Exploring the Clinical Characteristics and Outcomes of Rhinovirus Infection in Hospitalized Children Compared with Other Respiratory Viruses
Abstract
:1. Introduction
2. Materials and Methods
3. Results
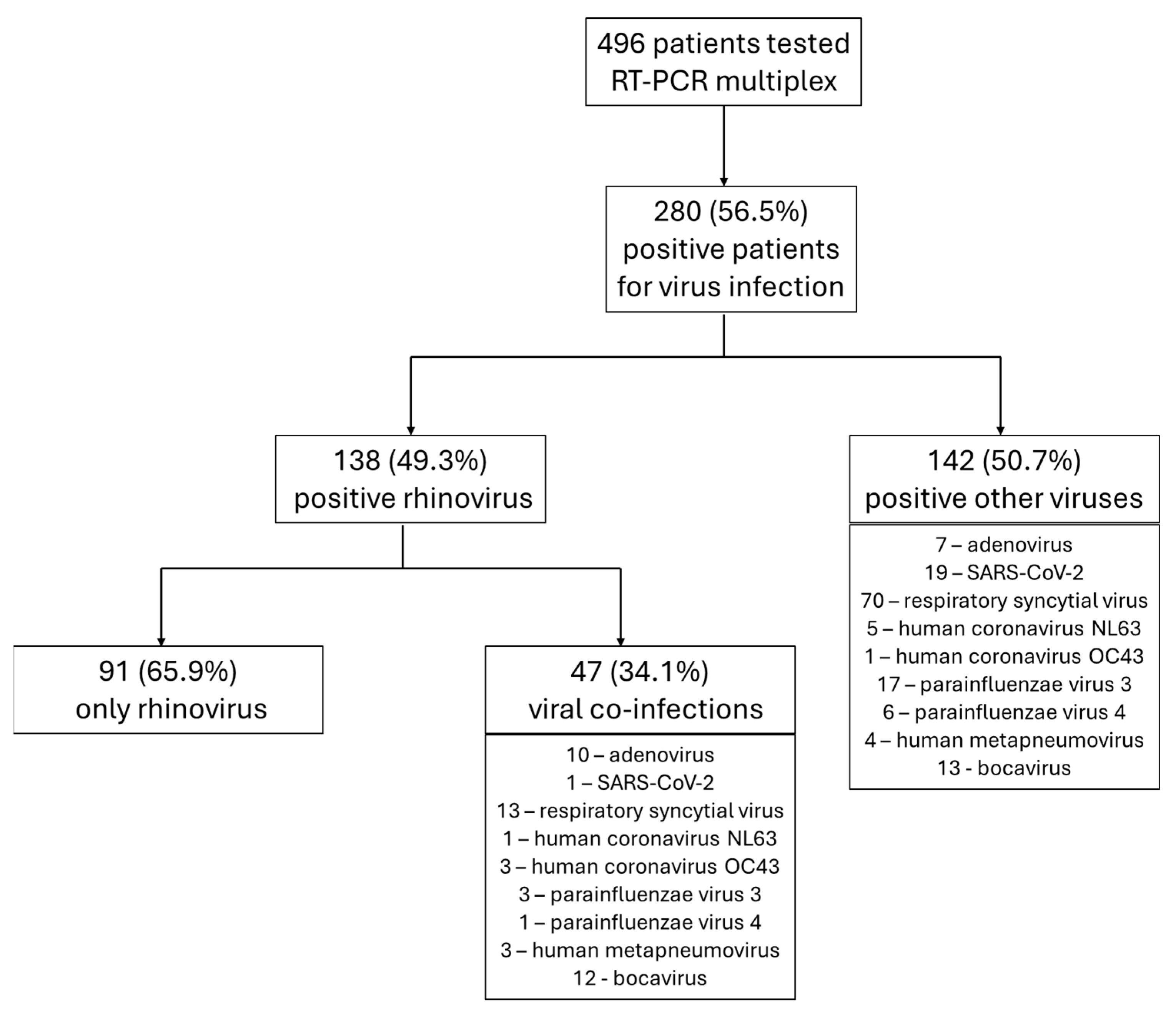
3.1. General Data Analysis
3.2. Specific and Comparative Characteristics of Rhinovirus-Positive Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, A.; Wilkinson, T.M.A. Respiratory viral infections in the elderly. Ther. Adv. Respir. Dis. 2021, 15, 1753466621995050. [Google Scholar] [CrossRef] [PubMed]
- Leotte, J.; Trombetta, H.; Faggion, H.Z.; Almeida, B.M.; Nogueira, M.B.; Vidal, L.R.; Raboni, S.M. Impact and seasonality of human rhinovirus infection in hospitalized patients for two consecutive years. J. Pediatr. 2017, 93, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Ljubin-Sternak, S.; Mestrovic, T. Rhinovirus-A True Respiratory Threat or a Common Inconvenience of Childhood? Viruses 2023, 15, 825. [Google Scholar] [CrossRef] [PubMed]
- Gern, J.E. The ABCs of rhinoviruses, wheezing, and asthma. J. Virol. 2010, 84, 7418–7426. [Google Scholar] [CrossRef] [PubMed]
- Vandini, S.; Biagi, C.; Fischer, M.; Lanari, M. Impact of Rhinovirus Infections in Children. Viruses 2019, 11, 521. [Google Scholar] [CrossRef]
- To, K.K.W.; Yip, C.C.Y.; Yuen, K.Y. Rhinovirus—From bench to bedside. J. Formos. Med. Assoc. 2017, 116, 496–504. [Google Scholar] [CrossRef]
- Winther, B. Rhinovirus infections in the upper airway. Proc. Am. Thorac. Soc. 2011, 8, 79–89. [Google Scholar] [CrossRef]
- Yin-Murphy, M.; Almond, J.W. Picornaviruses. In Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Cafferkey, J.; Coultas, J.A.; Mallia, P. Human rhinovirus infection and COPD: Role in exacerbations and potential for therapeutic targets. Expert Rev. Respir. Med. 2020, 14, 777–789. [Google Scholar] [CrossRef]
- Atmar, R.L. Uncommon(ly considered) manifestations of infection with rhinovirus, agent of the common cold. Clin. Infect. Dis. 2005, 41, 266–267. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Turner, R.B.; Braciale, T.; Heymann, P.W.; Borish, L. Pathogenesis of rhinovirus infection. Curr. Opin. Virol. 2012, 2, 287–293. [Google Scholar] [CrossRef]
- Miron, V.D.; Gunsahin, D.; Filimon, C.; Bar, G.; Craiu, M. Pediatric Emergencies and Hospital Admissions in the First Six Months of the COVID-19 Pandemic in a Tertiary Children’s Hospital in Romania. Children 2022, 9, 513. [Google Scholar] [CrossRef]
