. Signal 2018, 11, 557. [Google Scholar] [CrossRef]
- Chesler, L.; Schlieve, C.; Goldenberg, D.D.; Kenney, A.; Kim, G.; McMillan, A.; Matthay, K.K.; Rowitch, D.; Weiss, W.A. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006, 66, 8139–8146. [Google Scholar] [CrossRef]
- Segerstrom, L.; Baryawno, N.; Sveinbjornsson, B.; Wickstrom, M.; Elfman, L.; Kogner, P.; Johnsen, J.I. Effects of small molecule inhibitors of PI3K/Akt/mTOR signaling on neuroblastoma growth in vitro and in vivo. Int. J. Cancer 2011, 129, 2958–2965. [Google Scholar] [CrossRef]
- Le, X.; Antony, R.; Razavi, P.; Treacy, D.J.; Luo, F.; Ghandi, M.; Castel, P.; Scaltriti, M.; Baselga, J.; Garraway, L.A. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov. 2016, 6, 1134–1147. [Google Scholar] [CrossRef]
- Nawijn, M.C.; Alendar, A.; Berns, A. For better or for worse: The role of Pim oncogenes in tumorigenesis. Nat. Rev. Cancer 2011, 11, 23–34. [Google Scholar] [CrossRef]
- Mohlin, S.; Hansson, K.; Radke, K.; Martinez, S.; Blanco-Apiricio, C.; Garcia-Ruiz, C.; Welinder, C.; Esfandyari, J.; O’Neill, M.; Pastor, J.; et al. Anti-tumor effects of PIM/PI3K/mTOR triple kinase inhibitor IBL-302 in neuroblastoma. EMBO Mol. Med. 2019, 11, e10058. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.Y.; Park, K.J.; Kim, W.H.; Roh, G.S. A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci. 2016, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, G.; Chwa, J.; Oh, M.E.; Abeywardana, T.; Yang, Y.; Wang, Q.A.; Jiang, L. Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer. Br. J. Cancer 2020, 122, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Kao, Y.C.; Hsieh, C.H.; Tsai, S.Y.; Cheung, C.H.Y.; Huang, H.C.; Juan, H.F. Multiomics Reveals Induction of Neuroblastoma SK-N-BE(2)C Cell Death by Mitochondrial Division Inhibitor 1 through Multiple Effects. J. Proteome Res. 2024, 23, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Tivnan, A.; Fay, J.; Bryan, K.; Meehan, M.; Creevey, L.; Lynch, J.; Bray, I.M.; O’Meara, A.; Tracey, L.; et al. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br. J. Cancer 2012, 107, 967–976. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Carter, D.R.; Liu, B.; Mayoh, C.; Beckers, A.; Lalwani, A.; Nagy, Z.; De Brouwer, S.; Decaesteker, B.; Hung, T.T.; et al. Network Modeling of microRNA-mRNA Interactions in Neuroblastoma Tumorigenesis Identifies miR-204 as a Direct Inhibitor of MYCN. Cancer Res. 2018, 78, 3122–3134. [Google Scholar] [CrossRef]
- Bachetti, T.; Di Zanni, E.; Ravazzolo, R.; Ceccherini, I. miR-204 mediates post-transcriptional down-regulation of PHOX2B gene expression in neuroblastoma cells. Biochim. Biophys. Acta 2015, 1849, 1057–1065. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Chiangjong, W.; Panachan, J.; Keadsanti, S.; Newburg, D.S.; Morrow, A.L.; Hongeng, S.; Chutipongtanate, S. Development of red blood cell-derived extracellular particles as a biocompatible nanocarrier of microRNA-204 (REP-204) to harness anti-neuroblastoma effect. Nanomedicine 2024, 60, 102760. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Chen, L.; Gong, B.; Jia, D.; Sun, Q. Nuclear transport proteins: Structure, function, and disease relevance. Signal Transduct. Target. Ther. 2023, 8, 425. [Google Scholar] [CrossRef]
