Socioeconomic and Health Determinants of the Prevalence of COVID-19 in a Population of Children with Respiratory Diseases and Symptoms
Abstract
1. Introduction
- 1.
- Are asthma, bronchitis, or respiratory symptoms and socioeconomic factors risk factors for COVID-19?
- 2.
- Do selected socioeconomic factors differentiate the prevalence of COVID-19 among children with respiratory diseases and symptoms?
2. Materials and Methods
2.1. Study Group Attributes
2.2. Eligibility Criteria
2.3. Research Tool
- The education of the child’s father and mother, defined as low (in the case of primary or vocational education), middle (in the case of secondary education), or high (in the case of higher education);
- Self-assessment of the material situation (good, average, or bad);
- Whether the child has siblings (yes or no);
- The existence of indications of mold and dampness within the dwelling over the preceding 24 months (current, present but mitigated, or never present).
2.4. Statistical Analyses
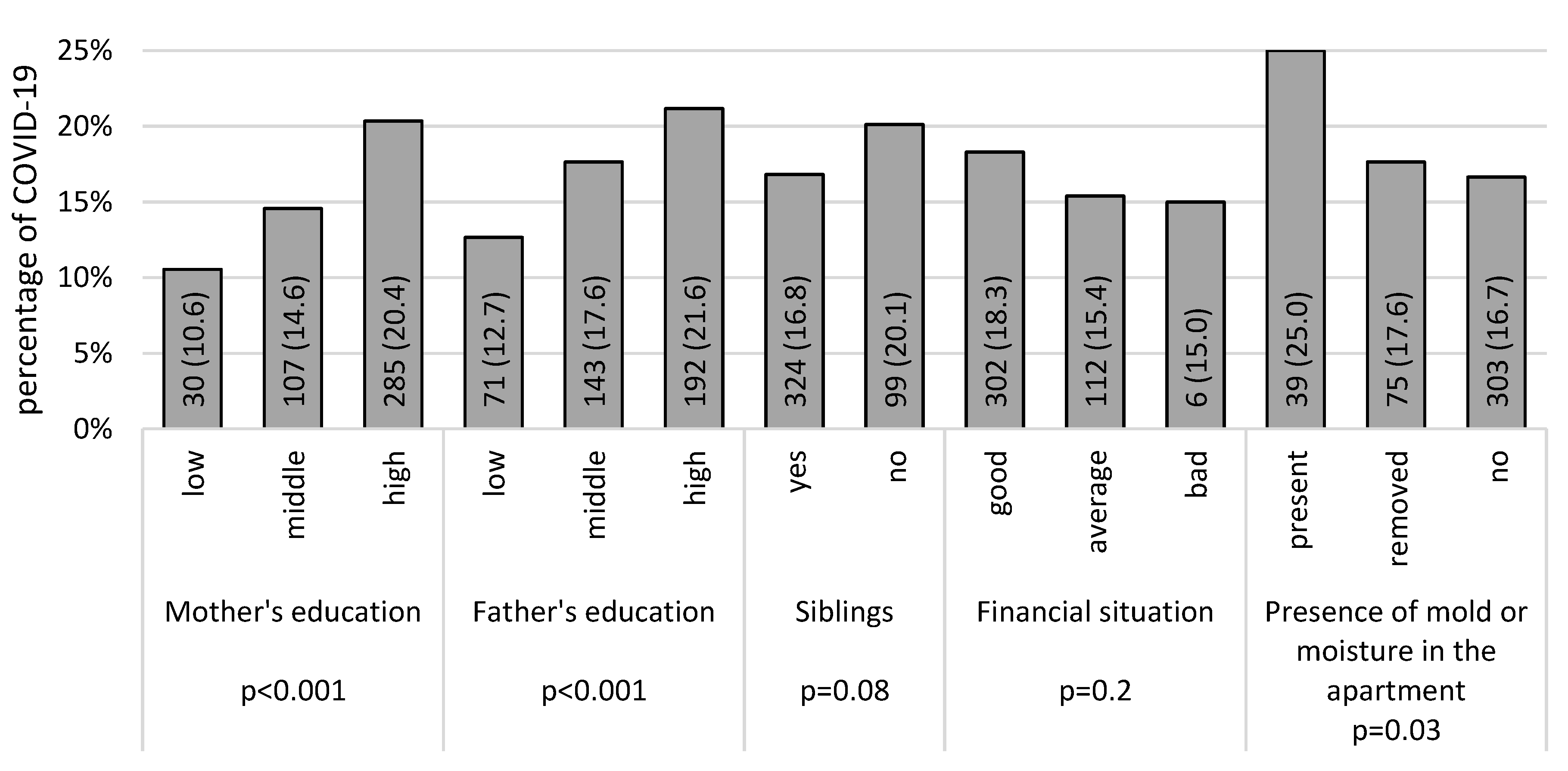
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodman Berlot, J.; Aldeco, M.; Lepej, D.; Praprotnik, M.; Šetina Šmid, S.; Zver, A.; Krivec, U. Better COVID-19 Outcomes in Children with Good Asthma Control. Appl. Microbiol. 2023, 3, 1204–1213. [Google Scholar] [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Badenes-Marques, G.; Gil-Fortuño, M.; Orrico-Sánchez, A.; Pac-Sa, M.R.; Perez-Olaso, O.; Sala-Trull, D.; Sánchez-Urbano, M.; Arnedo-Pena, A. Long COVID Prevalence and the Impact of the Third SARS-CoV-2 Vaccine Dose: A Cross-Sectional Analysis from the Third Follow-Up of the Borriana Cohort, Valencia, Spain (2020–2022). Vaccines 2023, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Forno, E. Asthma and COVID-19 in children: A systematic review and call for data. Pediatr. Pulmonol. 2020, 55, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Kalayci, O.; Sediva, A.; Untersmayr, E.; Munblit, D.; del Rio, P.R.; Vazquez-Ortiz, M.; Arasi, S.; Alvaro-Lozano, M.; Tsabouri, S.; et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics—The 2020 COVID-19 pandemic. Pediatr. Allergy Immunol. 2020, 5, 442–448. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Faner, R.; Sibila, O.; Badia, J.R.; Agusti, A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir. Med. 2020, 8, 436–438. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. BMJ 2020, 22, m1985. [Google Scholar] [CrossRef]
- Jackowska, T.; Kuchar, E.; Okarska-Napierała, M. COVID-19 in children. Almanac 2021, 16, 48–55. Available online: http://www.urpl.gov.pl/sites/default/files/zalaczniki/Almanach_Vol.%2016_n4_str.%2048-55.pdf (accessed on 14 September 2023).
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020, 18, 1775–1776. [Google Scholar] [CrossRef]