- Sandulescu, O.; Draganescu, A.; Pitigoi, D. Influenza redefined-clinical and epidemiological insight. Germs 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Sandulescu, M.; Sandulescu, O. Changing clinical patterns and ear-nose-throat complications of seasonal viral respiratory tract infections. Germs 2023, 13, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, A.C.; Miron, V.D.; Sandulescu, O.; Bilasco, A.; Streinu-Cercel, A.; Sandu, R.G.; Marinescu, A.; Gunsahin, D.; Hoffmann, K.I.; Horobet, D.S.; et al. Omicron in Infants-Respiratory or Digestive Disease? Diagnostics 2023, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A.; et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic—United States, 2020–2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.D.; Bar, G.; Filimon, C.; Craiu, M. From COVID-19 to Influenza-Real-Life Clinical Practice in a Pediatric Hospital. Diagnostics 2022, 12, 1208. [Google Scholar] [CrossRef]
- Miron, V.D.; Banica, L.; Sandulescu, O.; Paraschiv, S.; Surleac, M.; Florea, D.; Vlaicu, O.; Milu, P.; Streinu-Cercel, A.; Bilasco, A.; et al. Clinical and molecular epidemiology of influenza viruses from Romanian patients hospitalized during the 2019/20 season. PLoS ONE 2021, 16, e0258798. [Google Scholar] [CrossRef]
- Smedberg, J.R.; DiBiase, L.M.; Hawken, S.E.; Allen, A.; Mohan, S.; Santos, C.; Smedberg, T.; Barzin, A.H.; Wohl, D.A.; Miller, M.B. Reduction and persistence of co-circulating respiratory viruses during the SARS-CoV-2 pandemic. Am. J. Infect. Control. 2022, 50, 1064–1066. [Google Scholar] [CrossRef]
- Haapanen, M.; Renko, M.; Artama, M.; Kuitunen, I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children–A nationwide register study in Finland. EClinicalMedicine 2021, 34, 100807. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. SARS-CoV-2 coinfections: Could influenza and the common cold be beneficial? J. Med. Virol. 2020, 92, 2623–2630. [Google Scholar] [CrossRef]
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Lamson, D.M.; St George, K.; Walsh, T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.D.; Craiu, M. Red throat or acute pharyngitis-challenges in real life clinical practice. Germs 2021, 11, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Peltola, V.; Waris, M.; Osterback, R.; Susi, P.; Hyypia, T.; Ruuskanen, O. Clinical effects of rhinovirus infections. J. Clin. Virol. 2008, 43, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Min, L.; Zhang, X. Usefulness of procalcitonin (PCT), C-reactive protein (CRP), and white blood cell (WBC) levels in the differential diagnosis of acute bacterial, viral, and mycoplasmal respiratory tract infections in children. BMC Pulm. Med. 2021, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Debes, S.; Haug, J.B.; De Blasio, B.F.; Lindstrom, J.C.; Jonassen, C.M.; Dudman, S.G. Antibiotic Consumption in a Cohort of Hospitalized Adults with Viral Respiratory Tract Infection. Antibiotics 2023, 12, 788. [Google Scholar] [CrossRef]
- Diac, I.; Dogaroiu, C.; Keresztesi, A.A.; Horumba, M. Antimicrobial resistance trends-a single-center retrospective study of healthcare-associated pathogens-postmortem sampling from medico-legal autopsies in Bucharest. Germs 2022, 12, 352–360. [Google Scholar] [CrossRef]