- Galinski, B.; Luxemburg, M.; Landesman, Y.; Pawel, B.; Johnson, K.J.; Master, S.R.; Freeman, K.W.; Loeb, D.M.; Hebert, J.M.; Weiser, D.A. XPO1 inhibition with selinexor synergizes with proteasome inhibition in neuroblastoma by targeting nuclear export of IkB. Transl. Oncol. 2021, 14, 101114. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Wang, H.; Sun, M.; Lee, D.G.; Peng, J.; Thiele, C.J. Combining selinexor with alisertib to target the p53 pathway in neuroblastoma. Neoplasia 2022, 26, 100776. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Sasai, K.; Kawai, H.; Yuan, Z.M.; Bondaruk, J.; Suzuki, F.; Fujii, S.; Arlinghaus, R.B.; Czerniak, B.A.; Sen, S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 2004, 36, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Pouyssegur, J. Targeting the ERK signaling pathway in cancer therapy. Ann. Med. 2006, 38, 200–211. [Google Scholar] [CrossRef]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Colmet Daage, L.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Choi, J.; Shi, Z.; Guo, L.; Elizarraras, J.; Gu, A.; Cheng, F.; Pei, Y.; Lu, D.; et al. ERK Inhibitor Ulixertinib Inhibits High-Risk Neuroblastoma Growth In Vitro and In Vivo. Cancers 2022, 14, 5534. [Google Scholar] [CrossRef]
- Islam, M.S.; Takano, R.; Yokochi, T.; Akter, J.; Nakamura, Y.; Nakagawara, A.; Tatsumi, Y. Programmed expression of pro-apoptotic BMCC1 during apoptosis, triggered by DNA damage in neuroblastoma cells. BMC Cancer 2019, 19, 542. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Chandel, S.; Bhattacharya, A.; Gautam, A.; Zeng, W.; Alka, O.; Sachsenberg, T.; Gupta, G.D.; Narang, R.K.; Ravichandiran, V.; Singh, R. Investigation of the anti-cancer potential of epoxyazadiradione in neuroblastoma: Experimental assays and molecular analysis. J. Biomol. Struct. Dyn. 2023, 1–19. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Bernini, R.; Villanova, N.; Clemente, M.; Cicaloni, V.; Tinti, L.; Salvini, L.; Taddei, A.R.; Tiezzi, A.; Ovidi, E. In Vitro Anti-Proliferative and Apoptotic Effects of Hydroxytyrosyl Oleate on SH-SY5Y Human Neuroblastoma Cells. Int. J. Mol. Sci. 2022, 23, 12348. [Google Scholar] [CrossRef]
- Forbes, M.; Kempa, R.; Mastrobuoni, G.; Rayman, L.; Pietzke, M.; Bayram, S.; Arlt, B.; Spruessel, A.; Deubzer, H.E.; Kempa, S. L-Glyceraldehyde Inhibits Neuroblastoma Cell Growth via a Multi-Modal Mechanism on Metabolism and Signaling. Cancers 2024, 16, 1664. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Rajadurai, P.; Abas, F.; Othman, I.; Naidu, R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front. Mol. Biosci. 2021, 8, 645856. [Google Scholar] [CrossRef]
- Morretta, E.; Brullo, C.; Belvedere, R.; Petrella, A.; Spallarossa, A.; Monti, M.C. Targeting USP-7 by a Novel Fluorinated 5-Pyrazolyl-Urea Derivative. Int. J. Mol. Sci. 2023, 24, 9200. [Google Scholar] [CrossRef]
- Chittavanich, P.; Saengwimol, D.; Roytrakul, S.; Rojanaporn, D.; Chaitankar, V.; Srimongkol, A.; Anurathapan, U.; Hongeng, S.; Kaewkhaw, R. Ceftriaxone exerts antitumor effects in MYCN-driven retinoblastoma and neuroblastoma by targeting DDX3X for translation repression. Mol. Oncol. 2023, 18, 918–938. [Google Scholar] [CrossRef]