- Beurnier, A.; Jutant, E.-M.; Jevnikar, M.; Boucly, A.; Pichon, J.; Preda, M.; Frank, M.; Laurent, J.; Richard, C.; Monnet, X.; et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur. Respir. J. 2020, 56, 2001875. [Google Scholar] [CrossRef]
- Grandbastien, M.; Piotin, A.; Godet, J.; Abessolo-Amougou, I.; Ederlé, C.; Enache, I.; Fraisse, P.; Hoang, T.C.T.; Kassegne, L.; Labani, A.; et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J. Allergy Clin. Immunol. Pract. 2020, 8, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Sharron, M.P.; Wai, K.; Pillai, D.K.; Rastogi, D. Asthma as a comorbidity in COVID-19 pediatric ICU admissions in a large metropolitan children’s hospital. Pediatr. Pulmonol. 2023, 1, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Pan, J.; Katikireddi, S.V.; McCowan, C.; Kerr, S.; Agrawal, U.; Shah, S.A.; Simpson, C.R.; Ritchie, L.D.; Robertson, C.; et al. Risk of COVID-19 hospital admission among children aged 5-17 years with asthma in Scotland: A national incident cohort study. Lancet Respir. Med. 2022, 2, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.; Smith, C.; Silveira, L.; Jung, S.; Curran-Hays, S.; Jarjour, J.; Carpenter, L.B.; Pickard, K.B.; Mattiucci, M.; Fresia, J.B.; et al. Risk factors for severe COVID-19 in children. Pediatr. Infect. Dis. J. 2021, 4, e137–e145. [Google Scholar] [CrossRef] [PubMed]
- García, C.N. Socioeconomic, demographic and healthcare determinants of the COVID-19 pandemic: An ecological study of Spain. BMC Public Health 2021, 1, 606. [Google Scholar] [CrossRef]
- Ngepah, N. Socio-economic determinants of global COVID-19 mortalities: Policy lessons for current and future pandemics. Health Policy Plan. 2021, 4, 418–434. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, X.; Shi, W. Impacts of social and economic factors on the transmission of coronavirus disease 2019 (COVID-19) in China. J. Popul. Econ. 2020, 4, 1127–1172. [Google Scholar] [CrossRef]
- Azarpazhooh, M.R.; Morovatdar, N.; Avan, A.; Phan, T.G.; Divani, A.A.; Yassi, N.; Stranges, S.; Silver, B.; Biller, J.; Belasi, M.T.; et al. COVID-19 pandemic and burden of non-communicable diseases: An ecological study on data of 185 countries. J. Stroke Cerebrovasc. Dis. 2020, 9, 105089. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Warwick, A.N.; Hysi, P.G.; Kastner, A.; Dick, A.; Khaw, P.T.; Tufail, A.; Foster, P.J.; Khaw, K.T. Associations with COVID-19 hospitalisation among 406,793 adults: The UK Biobank prospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Novak, N.; Cabanillas, B. Viruses and asthma: The role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology 2020, 2, 83–93. [Google Scholar] [CrossRef]
