Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future
Abstract
1. Introduction
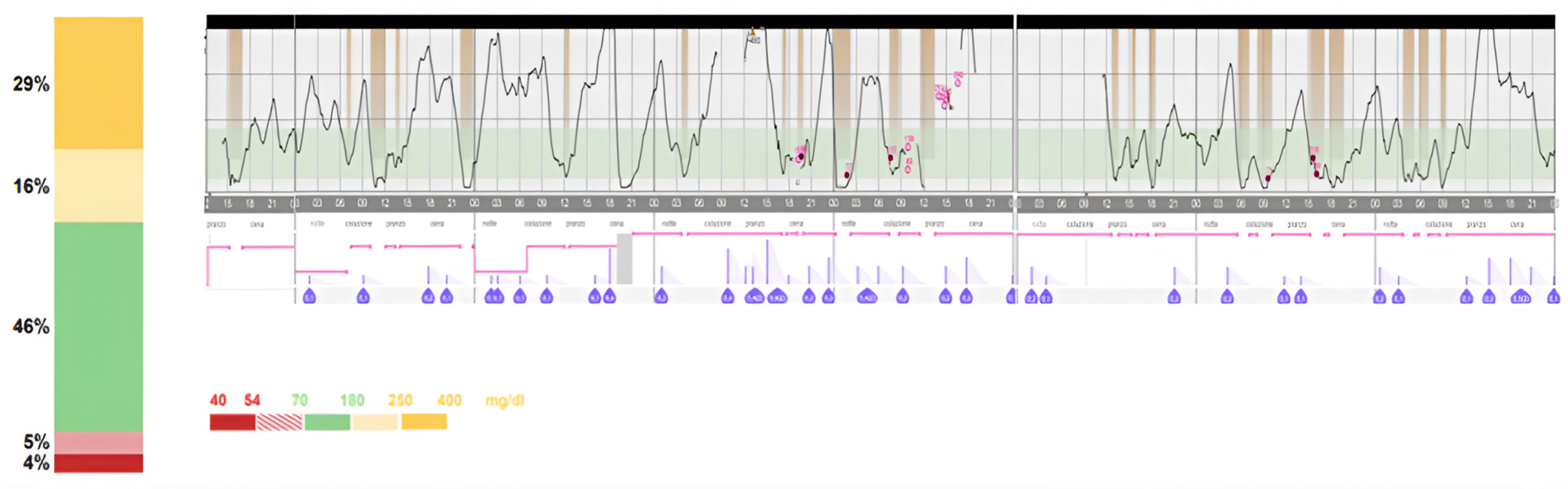
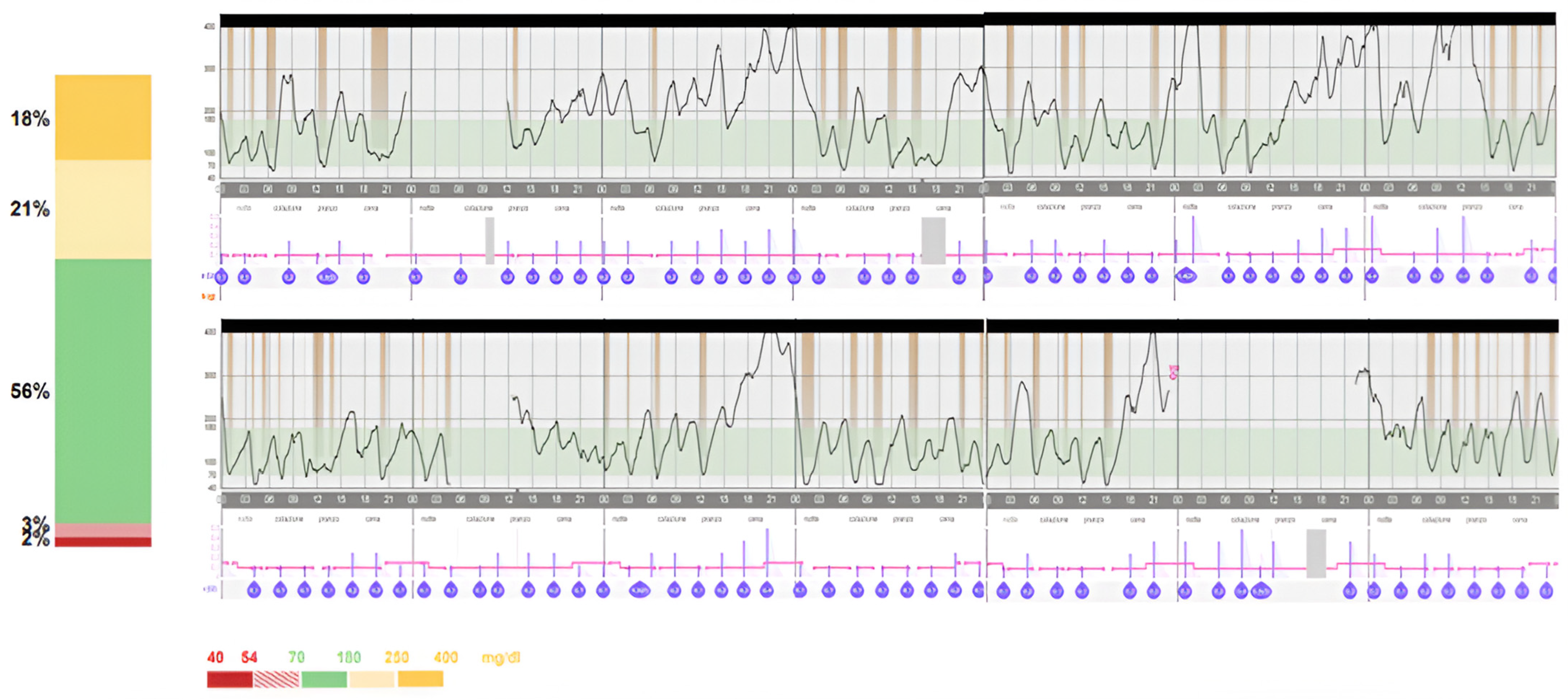
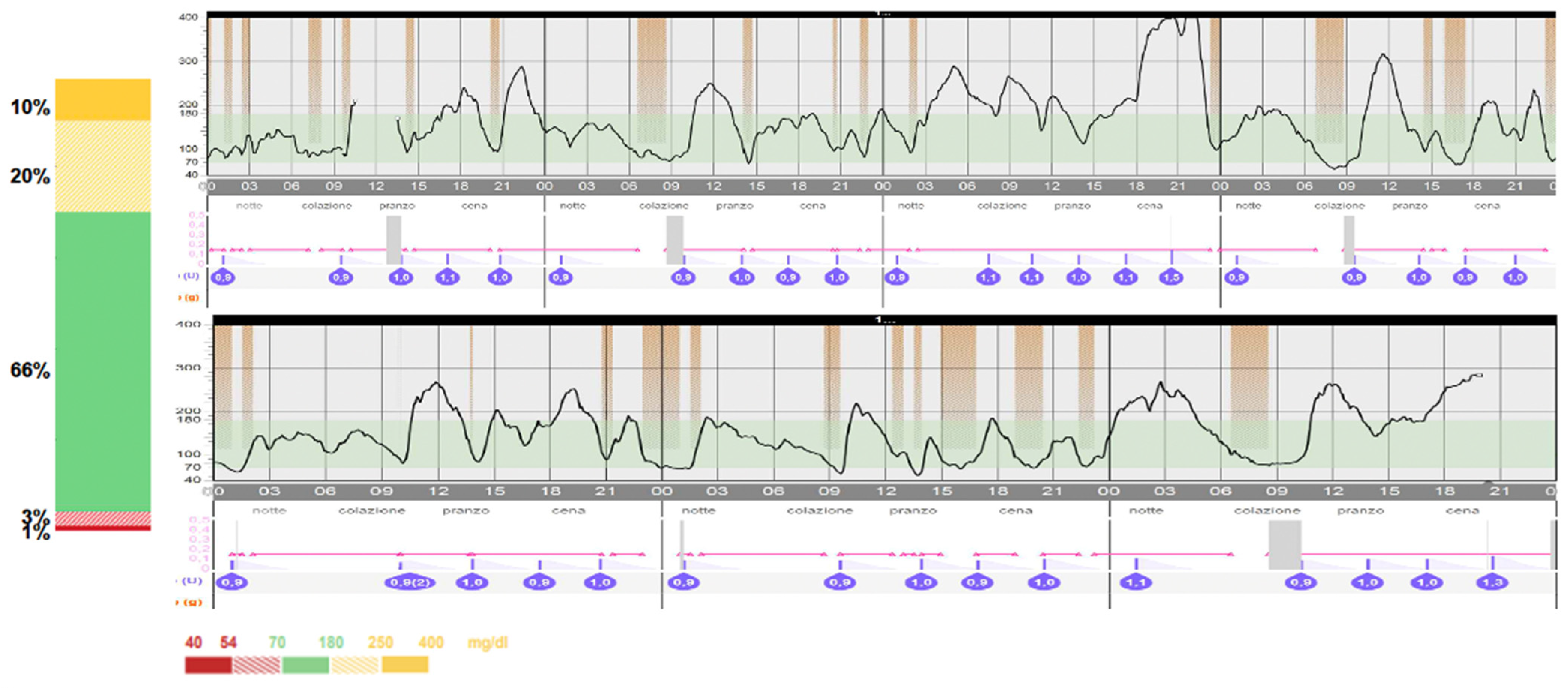
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letourneau, L.R.; Carmody, D.; Wroblewski, K.; Denson, A.M.; Sanyoura, M.; Naylor, R.N.; Philipson, L.H.; Greeley, S.A.W. Diabetes Presentation in Infancy: High Risk of Diabetic Ketoacidosis. Diabetes Care 2017, 40, e147–e148. [Google Scholar] [CrossRef] [PubMed]
- Grulich-Henn, J.; Wagner, V.; Thon, A.; Schober, E.; Marg, W.; Kapellen, T.M.; Haberland, H.; Raile, K.; Ellard, S.; Flanagan, S.E.; et al. Entities and frequency of neonatal diabetes: Data from the diabetes documentation and quality management system (DPV). Diabet. Med. 2010, 27, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Cavé, H. Neonatal diabetes mellitus: A disease linked to multiple mechanisms. Orphanet J. Rare Dis. 2007, 2, 12. [Google Scholar] [CrossRef]
- Aguilar-Bryan, L.; Bryan, J. Neonatal Diabetes Mellitus. Endocr. Rev. 2008, 29, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Panza, R.; Schettini, F.; Piccinno, E.; Laforgia, N. Safety and effectiveness of Medtronic MiniMed™ 780G in a neonate with transient neonatal diabetes mellitus: A case report. Acta Diabetol. 2024, 61, 529–532. [Google Scholar] [CrossRef]
- Kumarasiri, I.M.; Hoole, T.J.; Nimanthi, M.W.A.; Jayasundara, I.; Balasubramaniam, R.; Atapattu, N. Clinical and Genetic Characteristics and Outcome in Patients with Neonatal Diabetes Mellitus from a Low Middle-Income Country. J. Clin. Res. Pediatr. Endocrinol. 2024. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Codner, E.; Craig, M.E.; Huynh, T.; Maahs, D.M.; Mahmud, F.H.; Marcovecchio, L.; DiMeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr. Diabetes 2022, 23, 1270–1276. [Google Scholar] [CrossRef]