- Halakos, E.G.; Connell, A.J.; Glazewski, L.; Wei, S.; Mason, R.W. Bottom up proteomics reveals novel differentiation proteins in neuroblastoma cells treated with 13-cis retinoic acid. J. Proteom. 2019, 209, 103491. [Google Scholar] [CrossRef]
- Halakos, E.G.; Connell, A.J.; Glazewski, L.; Wei, S.; Mason, R.W. Bottom up proteomics identifies neuronal differentiation pathway networks activated by cathepsin inhibition treatment in neuroblastoma cells that are enhanced by concurrent 13-cis retinoic acid treatment. J. Proteom. 2021, 232, 104068. [Google Scholar] [CrossRef]
- Shilpa, G.; Renjitha, J.; Saranga, R.; Sajin, F.K.; Nair, M.S.; Joy, B.; Sasidhar, B.S.; Priya, S. Epoxyazadiradione Purified from the Azadirachta indica Seed Induced Mitochondrial Apoptosis and Inhibition of NFkappaB Nuclear Translocation in Human Cervical Cancer Cells. Phytother. Res. 2017, 31, 1892–1902. [Google Scholar] [CrossRef]
- Stickland, L.H. The inhibition of glucolysis by glyceraldehyde. Biochem. J. 1941, 35, 859–871. [Google Scholar] [CrossRef]
- Sakamoto, A.; Prasad, K.N. Effect of DL-glyceraldehyde on mouse neuroblastoma cells in culture. Cancer Res. 1972, 32, 532–534. [Google Scholar]
- Marengo, B.; Meta, E.; Brullo, C.; De Ciucis, C.; Colla, R.; Speciale, A.; Garbarino, O.; Bruno, O.; Domenicotti, C. Biological evaluation of pyrazolyl-urea and dihydro-imidazo-pyrazolyl-urea derivatives as potential anti-angiogenetic agents in the treatment of neuroblastoma. Oncotarget 2020, 11, 3459–3472. [Google Scholar] [CrossRef]
- Morretta, E.; Sidibe, A.; Spallarossa, A.; Petrella, A.; Meta, E.; Bruno, O.; Monti, M.C.; Brullo, C. Synthesis, functional proteomics and biological evaluation of new 5-pyrazolyl ureas as potential anti-angiogenic compounds. Eur. J. Med. Chem. 2021, 226, 113872. [Google Scholar] [CrossRef]
- Morretta, E.; Belvedere, R.; Petrella, A.; Spallarossa, A.; Rapetti, F.; Bruno, O.; Brullo, C.; Monti, M.C. Novel insights on the molecular mechanism of action of the anti-angiogenic pyrazolyl-urea GeGe-3 by functional proteomics. Bioorg Chem. 2021, 115, 105168. [Google Scholar] [CrossRef]
- Sardana, D.; Zhu, C.; Zhang, M.; Gudivada, R.C.; Yang, L.; Jegga, A.G. Drug repositioning for orphan diseases. Brief. Bioinform. 2011, 12, 346–356. [Google Scholar]
- Li, X.; Li, H.; Li, S.; Zhu, F.; Kim, D.J.; Xie, H.; Li, Y.; Nadas, J.; Oi, N.; Zykova, T.A.; et al. Ceftriaxone, an FDA-approved cephalosporin antibiotic, suppresses lung cancer growth by targeting Aurora B. Carcinogenesis 2012, 33, 2548–2557. [Google Scholar] [CrossRef]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Neuman, T.; Stephens, R.W.; Salonen, E.M.; Timmusk, T.; Vaheri, A. Induction of morphological differentiation of human neuroblastoma cells is accompanied by induction of tissue-type plasminogen activator. J. Neurosci. Res. 1989, 23, 274–281. [Google Scholar] [CrossRef]
- Moreno, L.; Caron, H.; Geoerger, B.; Eggert, A.; Schleiermacher, G.; Brock, P.; Valteau-Couanet, D.; Chesler, L.; Schulte, J.H.; De Preter, K.; et al. Accelerating drug development for neuroblastoma—New Drug Development Strategy: An Innovative Therapies for Children with Cancer, European Network for Cancer Research in Children and Adolescents and International Society of Paediatric Oncology Europe Neuroblastoma project. Expert Opin. Drug Discov. 2017, 12, 801–811. [Google Scholar]