- Morrison, C.B.; Edwards, C.E.; Shaffer, K.M.; Araba, K.C.; Wykoff, J.A.; Williams, D.R.; Asakura, T.; Dang, H.; Morton, L.C.; Gilmore, R.C.; et al. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc. Natl. Acad. Sci. USA 2022, 16, e2119680119. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; McGarvey, L.; Song, W.-J.; Chang, A.B.; Lai, K.; Canning, B.J.; Birring, S.S.; Smith, J.A.; Mazzone, S.B. Cough hypersensitivity and chronic cough. Nat. Rev. Dis. Primers 2022, 1, 45. [Google Scholar] [CrossRef] [PubMed]
- O’grady, K.-A.F.; Drescher, B.J.; Goyal, V.; Phillips, N.; Acworth, J.; Marchant, J.M.; Chang, A.B. Chronic cough postacute respiratory illness in children: A cohort study. Arch. Dis. Child. 2017, 102, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Mania, A.; Pokorska-Śpiewak, M.; Figlerowicz, M.; Pawłowska, M.; Mazur-Melewska, K.; Faltin, K.; Talarek, E.; Zawadka, K.; Dobrzeniecka, A.; Ciechanowski, P.; et al. Pneumonia, gastrointestinal symptoms, comorbidities, and coinfections as factors related to a lengthier hospital stay in children with COVID-19-analysis of a paediatric part of Polish register SARSTer. Infect. Dis. 2022, 3, 196–204. [Google Scholar] [CrossRef]
- Mania, A.; Faltin, K.; Mazur-Melewska, K.; Małecki, P.; Jończyk-Potoczna, K.; Lubarski, K.; Lewandowska, Z.; Cwalińska, A.; Rosada-Kurasińska, J.; Bartkowska-Śniatkowska, A.; et al. Clinical Picture and Risk Factors of Severe Respiratory Symptoms in COVID-19 in Children. Viruses 2021, 12, 2366. [Google Scholar] [CrossRef]
- Morante-García, W.; Zapata-Boluda, R.M.; García-González, J.; Campuzano-Cuadrado, P.; Calvillo, C.; Alarcón-Rodríguez, R. Influence of social determinants of health on COVID-19 infection in socially vulnerable groups. Int. J. Environ. Res. Public Health 2022, 3, 1294. [Google Scholar] [CrossRef] [PubMed]
- Baena-Díez, J.M.; Barroso, M.; Cordeiro-Coelho, S.I.; Díaz, J.L.; Grau, M. Impact of COVID-19 outbreak by income: Hitting hardest the most deprived. J. Public Health 2020, 42, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Corburn, J.; Vlahov, D.; Mberu, B.; Riley, L.; Caiaffa, W.T.; Rashid, S.F.; Ko, A.; Patel, S.; Jukur, S.; Martínez-Herrera, E.; et al. Slum Health: Arresting COVID-19 and improving well-being in urban informal settlements. J. Urban Health 2020, 97, 348–357. [Google Scholar] [CrossRef]
- Gibson, L.; Rush, D. Novel Coronavirus in Cape Town Informal Settlements: Feasibility of Using Informal Dwelling Outlines to Identify High Risk Areas for COVID-19 Transmission from A Social Distancing Perspective. JMIR Public Health Surveill. 2020, 6, e18844. [Google Scholar] [CrossRef]
- Quaife, M.; van Zandvoort, K.; Gimma, A.; Shah, K.; McCreesh, N.; Prem, K.; Barasa, E.; Mwanga, D.; Kangwana, B.; Pinchoff, J.; et al. The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med. 2020, 18, 316. [Google Scholar] [CrossRef]