- Schaut, M.; Schaefer, M.; Trost, U.; Sander, A. Integrated antibiotic clinical decision support system (CDSS) for appropriate choice and dosage: An analysis of retrospective data. Germs 2022, 12, 203–213. [Google Scholar] [CrossRef]
- Săndulescu, O.; Viziteu, I.; Streinu-Cercel, A.; Miron, V.D.; Preoțescu, L.L.; Chirca, N.; Albu, S.E.; Craiu, M.; Streinu-Cercel, A. Novel Antimicrobials, Drug Delivery Systems and Antivirulence Targets in the Pipeline—From Bench to Bedside. Appl. Sci. 2022, 12, 11615. [Google Scholar] [CrossRef]
- Rao, S.; Lamb, M.M.; Moss, A.; Mistry, R.D.; Grice, K.; Ahmed, W.; Santos-Cantu, D.; Kitchen, E.; Patel, C.; Ferrari, I.; et al. Effect of Rapid Respiratory Virus Testing on Antibiotic Prescribing Among Children Presenting to the Emergency Department with Acute Respiratory Illness: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2111836. [Google Scholar] [CrossRef]
- Tarciuc, P.; Plesca, D.A.; Duduciuc, A.; Gimiga, N.; Tataranu, E.; Herdea, V.; Ion, L.M.; Diaconescu, S. Self-Medication Patterns during a Pandemic: A Qualitative Study on Romanian Mothers’ Beliefs toward Self-Treatment of Their Children. Healthcare 2022, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Plesca, V.S.; Miron, V.D.; Marinescu, A.G.; Draganescu, A.C.; Plesca, A.D.; Sandulescu, O.; Voiosu, C.; Hainarosie, R.; Streinu-Cercel, A. Hospitalizations for Acute Otitis and Sinusitis in Patients Living with HIV: A Retrospective Analysis of a Tertiary Center in Romania. J. Clin. Med. 2024, 13, 3346. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Elena, A.X.; Kunath, M.A.; Berendonk, T.U.; Klumper, U. Reduced selection for antibiotic resistance in community context is maintained despite pressure by additional antibiotics. ISME Commun. 2023, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- van Houten, C.B.; Cohen, A.; Engelhard, D.; Hays, J.P.; Karlsson, R.; Moore, E.; Fernandez, D.; Kreisberg, R.; Collins, L.V.; de Waal, W.; et al. Antibiotic misuse in respiratory tract infections in children and adults–A prospective, multicentre study (TAILORED Treatment). Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 505–514. [Google Scholar] [CrossRef]
- Wildenbeest, J.G.; van der Schee, M.P.; Hashimoto, S.; Benschop, K.S.; Minnaar, R.P.; Sprikkelman, A.B.; Haarman, E.G.; van Aalderen, W.M.; Sterk, P.J.; Pajkrt, D.; et al. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin. Microbiol. Infect. 2016, 22, 736.e9–736.e15. [Google Scholar] [CrossRef]
- Plesca, V.S.; Streinu-Cercel, A.; Sandulescu, O.; Draganescu, A.C.; Hainarosie, R.; Plesca, A.D. Incidence and Characteristics of Pediatric Patients with Acute Otitis Hospitalized in a Romanian Infectious Diseases Hospital. Children 2024, 11, 832. [Google Scholar] [CrossRef]