- Johnsen, J.I.; Dyberg, C.; Fransson, S.; Wickstrom, M. Molecular mechanisms and therapeutic targets in neuroblastoma. Pharmacol. Res. 2018, 131, 164–176. [Google Scholar] [CrossRef]
- McKerrow, J.H. Update on drug development targeting parasite cysteine proteases. PLoS Negl. Trop. Dis. 2018, 12, e0005850. [Google Scholar] [CrossRef]
- Tucker, E.R.; Poon, E.; Chesler, L. Targeting MYCN and ALK in resistant and relapsing neuroblastoma. Cancer Drug Resist. 2019, 2, 803–812. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Cai, Y.; Cai, Y.; Yuan, X.; Wang, L.; Wu, Z.; Wu, Y. Annexin A2 could enhance multidrug resistance by regulating NF-kappaB signaling pathway in pediatric neuroblastoma. J. Exp. Clin. Cancer Res. 2017, 36, 111. [Google Scholar] [CrossRef]
- Galenkamp, K.M.; Carriba, P.; Urresti, J.; Planells-Ferrer, L.; Coccia, E.; Lopez-Soriano, J.; Barneda-Zahonero, B.; Moubarak, R.S.; Segura, M.F.; Comella, J.X. TNFalpha sensitizes neuroblastoma cells to FasL-, cisplatin- and etoposide-induced cell death by NF-kappaB-mediated expression of Fas. Mol. Cancer 2015, 14, 62. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Guo, H.; Lin, H.; Li, M.; Yang, T.; Wang, H.Y.; Li, D.; Liu, J.; Li, L.; et al. PTBP2-Mediated Alternative Splicing of IRF9 Controls Tumor-Associated Monocyte/Macrophage Chemotaxis and Repolarization in Neuroblastoma Progression. Research 2023, 6, 0033. [Google Scholar] [CrossRef]
- Levin, V.A.; Panchabhai, S.C.; Shen, L.; Kornblau, S.M.; Qiu, Y.; Baggerly, K.A. Different changes in protein and phosphoprotein levels result from serum starvation of high-grade glioma and adenocarcinoma cell lines. J. Proteome Res. 2010, 9, 179–191. [Google Scholar] [CrossRef]
- Chae, S.Y.; Nam, D.; Hyeon, D.Y.; Hong, A.; Lee, T.D.; Kim, S.; Im, D.; Hong, J.; Kang, C.; Lee, J.W.; et al. DNA repair and cholesterol-mediated drug efflux induce dose-dependent chemoresistance in nutrient-deprived neuroblastoma cells. iScience 2021, 24, 102325. [Google Scholar] [CrossRef]
- Yang, C.; Tan, J.; Zhu, J.; Wang, S.; Wei, G. YAP promotes tumorigenesis and cisplatin resistance in neuroblastoma. Oncotarget 2017, 8, 37154–37163. [Google Scholar] [CrossRef]
- Merlos Rodrigo, M.A.; Buchtelova, H.; de Los Rios, V.; Casal, J.I.; Eckschlager, T.; Hrabeta, J.; Belhajova, M.; Heger, Z.; Adam, V. Proteomic Signature of Neuroblastoma Cells UKF-NB-4 Reveals Key Role of Lysosomal Sequestration and the Proteasome Complex in Acquiring Chemoresistance to Cisplatin. J. Proteome Res. 2019, 18, 1255–1263. [Google Scholar] [CrossRef]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular pathogenesis and therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef]
- Acosta, S.; Lavarino, C.; Paris, R.; Garcia, I.; de Torres, C.; Rodriguez, E.; Beleta, H.; Mora, J. Comprehensive characterization of neuroblastoma cell line subtypes reveals bilineage potential similar to neural crest stem cells. BMC Dev. Biol. 2009, 9, 12. [Google Scholar] [CrossRef]