- Wojczyk, M.; Niewiadomska, E.; Kowalska, M. The Incidence Proportion of SARS-CoV-2 Infections and the Percentage of Deaths among Infected Healthcare Workers in Poland. J. Clin. Med. 2023, 12, 3714. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Asaba, K. Educational attainment decreases the risk of COVID-19 severity in the European Population: A two-sample mendelian randomization study. Front. Public Health 2021, 9, 673451. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; O’donnell, C.A.; Jani, B.D.; Demou, E.; Ho, F.K.; Celis-Morales, C.; Nicholl, B.I.; Mair, F.S.; Welsh, P.; Sattar, N.; et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: Prospective cohort study using UK Biobank. BMC Med. 2020, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, P.; Miani, A.; Setti, L.; De Gennaro, G.; Rodo, X.; Artinano, B.; Vara, E.; Rancan, L.; Arias, J.; Passarini, F.; et al. The role of outdoor and indoor air quality in the spread of SARS-CoV-2: Overview and recommendations by the research group on COVID-19 and particulate matter (RESCOP commission). Environ. Res. 2022, 211, 113038. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef] [PubMed]
- Wypych-Ślusarska, A.; Krupa-Kotara, K.; Niewiadomska, E. Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children? Int. J. Environ. Res. Public Health 2022, 19, 15366. [Google Scholar] [CrossRef] [PubMed]
- De Linares, C.; Navarro, D.; Puigdemunt, R.; Belmonte, J. Aspergillus Conidia and Allergens in Outdoor Environment: A Health Hazard? J. Fungi 2023, 9, 624. [Google Scholar] [CrossRef]
- Wypych-Ślusarska, A.; Grot, M.; Kujawińska, M.; Nigowski, M.; Krupa-Kotara, K.; Oleksiuk, K.; Głogowska-Ligus, J.; Grajek, M. Respiratory Symptoms, Allergies, and Environmental Exposures in Children with and without Asthma. Int. J. Environ. Res. Public Health 2022, 19, 11180. [Google Scholar] [CrossRef]
- Reed-Thryselius, S.; Fuss, L.; Rausch, D. The relationships between socioeconomic status, COVID-19 risk perceptions, and the adoption of protective measures in a mid-western city in the United States. J. Community Health 2022, 3, 464–474. [Google Scholar] [CrossRef]

| Disease/Respiratory Symptom | Total | Habitation | p-Value | Sex | p-Value | Age | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Town n = 1617 | Village n = 837 | Boy n = 1299 | Girl n = 1155 | 5–8 n = 637 | 9–12 n = 1237 | 13–15 n = 563 | |||||
| Chronic cough | 272 (11.1) | 193 (11.9) | 79 (9.5) | 0.06 | 167 (12.9) | 105 (9.1) | 0.002 | 88 (13.8) | 129 (10.4) | 51 (9.1) | 0.02 |
| Waking up at night in the last 12 months due to cough | 363 (14.8) | 236 (14.6) | 127 (15.2) | 0.7 | 202 (15.6) | 161 (19.5) | 0.2 | 114 (17.9) | 187 (15.1) | 59 (7.5) | 0.001 |
| Wheezing ever | 635 (26.0) | 421 (26.2) | 214 (25.7) | 0.8 | 389 (30.1) | 246 (21.4) | <0.001 | 168 (26.5) | 342 (27.8) | 115 (20.6) | 0.004 |
