Audiological and Subjective Benefits in a Child with Microtia and Atresia After Sequential Bilateral Implantation with Active Bone Conduction Devices: A Case Study
Abstract
1. Introduction
Device Description
2. Case Report
2.1. Audiological Tests
2.2. Sound Source Localization
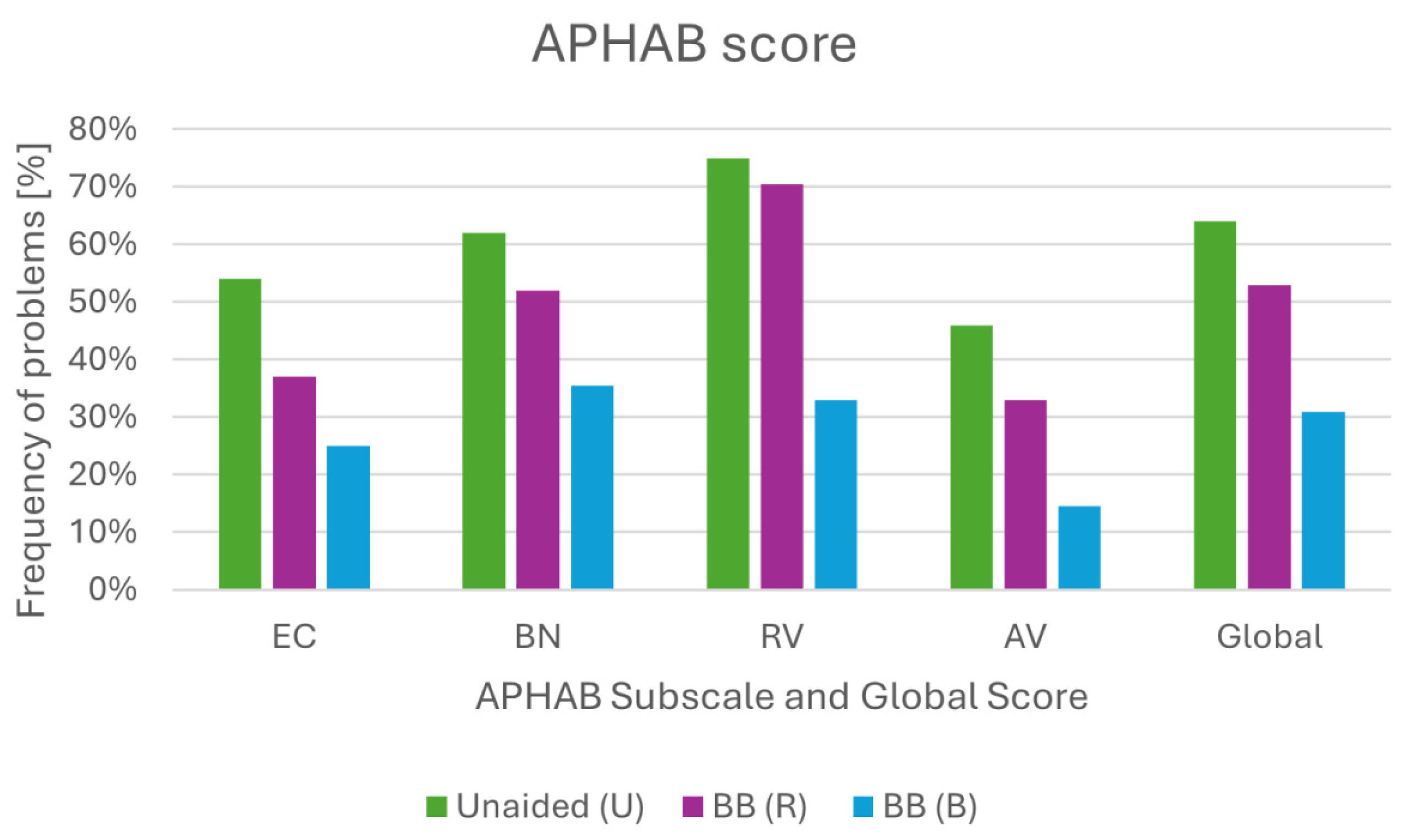
2.3. Questionnaire Assessment
2.4. Sound-Field Hearing Thresholds (SFHTs) and Word Recognition Scores (WRSs) Results
2.5. Sound Source Localization Results
2.6. Questionnaire Assessment Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kılıç, S.; Bouzaher, M.H.; Cohen, M.S.; Lieu, J.E.C.; Kenna, M.; Anne, S. Comprehensive medical evaluation of pediatric bilateral sensorineural hearing loss. Laryngoscope Investig. Otolaryngol. 2021, 6, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Luquetti, D.V.; Heike, C.L.; Hing, A.V.; Cunningham, M.L.; Cox, T.C. Microtia: Epidemiology and genetics. Am. J. Med. Genet. Part A 2012, 158A, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Cywka, K.B.; Król, B.; Skarżyński, P.H. Effectiveness of Bone Conduction Hearing Aids in Young Children with Congenital Aural Atresia and Microtia. Med. Sci. Monit. 2021, 27, e933915-1–e933915-8. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.; Noble, W. Optimizing Sound Localization with Hearing Aids. Trends Amplif. 1998, 3, 51–73. [Google Scholar] [CrossRef]
- Hirsh, I.J. The Relation Between Localization and Intelligibility. J. Acoust. Soc. Am. 1950, 22, 196–200. [Google Scholar] [CrossRef]
- Kara, A.; Iseri, M.; Durgut, M.; Topdag, M.; Ozturk, M. Comparing audiological test results obtained from a sound processor attached to a Softband with direct and magnetic passive bone conduction hearing implant systems. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4193–4198. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wang, P.; Fan, Y.; Chen, X. Hearing improvement with softband and implanted bone-anchored hearing devices and modified implantation surgery in patients with bilateral microtia-atresia. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 120–125. [Google Scholar] [CrossRef]
- Pittman, A.L. Bone Conduction Amplification in Children: Stimulation via a Percutaneous Abutment versus a Transcutaneous Softband. Ear Hear. 2019, 40, 1307–1315. [Google Scholar] [CrossRef]
- Cywka, K.B.; Skarżyński, H.; Król, B.; Skarżyński, P.H. The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits. J. Clin. Med. 2021, 10, 5916. [Google Scholar] [CrossRef]
- Reinfeldt, S.; Håkansson, B.; Taghavi, H.; Eeg-Olofsson, M. New developments in bone-conduction hearing implants: A review. Med. Devices 2015, 8, 79–93. [Google Scholar] [CrossRef]