- Gartlgruber, M.; Sharma, A.K.; Quintero, A.; Dreidax, D.; Jansky, S.; Park, Y.G.; Kreth, S.; Meder, J.; Doncevic, D.; Saary, P.; et al. Super enhancers define regulatory subtypes and cell identity in neuroblastoma. Nat. Cancer 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Lopez-Carrasco, A.; Berbegall, A.P.; Martin-Vano, S.; Blanquer-Maceiras, M.; Castel, V.; Navarro, S.; Noguera, R. Intra-Tumour Genetic Heterogeneity and Prognosis in High-Risk Neuroblastoma. Cancers 2021, 13, 5173. [Google Scholar] [CrossRef]
- van Groningen, T.; Akogul, N.; Westerhout, E.M.; Chan, A.; Hasselt, N.E.; Zwijnenburg, D.A.; Broekmans, M.; Stroeken, P.; Haneveld, F.; Hooijer, G.K.J.; et al. A NOTCH feed-forward loop drives reprogramming from adrenergic to mesenchymal state in neuroblastoma. Nat. Commun. 2019, 10, 1530. [Google Scholar] [CrossRef]
- Shi, H.; Tao, T.; Abraham, B.J.; Durbin, A.D.; Zimmerman, M.W.; Kadoch, C.; Look, A.T. ARID1A loss in neuroblastoma promotes the adrenergic-to-mesenchymal transition by regulating enhancer-mediated gene expression. Sci. Adv. 2020, 6, eaaz3440. [Google Scholar] [CrossRef]
- Gomez, R.L.; Ibragimova, S.; Ramachandran, R.; Philpott, A.; Ali, F.R. Tumoral heterogeneity in neuroblastoma. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188805. [Google Scholar] [CrossRef]
- Braekeveldt, N.; von Stedingk, K.; Fransson, S.; Martinez-Monleon, A.; Lindgren, D.; Axelson, H.; Levander, F.; Willforss, J.; Hansson, K.; Ora, I.; et al. Patient-Derived Xenograft Models Reveal Intratumor Heterogeneity and Temporal Stability in Neuroblastoma. Cancer Res. 2018, 78, 5958–5969. [Google Scholar] [CrossRef]
- Perkel, J.M. Single-cell proteomics takes centre stage. Nature 2021, 597, 580–582. [Google Scholar] [CrossRef]
- Arias-Hidalgo, C.; Juanes-Velasco, P.; Landeira-Vinuela, A.; Garcia-Vaquero, M.L.; Montalvillo, E.; Gongora, R.; Hernandez, A.P.; Fuentes, M. Single-Cell Proteomics: The Critical Role of Nanotechnology. Int. J. Mol. Sci. 2022, 23, 6707. [Google Scholar] [CrossRef]
- Lohani, V.; Akhiya, A.R.; Kundu, S.; Akhter, M.Q.; Bag, S. Single-Cell Proteomics with Spatial Attributes: Tools and Techniques. ACS Omega 2023, 8, 17499–17510. [Google Scholar] [CrossRef]
- Torok, S.; Vegvari, A.; Rezeli, M.; Fehniger, T.E.; Tovari, J.; Paku, S.; Laszlo, V.; Hegedus, B.; Rozsas, A.; Dome, B.; et al. Localization of sunitinib, its metabolites and its target receptors in tumour-bearing mice: A MALDI-MS imaging study. Br. J. Pharmacol. 2015, 172, 1148–1163. [Google Scholar] [CrossRef]
- Ryu, S.; Hayashi, M.; Aikawa, H.; Okamoto, I.; Fujiwara, Y.; Hamada, A. Heterogeneous distribution of alectinib in neuroblastoma xenografts revealed by matrix-assisted laser desorption ionization mass spectrometry imaging: A pilot study. Br. J. Pharmacol. 2018, 175, 29–37. [Google Scholar] [CrossRef]
- Wu, Z.; Hundsdoerfer, P.; Schulte, J.H.; Astrahantseff, K.; Boral, S.; Schmelz, K.; Eggert, A.; Klein, O. Discovery of Spatial Peptide Signatures for Neuroblastoma Risk Assessment by MALDI Mass Spectrometry Imaging. Cancers 2021, 13, 3184. [Google Scholar] [CrossRef]
- Chen, Y.N.; LaMarche, M.J.; Chan, H.M.; Fekkes, P.; Garcia-Fortanet, J.; Acker, M.G.; Antonakos, B.; Chen, C.H.; Chen, Z.; Cooke, V.G.; et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 2016, 535, 148–152. [Google Scholar] [CrossRef]