| Wheezing in the last 12 months | 246 (10.1) | 162 (10.1) | 84 (10.1) | 0.9 | 148 (11.5) | 98 (8.5) | 0.01 | 89 (14.1) | 116 (9.5) | 37 (6.6) | <0.001 |
| Waking up at night due to wheezing | 157 (6.4) | 107 (6.6) | 50 (6.0) | 0.5 | 87 (6.7) | 70 (6.1) | 0.5 | 64 (10.1) | 65 (5.3) | 24 (4.3) | <0.001 |
| Dyspnea ever | 368 (15.0) | 248 (15.4) | 120 (14.3) | 0.5 | 227 (17.5) | 141 (12.2) | <0.001 | 91 (14.3) | 191 (15.5) | 83 (14.8) | 0.7 |
| Dyspnea in the last 12 months | 133 (5.4) | 94 (5.8) | 39 (4.7) | 0.2 | 80 (6.2) | 53 (4.6) | 0.08 | 43 (6.8) | 64 (5.2) | 23 (4.1) | 0.1 |
| Bronchitis | 465 (18.9) | 314 (19.4) | 151 (18.0) | 0.4 | 300 (23.1) | 165 (14.3) | <0.001 | 127 (19.9) | 239 (19.3) | 95 (16.9) | 0.3 |
| Asthma | 372 (15.2) | 250 (15.5) | 122 (14.7) | 0.6 | 229 (17.7) | 143 (12.5) | <0.001 | 94 (14.8) | 192 (15.6) | 84 (15.0) | 0.8 |
| COVID-19 | 423 (17.4) | 316 (19.7) | 107 (12.9) | <0.001 | 227 (17.7) | 196 (17.1) | 0.7 | 98 (15.6) | 223 (18.2) | 100 (18.0) | 0.3 |
| Determinants of COVID-19 (* Reference Group) | OR (95%CI) Crude | p-Value | OR (95%CI) Adjusted by Sex and Age | p-Value |
|---|---|---|---|---|
| Place of residence | ||||
| Village/city * | 1.7 (1.3–2.1) | <0.001 | 1.7 (1.3–2.1) | <0.001 |
| Mother’s education | ||||
| Low */middle | 1.4 (0.8–2.1) | 0.09 | 1.5 (1.0–2.3) | 0.06 |
| Middle */high | 1.5 (1.1–1.9) | <0.001 | 1.5 (1.2–1.9) | 0.001 |
| Low */high | 2.2 (1.3–3.0) | <0.001 | 2.3 (1.6–3.5) | <0.001 |
| Father’s education | ||||
| Low */middle | 1.5 (1.0–1.9) | 0.01 | 1.5 (1.1–2.0) | 0.01 |
| Middle */high | 1.3 (1.0–1.6) | 0.04 | 1.3 (1.0–1.7) | 0.03 |
| Low */high | 1.9 (1.3–2.4) | <0.001 | 1.9 (1.4–2.6) | <0.001 |
| Presence of mold or moisture in the apartment | ||||
| None */removed | 1.1 (0.8–1.4) | 0.6 | 1.0 (0.8–1.4) | 0.7 |
| Removed */present | 1.6 (0.9–2.2) | 0.05 | 1.6 (1.0–2.5) | 0.02 |
| None */present | 1.7 (1.0–2.3) | 0.008 | 1.7 (1.2–2.5) | 0.007 |
| Bronchitis | ||||
| No/yes * | 1.5 (1.2–2.0) | <0.001 | 1.5 (1.2–2.0) | <0.001 |
| Chronic cough | ||||
| No/yes * | 1.8 (1.3–2.4) | <0.001 | 1.8 (1.4–2.4) | <0.001 |
| Asthma | ||||
| No/yes * | 1.3 (0.9–1.6) | 0.1 | 1.2 (0.9–1.6) | 0.1 |
| Disease/ Respiratory Symptom | Yes | No | |||||
|---|---|---|---|---|---|---|---|
| Asthma | COVID-19 Yes | COVID-19 No | p-Value | COVID-19 Yes | COVID-19 No | p-Value | |
| Mother’s education | Low | 5 (12.2) | 36 (87.8) | 0.8 | 24 (10.1) | 214 (89.9) | <0.001 |
| Middle | 18 (16.1) | 95 (83.9) | 89 (14.3) | 531 (85.7) | |||
| High | 51 (24.2) | 160 (75.8) | 233 (19.7) | 951 (80.3) | |||
| Father’s education | Low | 13 (15.1) | 73 (84.9) | 0.2 | 57 (12.1) | 412 (87.9) | <0.001 |
| Middle | 27 (19.6) | 111 (80.4) | 116 (17.3) | 553 (82.7) | |||