- Skarżyński, P.H.; Ratuszniak, A.; Król, B.; Kozieł, M.; Osińska, K.; Cywka, K.; Sztabnicka, A.; Skarżyński, H. The Bonebridge in Adults with Mixed and Conductive Hearing Loss: Audiological and Quality of Life Outcomes. Audiol. Neurootol. 2019, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Utrilla, C.; Gavilán, J.; García-Raya, P.; Calvino, M.; Lassaletta, L. MRI after Bonebridge implantation: A comparison of two implant generations. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, H.; Lorens, A.; Kruszynska, M.; Obrycka, A.; Pastuszak, D.; Skarzynski, P.H. The hearing benefit of cochlear implantation for individuals with unilateral hearing loss, but no tinnitus. Acta Otolaryngol. 2017, 137, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Alexander, G.C. The Abbreviated Profile of Hearing Aid Benefit. Ear Hear. 1995, 16, 176–186. [Google Scholar] [CrossRef]
- Bravo-Torres, S.; Der-Mussa, C.; Fuentes-López, E. Active transcutaneous bone conduction implant: Audiological results in paediatric patients with bilateral microtia associated with external auditory canal atresia. Int. J. Audiol. 2018, 57, 53–60. [Google Scholar] [CrossRef]
- Rahne, T.; Seiwerth, I.; Götze, G.; Heider, C.; Radetzki, F.; Herzog, M.; Plontke, S.K. Functional results after Bonebridge implantation in adults and children with conductive and mixed hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 3263–3269. [Google Scholar] [CrossRef]
- Ratuszniak, A.; Skarzynski, P.H.; Gos, E.; Skarzynski, H. The Bonebridge implant in older children and adolescents with mixed or conductive hearing loss: Audiological outcomes. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 97–102. [Google Scholar] [CrossRef]
- Baumgartner, W.-D.; Hamzavi, J.-S.; Böheim, K.; Wolf-Magele, A.; Schlögel, M.; Riechelmann, H.; Zorowka, P.; Koci, V.; Keck, T.; Potzinger, P.; et al. A New Transcutaneous Bone Conduction Hearing Implant: Short-Term Safety and Efficacy in Children. Otol. Neurotol. 2016, 37, 713–720. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Ren, R.; Han, D.M.; Li, Y.; Ma, X.B.; Wang, D.N.; Li, Y.L. The implantation of Bonebridge in bilateral congenital malformation of external and middle ear. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 52, 512–516. [Google Scholar] [CrossRef]
- Willenborg, K.; Lenarz, T.; Busch, S. Surgical and audiological outcomes with a new transcutaneous bone conduction device with reduced transducer thickness in children. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 4381–4389. [Google Scholar] [CrossRef]
- Šikolová, S.; Urík, M.; Hošnová, D.; Kruntorád, V.; Bartoš, M.; Motyka, O.; Jabandžiev, P. Two Bonebridge bone conduction hearing implant generations: Audiological benefit and quality of hearing in children. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, I.; Fröhlich, L.; Schilde, S.; Götze, G.; Plontke, S.K.; Rahne, T. Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zernotti, M.E.; Chiaraviglio, M.M.; Mauricio, S.B.; Tabernero, P.A.; Zernotti, M.; Di Gregorio, M.F. Audiological outcomes in patients with congenital aural atresia implanted with transcutaneous active bone conduction hearing implant. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, Y.; Wang, P.; Fan, Y.; Chen, Y.; Zhu, Y.; Chen, X. Aesthetic and hearing rehabilitation in patients with bilateral microtia-atresia. Int. J. Pediatr. Otorhinolaryngol. 2017, 101, 150–157. [Google Scholar] [CrossRef]