- Shaw-Smith, C.; De Franco, E.; Allen, H.L.; Batlle, M.; Flanagan, S.E.; Borowiec, M.; Taplin, C.E.; Velden, J.v.A.-V.d.; Cruz-Rojo, J.; de Nanclares, G.P.; et al. GATA4 Mutations Are a Cause of Neonatal and Childhood-Onset Diabetes. Diabetes 2014, 63, 2888–2894. [Google Scholar] [CrossRef]
- Castañeda, J.; Arrieta, A.; Heuvel, T.v.D.; Battelino, T.; Cohen, O. Time in Tight Glucose Range in Type 1 Diabetes: Predictive Factors and Achievable Targets in Real-World Users of the MiniMed 780G System. Diabetes Care 2024, 47, 790–797. [Google Scholar] [CrossRef]
- Bahillo-Curieses, P.; Velasco, P.F.; Pérez-López, P.; Martínez, A.M.V.; Marca, M.d.l.O.N.d.l.; Díaz-Soto, G. Utility of time in tight range (TITR) in evaluating metabolic control in pediatric and adult patients with type 1 diabetes in treatment with advanced hybrid closed-loop systems. Endocrine 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sako, S.; Tsunogai, T.; Oishi, K. Thiamine-Responsive Megaloblastic Anemia Syndrome. 2003 Oct 24 [updated 2022 Jul 28]. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2024. [Google Scholar] [PubMed]
- Zmysłowska, A.; Młynarski, W. Megaloblastic anaemia in infancy or early childhood and diabetes as leading symptoms of the TRMA syndrome. Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 37–39. [Google Scholar] [PubMed]
- Lu, H.; Vaucher, J.; Tran, C.; Vollenweider, P.; Castioni, J. L’anémie mégaloblastique thiamine dépendante ou syndrome de Rogers: Une revue de la littérature. La Rev. Médecine Interne 2019, 40, 20–27. [Google Scholar] [CrossRef]
- Pulkkinen, M.-A.; Varimo, T.J.; Hakonen, E.T.; Harsunen, M.H.; Hyvönen, M.E.; Janér, J.N.; Kiiveri, S.M.; Laakkonen, H.M.; Laakso, S.M.; Wehkalampi, K.; et al. MiniMed 780G™ in 2- to 6-Year-Old Children: Safety and Clinical Outcomes After the First 12 Weeks. Diabetes Technol. Ther. 2023, 25, 100–107. [Google Scholar] [CrossRef]