- Stewart, C.J.; Hasegawa, K.; Wong, M.C.; Ajami, N.J.; Petrosino, J.F.; Piedra, P.A.; Espinola, J.A.; Tierney, C.N.; Camargo, C.A., Jr.; Mansbach, J.M. Respiratory Syncytial Virus and Rhinovirus Bronchiolitis Are Associated with Distinct Metabolic Pathways. J. Infect. Dis. 2018, 217, 1160–1169. [Google Scholar] [CrossRef]
- Draganescu, A.C.; Miron, V.D.; Streinu-Cercel, A.; Florea, D.; Vlaicu, O.; Bilasco, A.; Otelea, D.; Luminos, M.L.; Pitigoi, D.; Streinu-Cercel, A.; et al. Circulation of influenza A viruses among patients hospitalized for severe acute respiratory infection in a tertiary care hospital in Romania in the 2018/19 season: Results from an observational descriptive epidemiological study. Medicine 2021, 100, e28460. [Google Scholar] [CrossRef]
- Chantzi, F.M.; Papadopoulos, N.G.; Bairamis, T.; Tsiakou, M.; Bournousouzis, N.; Constantopoulos, A.G.; Liapi, G.; Xatzipsalti, M.; Kafetzis, D.A. Human rhinoviruses in otitis media with effusion. Pediatr. Allergy Immunol. 2006, 17, 514–518. [Google Scholar] [CrossRef]
- Plesca, V.S.; Marinescu, A.G.; Voiosu, C.; Draganescu, A.C.; Streinu-Cercel, A.; Vilaia, A.; Hainarosie, R.; Plesca, D.A.; Sandulescu, O. Occurrence of acute otitis and sinusitis in patients hospitalized for influenza. Germs 2024, 14, 38–44. [Google Scholar] [CrossRef]
- Rankin, D.A.; Spieker, A.J.; Perez, A.; Stahl, A.L.; Rahman, H.K.; Stewart, L.S.; Schuster, J.E.; Lively, J.Y.; Haddadin, Z.; Probst, V.; et al. Circulation of Rhinoviruses and/or Enteroviruses in Pediatric Patients with Acute Respiratory Illness Before and During the COVID-19 Pandemic in the US. JAMA Netw. Open 2023, 6, e2254909. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Only Rhinovirus Infection | Negative Patients | p-Value | Other Respiratory Viruses | p-Value | Rhinovirus Co-Infection | p-Value |
|---|---|---|---|---|---|---|---|
| N = 91 | N = 216 | N = 142 | N = 47 | ||||
| Demographic data | |||||||
| Male | 49 (53.8) | 114 (52.8) | 0.862 | 78 (54.9) | 0.862 | 28 (59.6) | 0.521 |
| Age (months), median (IQR) | 12 (2, 53) | 6.5 (0, 58.5) | <0.001 | 3 (0, 24.5) | <0.001 | 13 (4, 32) | 0.457 |
| Clinical features | |||||||
| Malaise | 38 (41.8) | 73 (33.8) | 0.184 | 56 (39.4) | 0.729 | 19 (40.4) | 0.887 |
| Fever | 43 (47.3) | 92 (42.6) | 0.454 | 68 (47.9) | 0.920 | 30 (63.8) | 0.064 |
| Cough | 58 (63.7) | 57 (26.4) | <0.001 | 121 (85.2) | <0.001 | 40 (85.1) | 0.009 |
| Nasal congestion | 39 (42.9) | 27 (12.5) | <0.001 | 83 (58.5) | 0.020 | 23 (48.9) | 0.497 |
| Dyspnea | 47 (51.6) | 46 (21.3) | <0.001 | 95 (66.9) | 0.019 | 33 (70.2) | 0.036 |
| Diarrhea | 18 (19.8) | 57 (26.4) | 0.219 | 18 (12.7) | 0.143 | 6 (12.8) | 0.303 |
| Vomiting | 17 (18.7) | 34 (15.7) | 0.105 | 15 (10.6) | 0.079 | 2 (4.3) | 0.019 |
| Laboratory findings | |||||||
| Increased WBC | 38 (41.8) | 66 (30.6) | 0.058 | 24 (16.9) | <0.001 | 14 (29.8) | 0.169 |
| Decreased WBC | 3 (3.3) | 16 (7.4) | 0.172 | 10 (7.0) | 0.223 | 0 (0.0) | NA |