- Li, Y.; Dong, J.; Qin, J.J. Small molecule inhibitors targeting heat shock protein 90: An updated review. Eur. J. Med. Chem. 2024, 275, 116562. [Google Scholar] [CrossRef]
- Regan, P.L.; Jacobs, J.; Wang, G.; Torres, J.; Edo, R.; Friedmann, J.; Tang, X.X. Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma. Int. J. Oncol. 2011, 38, 105–112. [Google Scholar]
- Hsieh, C.H.; Huang, C.T.; Cheng, Y.S.; Hsu, C.H.; Hsu, W.M.; Chung, Y.H.; Liu, Y.L.; Yang, T.S.; Chien, C.Y.; Lee, Y.H.; et al. Homoharringtonine as a PHGDH inhibitor: Unraveling metabolic dependencies and developing a potent therapeutic strategy for high-risk neuroblastoma. Biomed. Pharmacother. 2023, 166, 115429. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef]
- DeGregori, J.; Johnson, D.G. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr. Mol. Med. 2006, 6, 739–748. [Google Scholar]
- Ejeskar, K.; Krona, C.; Caren, H.; Zaibak, F.; Li, L.; Martinsson, T.; Ioannou, P.A. Introduction of in vitro transcribed ENO1 mRNA into neuroblastoma cells induces cell death. BMC Cancer 2005, 5, 161. [Google Scholar] [CrossRef]
- Omenn, G.S. Strategies for plasma proteomic profiling of cancers. Proteomics 2006, 6, 5662–5673. [Google Scholar] [CrossRef]
| a. Molecular risk factors in NB | Relevant protein targets in this review |
| MYCN network or MYCN status | P300 [49], PHGDH [51], PKM2 [61], HK2 [61], Alix [62], TSG101 [62], FLOT1 [62], VPS35 [62], DDX3X [135], |
| RTK/ALK signaling | IGF1R [53], SHP2 [53,56], CDK16 [53], LMNA [54], IRS2 [55], DUSP4 [105] |
| Wnt/β-catenin signaling | hnRNPH3, hnRNPA2B1 [58] |
| RAS/MAPK pathway | SHP2 [53,56], PRUNE2 [127], MK167 [127], DUSP4 [105] |
| ATR activity | RAD51 [101], BRCA1/2 [101], E2F3 [101], DCK [101], ATM [102], DNAPK [102] |
| PI3K/Akt/mTOR pathway | IRS2 [55], Caspase3 [110], CDK6 [110], PIKK Family [102] |
| b. Risk stratification in NB | Relevant protein targets in this review |
| High risk-related | P300 [49], PHGDH [51,113], MATR3 [52], SHP2 [53,56], PAI1 [57], SPARC [57], POSTN [57], LEG1 [57], IRS2 [55], SEMA3A [60], SHMT2 [60], PKM2 [61], HK2 [61], NCAM [63,64], NCL [63], LGALS3BP [63], MYH9 [63], FN1 [63], LTBP1 [63], DDX3X [135], ANXA2 [152] |
| Favorable outcome-related | ACADM [50], LUM [63], VASP [63], DCN [63], CALR [63], AKAP12 [63], PRUNE2 [127], Caspase3 [110], PTBP2 [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Wang, Y.; Zhang, M.; Tao, T.; Sun, Z. Unveiling Tumorigenesis Mechanisms and Drug Therapy in Neuroblastoma by Mass Spectrometry Based Proteomics. Children 2024, 11, 1323. https://doi.org/10.3390/children11111323
Ren K, Wang Y, Zhang M, Tao T, Sun Z. Unveiling Tumorigenesis Mechanisms and Drug Therapy in Neuroblastoma by Mass Spectrometry Based Proteomics. Children. 2024; 11(11):1323. https://doi.org/10.3390/children11111323
Chicago/Turabian StyleRen, Keyi, Yu Wang, Minmin Zhang, Ting Tao, and Zeyu Sun. 2024. "Unveiling Tumorigenesis Mechanisms and Drug Therapy in Neuroblastoma by Mass Spectrometry Based Proteomics" Children 11, no. 11: 1323. https://doi.org/10.3390/children11111323
APA StyleRen, K., Wang, Y., Zhang, M., Tao, T., & Sun, Z. (2024). Unveiling Tumorigenesis Mechanisms and Drug Therapy in Neuroblastoma by Mass Spectrometry Based Proteomics. Children, 11(11), 1323. https://doi.org/10.3390/children11111323