- Varimo, T.; Pulkkinen, M.; Hakonen, E.; Hero, M.; Miettinen, P.J.; Tuomaala, A. First year on commercial hybrid closed-loop system—Experience on 111 children and adolescents with type 1 diabetes. Pediatr. Diabetes 2021, 22, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ekhlaspour, L.; Schoelwer, M.J.; Forlenza, G.P.; DeBoer, M.D.; Norlander, L.; Hsu, L.; Kingman, R.; Boranian, E.; Berget, C.; Emory, E.; et al. Safety and Performance of the Tandem t:slim X2 with Control-IQ Automated Insulin Delivery System in Toddlers and Preschoolers. Diabetes Technol. Ther. 2021, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, R.; Iafusco, D.; Rabbone, I.; Diedenhofen, G.; Bizzarri, C.; Patera, P.I.; Reinstadler, P.; Costantino, F.; Calcaterra, V.; Iughetti, L.; et al. Differences between transient neonatal diabetes mellitus subtypes can guide diagnosis and therapy. Eur. J. Endocrinol. 2021, 184, 575–585. [Google Scholar] [CrossRef]
- Karges, B.; Meissner, T.; Icks, A.; Kapellen, T.; Holl, R.W. Management of diabetes mellitus in infants. Nat. Rev. Endocrinol. 2011, 8, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, F.; Debeaufort, C.; Krogvold, L.; Patton, S.; Piloya, T.; Smart, C.; Van Name, M.; Weissberg-Benchell, J.; Silva, J.; Dimeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Managing diabetes in preschoolers. Pediatr. Diabetes 2022, 23, 1496–1511. [Google Scholar] [CrossRef]
- Greeley, S.A.W.; Polak, M.; Njølstad, P.R.; Barbetti, F.; Williams, R.; Castano, L.; Raile, K.; Chi, D.V.; Habeb, A.; Hattersley, A.T.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1188–1211. [Google Scholar] [CrossRef]
- Beltrand, J.; Baptiste, A.; Busiah, K.; Bouazza, N.; Godot, C.; Boucheron, A.; Djerada, Z.; Gozalo, C.; Berdugo, M.; Tréluyer, J.; et al. Glibenclamide oral suspension: Suitable and effective in patients with neonatal diabetes. Pediatr. Diabetes 2019, 20, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bowman, P.; Sulen, Å.; Barbetti, F.; Beltrand, J.; Svalastoga, P.; Codner, E.; Tessmann, E.H.; Juliusson, P.B.; Skrivarhaug, T.; Pearson, E.R.; et al. Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: An international cohort study. Lancet Diabetes Endocrinol. 2018, 6, 637–646, Erratum in: Lancet Diabetes Endocrinol. 2018, 6, e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, M.Y.; Gloyn, A.L.; Maahs, D.M.; Prahalad, P. Management of Neonatal Diabetes due to a KCNJ11 Mutation with Automated Insulin Delivery System and Remote Patient Monitoring. Case Rep. Endocrinol. 2023, 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rabbone, I.; Barbetti, F.; Gentilella, R.; Mossetto, G.; Bonfanti, R.; Maffeis, C.; Iafusco, D.; Piccinno, E. Insulin therapy in neonatal diabetes mellitus: A review of the literature. Diabetes Res. Clin. Pract. 2017, 129, 126–135. [Google Scholar] [CrossRef]
- London, S.; De Franco, E.; Elias-Assad, G.; Barhoum, M.N.; Felszer, C.; Paniakov, M.; Weiner, S.A.; Tenenbaum-Rakover, Y. Case Report: Neonatal Diabetes Mellitus Caused by a Novel GLIS3 Mutation in Twins. Front. Endocrinol. 2021, 12, 673755. [Google Scholar] [CrossRef]
- Rapini, N.; Martino, M.; Arnaldi, C.; Deodati, A.; Anagnostopoulou, L.; Amodeo, M.E.; Ciampalini, P.; Pampanini, V.; Lorubbio, A.; Tosini, D.; et al. Efficacy and safety of advanced hybrid closed loop systems in children with type 1 diabetes younger than 6 years. Front. Endocrinol. 2024, 15, 1382920. [Google Scholar] [CrossRef]
- Abraham, M.B.; Smith, G.J.; Dart, J.; Davis, E.A.; Jones, T.W. Clinical Outcomes with MiniMedTM 780G Advanced Hybrid Closed-Loop Therapy in 2- to 6-Year-Old Children with Type 1 Diabetes. Diabetes Technol. Ther. 2024, 26, 341–345. [Google Scholar] [CrossRef]
- Tornese, G.; Carletti, C.; Lanzetta, M.A.; Tamaro, G.; Barbi, E.; Faleschini, E. Safety of Real-life Usage of Advanced Hybrid Closed-Loop System MiniMed 780G in Children With Type 1 Diabetes Younger Than 7 Years Old. Diabetes Care 2023, 46, e123–e125. [Google Scholar] [CrossRef]