- Sprinzl, G.; Lenarz, T.; Ernst, A.; Hagen, R.; Wolf-Magele, A.; Mojallal, H.; Todt, I.; Mlynski, R.; Wolframm, M.D. First European Multicenter Results with a New Transcutaneous Bone Conduction Hearing Implant System: Short-Term Safety and Efficacy. Otol. Neurotol. 2013, 34, 1076–1083. [Google Scholar] [CrossRef]
- Priwin, C.; Stenfelt, S.; Granström, G.; Tjellström, A.; Håkansson, B. Bilateral bone-anchored hearing aids (BAHAs): An audiometric evaluation. Laryngoscope 2004, 114, 77–84. [Google Scholar] [CrossRef]
- Zeitooni, M.; Mäki-Torkko, E.; Stenfelt, S. Binaural Hearing Ability with Bilateral Bone Conduction Stimulation in Subjects with Normal Hearing: Implications for Bone Conduction Hearing Aids. Ear Hear. 2016, 37, 690–702. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Loveman, E.; Baguley, D.M.; Mitchell, T.E.; Sheehan, P.Z.; Harris, P.; Proops, D.W.; Jones, J.; Clegg, A.J.; Welch, K. Bone-anchored hearing aids for people with bilateral hearing impairment: A systematic review. Clin. Otolaryngol. 2011, 36, 419–441. [Google Scholar] [CrossRef]
- Dutt, S.N.; McDermott, A.-L.; Burrell, S.P.; Cooper, H.R.; Reid, A.P.; Proops, D.W. Speech intelligibility with bilateral bone-anchored hearing aids: The Birmingham experience. J. Laryngol. Otol. 2002, 116, 47–51. [Google Scholar] [CrossRef]
- Dun, C.A.J.; Agterberg, M.J.H.; Cremers, C.W.R.J.; Hol, M.K.S.; Snik, A.F.M. Bilateral bone conduction devices: Improved hearing ability in children with bilateral conductive hearing loss. Ear Hear. 2013, 34, 806–808. [Google Scholar] [CrossRef]
- Bosman, A.J.; Snik, A.F.; van der Pouw, C.T.; Mylanus, E.A.; Cremers, C.W. Audiometric evaluation of bilaterally fitted bone-anchored hearing aids. Audiol. Off. Organ Int. Soc. Audiol. 2001, 40, 158–167. [Google Scholar] [CrossRef]
- Canale, A.; Urbanelli, A.; Gragnano, M.; Bordino, V.; Albera, A. Comparison of Active Bone Conduction Hearing Implant Systems in Unilateral and Bilateral Conductive or Mixed Hearing Loss. Brain Sci. 2023, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- den Besten, C.A.; Bosman, A.J.; Nelissen, R.C.; Mylanus, E.A.M.; Hol, M.K.S. Controlled Clinical Trial on Bone-Anchored Hearing Implants and a Surgical Technique With Soft-tissue Preservation. Otol. Neurotol. 2016, 37, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ping, L.; Yang, T.; Niu, X.; Chen, Y.; Xia, X.; Gao, R.; Fan, Y.; Chen, X. Comparative effects of unilateral and bilateral bone conduction hearing devices on functional hearing and sound localization abilities in patients with bilateral microtia-atresia. Acta Otolaryngol. 2020, 140, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; Andries, E.; Kurz, A.; Tȧvora-Vieira, D.; Calvino, M.; Amann, E.; Anderson, I.; Lorens, A. Towards a Consensus on an ICF-Based Classification System for Horizontal Sound-Source Localization. J. Pers. Med. 2022, 12, 1971. [Google Scholar] [CrossRef]