| Increased neutrophils | 34 (37.4) | 64 (29.6) | 0.184 | 22 (15.5) | <0.001 | 13 (27.7) | 0.254 |
| Decreased neutrophils | 1 (1.1) | 14 (6.5) | 0.076 | 6 (4.2) | 0.251 | 1 (2.1) | NA |
| Increased monocyte | 55 (60.4) | 125 (57.9) | 0.680 | 78 (54.9) | 0.406 | 31 (66.0) | 0.527 |
| Increased lymphocyte | 9 (9.9) | 24 (11.1) | 0.751 | 17 (12.0) | 0.624 | 4 (8.5) | 0.828 |
| Decreased lymphocyte | 22 (24.2) | 36 (16.7) | 0.124 | 34 (23.9) | 0.920 | 9 (19.1) | 0.502 |
| Elevated CRP | 51 (56.0) | 96 (44.4) | 0.063 | 55 (38.7) | 0.009 | 20 (42.6) | 0.132 |
| Treatment | |||||||
| Antibiotics | 34 (37.4) | 115 (53.2) | 0.011 | 71 (50.0) | 0.058 | 21 (44.7) | 0.350 |
| Aerosol therapy | 58 (63.7) | 52 (24.9) | <0.001 | 114 (80.3) | 0.005 | 33 (70.2) | 0.446 |
| Isotonic saline solution * | 9 (9.9) | 7 (3.2) | 0.023 | 19 (13.4) | 0.423 | 5 (10.6) | 0.892 |
| Hypertonic saline solution * | 15 (16.5) | 25 (11.6) | 0.243 | 12 (8.5) | 0.061 | 2 (4.3) | 0.041 |
| Salbutamol * | 35 (38.5) | 25 (11.6) | <0.001 | 30 (21.1) | 0.003 | 14 (29.8) | 0.312 |
| Adrenaline * | 24 (26.4) | 20 (9.3) | <0.001 | 79 (55.6) | <0.001 | 21 (44.7) | 0.029 |
| Cortisone # | 39 (42.9) | 50 (23.1) | <0.001 | 51 (35.9) | 0.287 | 26 (55.3) | 0.164 |
| Complications | |||||||
| AOM | 10 (11.0) | 9 (4.2) | 0.023 | 8 (5.6) | 0.135 | 5 (10.6) | 0.920 |
| Acute bronchitis or bronchiolitis | 43 (47.3) | 11 (5.1) | <0.001 | 83 (58.5) | 0.094 | 32 (68.1) | 0.019 |
| ARF | 36 (39.6) | 28 (13.0) | <0.001 | 74 (52.1) | 0.060 | 28 (59.6) | 0.025 |
| Acute laryngitis | 6 (6.6) | 4 (1.9) | 0.042 | 5 (3.5) | 0.346 | 4 (8.5) | 0.734 |
| Acute pneumonia | 7 (7.7) | 16 (7.4) | 0.920 | 13 (9.2) | 0.718 | 5 (10.6) | 0.751 |
| Acute dehydration | 35 (38.5) | 81 (37.5) | 0.862 | 50 (35.2) | 0.617 | 21 (44.7) | 0.479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covaci, S.; Filimon, C.; Craiu, M. Exploring the Clinical Characteristics and Outcomes of Rhinovirus Infection in Hospitalized Children Compared with Other Respiratory Viruses. Children 2024, 11, 1303. https://doi.org/10.3390/children11111303
Covaci S, Filimon C, Craiu M. Exploring the Clinical Characteristics and Outcomes of Rhinovirus Infection in Hospitalized Children Compared with Other Respiratory Viruses. Children. 2024; 11(11):1303. https://doi.org/10.3390/children11111303
Chicago/Turabian StyleCovaci, Sigrid, Claudiu Filimon, and Mihai Craiu. 2024. "Exploring the Clinical Characteristics and Outcomes of Rhinovirus Infection in Hospitalized Children Compared with Other Respiratory Viruses" Children 11, no. 11: 1303. https://doi.org/10.3390/children11111303
APA StyleCovaci, S., Filimon, C., & Craiu, M. (2024). Exploring the Clinical Characteristics and Outcomes of Rhinovirus Infection in Hospitalized Children Compared with Other Respiratory Viruses. Children, 11(11), 1303. https://doi.org/10.3390/children11111303