- Gaudieri, P.A.; Chen, R.; Greer, T.F.; Holmes, C.S. Cognitive Function in Children With Type 1 Diabetes. Diabetes Care 2008, 31, 1892–1897. [Google Scholar] [CrossRef]
- Blasetti, A.; Chiuri, R.M.; Tocco, A.M.; Di Giulio, C.; Mattei, P.A.; Ballone, E.; Chiarelli, F.; Verrotti, A. The Effect of Recurrent Severe Hypoglycemia on Cognitive Performance in Children With Type 1 Diabetes. J. Child Neurol. 2011, 26, 1383–1391. [Google Scholar] [CrossRef]
- Cato, M.A.; Mauras, N.; Mazaika, P.; Kollman, C.; Cheng, P.; Aye, T.; Ambrosino, J.; Beck, R.W.; Ruedy, K.J.; Reiss, A.L.; et al. Longitudinal Evaluation of Cognitive Functioning in Young Children with Type 1 Diabetes over 18 Months. J. Int. Neuropsychol. Soc. 2016, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Aye, T.; Barnea-Goraly, N.; Ambler, C.; Hoang, S.; Schleifer, K.; Park, Y.; Drobny, J.; Wilson, D.M.; Reiss, A.L.; Buckingham, B.A. White Matter Structural Differences in Young Children With Type 1 Diabetes: A Diffusion Tensor Imaging Study. Diabetes Care 2012, 35, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Barnea-Goraly, N.; Raman, M.; Mazaika, P.; Marzelli, M.; Hershey, T.; Weinzimer, S.A.; Aye, T.; Buckingham, B.; Mauras, N.; White, N.H.; et al. Alterations in White Matter Structure in Young Children With Type 1 Diabetes. Diabetes Care 2014, 37, 332–340. [Google Scholar] [CrossRef]
- Fox, L.A.; Hershey, T.; Mauras, N.; Arbeláez, A.M.; Tamborlane, W.V.; Buckingham, B.; Tsalikian, E.; Englert, K.; Raman, M.; Jo, B.; et al. Persistence of abnormalities in white matter in children with type 1 diabetes. Diabetologia 2018, 61, 1538–1547. [Google Scholar] [CrossRef]
- Cameron, F.J.; Northam, E.A.; Ryan, C.M. The effect of type 1 diabetes on the developing brain. Lancet Child Adolesc. Health 2019, 3, 427–436. [Google Scholar] [CrossRef]