- Chen, P.; Yang, L.; Yang, J.; Wang, D.; Li, Y.; Zhao, C.; Liu, Y.; Gao, M.; Zhu, J.; Li, S.; et al. Simultaneous bilateral transcutaneous bone conduction device implantation: Sound localisation and speech perception in children with bilateral conductive hearing loss. J. Laryngol. Otol. 2022, 136, 939–946. [Google Scholar] [CrossRef]
- Dusu, K.; Walsh, M.; Eze, N. Paediatric Bonebridge: Bilateral simultaneous asymmetrical implantation. J. Laryngol. Otol. 2019, 133, 719–722. [Google Scholar] [CrossRef]
- Siegel, L.; Araslanova, R.; Stepniak, C.; Zimmerman, K.; Agrawal, S.K. Achondroplasia and severe sensorineural hearing loss: The role of active bone conduction implants. Cochlear Implants Int. 2022, 23, 291–299. [Google Scholar] [CrossRef]
- Canale, A.; Ndrev, D.; Sapino, S.; Bianchi, C.; Bordino, V.; Albera, A. Speech in Noise with Bilateral Active Bone Conduction Implant for Conductive and Mixed Hearing Loss. Otol. Neurotol. 2022, 43, 1000–1004. [Google Scholar] [CrossRef]
- Volgger, V.; Schießler, I.T.; Müller, J.; Schrötzlmair, F.; Pollotzek, M.; Hempel, J.M. Audiological results and subjective benefit of an active transcutaneous bone-conduction device in patients with congenital aural atresia. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 2345–2352. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cywka, K.; Ratuszniak, A.; Skarżyński, P.H. Audiological and Subjective Benefits in a Child with Microtia and Atresia After Sequential Bilateral Implantation with Active Bone Conduction Devices: A Case Study. Children 2024, 11, 1285. https://doi.org/10.3390/children11111285
Cywka K, Ratuszniak A, Skarżyński PH. Audiological and Subjective Benefits in a Child with Microtia and Atresia After Sequential Bilateral Implantation with Active Bone Conduction Devices: A Case Study. Children. 2024; 11(11):1285. https://doi.org/10.3390/children11111285
Chicago/Turabian StyleCywka, Katarzyna, Anna Ratuszniak, and Piotr Henryk Skarżyński. 2024. "Audiological and Subjective Benefits in a Child with Microtia and Atresia After Sequential Bilateral Implantation with Active Bone Conduction Devices: A Case Study" Children 11, no. 11: 1285. https://doi.org/10.3390/children11111285
APA StyleCywka, K., Ratuszniak, A., & Skarżyński, P. H. (2024). Audiological and Subjective Benefits in a Child with Microtia and Atresia After Sequential Bilateral Implantation with Active Bone Conduction Devices: A Case Study. Children, 11(11), 1285. https://doi.org/10.3390/children11111285