- Mauras, N.; Buckingham, B.; White, N.H.; Tsalikian, E.; Weinzimer, S.A.; Jo, B.; Cato, A.; Fox, L.A.; Aye, T.; Arbelaez, A.M.; et al. Impact of Type 1 Diabetes in the Developing Brain in Children: A Longitudinal Study. Diabetes Care 2021, 44, 983–992. [Google Scholar] [CrossRef]
- Jaser, S.S.; Jordan, L.C. Brain Health in Children with Type 1 Diabetes: Risk and Protective Factors. Curr. Diabetes Rep. 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L. 6. Glycemic Targets: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S97–S110. [Google Scholar] [CrossRef]
- Olinder, A.L.; DeAbreu, M.; Greene, S.; Haugstvedt, A.; Lange, K.; Majaliwa, E.S.; Pais, V.; Pelicand, J.; Town, M.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes education in children and adolescents. Pediatr. Diabetes 2022, 23, 1229–1242. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzotta, F.; Sarale, N.; Spacco, G.; Tantari, G.; Bertelli, E.; Bracciolini, G.; Secco, A.; d’Annunzio, G.; Maghnie, M.; Minuto, N.; et al. Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future. Children 2024, 11, 1225. https://doi.org/10.3390/children11101225
Pezzotta F, Sarale N, Spacco G, Tantari G, Bertelli E, Bracciolini G, Secco A, d’Annunzio G, Maghnie M, Minuto N, et al. Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future. Children. 2024; 11(10):1225. https://doi.org/10.3390/children11101225
Chicago/Turabian StylePezzotta, Federico, Nicola Sarale, Giordano Spacco, Giacomo Tantari, Enrica Bertelli, Giulia Bracciolini, Andrea Secco, Giuseppe d’Annunzio, Mohamad Maghnie, Nicola Minuto, and et al. 2024. "Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future" Children 11, no. 10: 1225. https://doi.org/10.3390/children11101225
APA StylePezzotta, F., Sarale, N., Spacco, G., Tantari, G., Bertelli, E., Bracciolini, G., Secco, A., d’Annunzio, G., Maghnie, M., Minuto, N., & Bassi, M. (2024). Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future. Children, 11(10), 1225. https://doi.org/10.3390/children11101225