| High | 32 (25.4) | 94 (74.6) | 164 (20.9) | 619 (79.1) | |||
| Presence of mold or moisture | Current | 8 (27.6) | 21 (12.4) | 0.6 | 31 (24.4) | 96 (75.6) | 0.05 |
| Removed | 12 (18.2) | 54 (81.8) | 62 (17.5) | 292 (82.5) | |||
| No | 55 (20.4) | 215 (79.6) | 247 (16.0) | 1295 (84.0) | |||
| Place of residence | Village | 107 (12.9) | 720 (87.1) | <0.001 | 88 (12.5) | 613 (87.5) | <0.001 |
| City | 316 (19.7) | 1283 (80.3) | 258 (19.2) | 1087 (18.8) | |||
| Chronic cough | COVID-19 Yes | COVID-19 No | p-Value | COVID-19 Yes | COVID-19 No | p-Value | |
| Mother’s education | Low | 9 (16.4) | 46 (83.6) | 0.2 | 21 (9.2) | 208 (90.8) | <0.001 |
| Middle | 22 (26.2) | 62 (73.8) | 85 (13.1) | 563 (86.9) | |||
| High | 38 (29.7) | 90 (70.3) | 246 (19.4) | 1024 (80.6) | |||
| Father’s education | Low | 16 (21.0) | 60 (78.9) | 0.2 | 55 (11.3) | 430 (88.7) | <0.001 |
| Middle | 26 (26.5) | 72 (73.5) | 116 (16.3) | 594 (83.7) | |||
| High | 26 (33.3) | 52 (66.7) | 171 (20.5) | 661 (79.5) | |||
| Presence of mold or moisture | Current | 10 (37.0) | 17 (63.0) | 0.3 | 29 (22.5) | 100 (77.5) | 0.1 |
| Removed | 12 (24.0) | 38 (76.0) | 63 (16.8) | 312 (83.2) | |||
| No | 44 (23.5) | 143 (76.5) | 258 (15.9) | 1370 (84.1) | |||
| Place of residence | Village | 12 (15.2) | 67 (84.8) | 0.009 | 94 (12.6) | 652 (87.4) | <0.001 |
| City | 58 (30.5) | 132 (69.5) | 258 (18.3) | 1149 (81.7) | |||
| Bronchitis | COVID-19 Yes | COVID-19 No | p-Value | COVID-19 Yes | COVID-19 No | p-Value | |
| Mother’s education | Low | 9 (16.1) | 47 (83.9) | 0.08 | 21 (9.2) | 207 (90.8) | <0.001 |
| Middle | 25 (18.5) | 110 (81.5) | 82 (13.7) | 517 (86.3) | |||
| High | 70 (26.6) | 193 (73.4) | 215 (18.9) | 922 (81.1) | |||
| Father’s education | Low | 17 (15.6) | 93 (84.5) | 0.2 | 57 (12.0) | 397 (88.0) | 0.001 |
| Middle | 36 (22.2) | 126 (77.8) | 104 (16.5) | 542 (83.5) | |||
| High | 49 (29.9) | 115 (70.1) | 148 (19.8) | 599 (80.2) | |||
| Presence of mold or moisture | Current | 14 (35.9) | 25 (64.1) | 0.1 | 25 (21.4) | 92 (78.6) | 0.2 |
| Removed | 18 (20.9) | 68 (79.1) | 57 (16.8) | 282 (83.2) | |||
| No | 70 (21.5) | 256 (78.5) | 233 (15.6) | 1260 (84.4) | |||
| Place of residence | Village | 27 (18.1) | 122 (82.8) | 0.08 | 80 (11.8) | 598 (88.2) | <0.001 |
| City | 78 (25.3) | 231 (74.7) | 238 (18.4) | 1052 (81.6) | |||
| Determinants of COVID-19 (* Reference Group) | Model 1 Bronchitis OR (95%CI) | p-Value | Model 2 Chronic Cough OR (95%CI) | p-Value | Model 3 Asthma OR (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Place of residence | ||||||
| Village/city * | 1.7 (1.3–2.1) | <0.001 | 1.1 (0.7–1.7) | 0.6 | 2.4 (1.3–4.6) | <0.001 |
| Mother’s education | ||||||
| Low/middle * | 1.4 (0.8–2.1) | 0.2 | 1.4 (0.9–2.5) | 0.1 | 1.4 (0.8–2.4) | 0.2 |
| Middle */high | 1.5 (1.1–1.9) | 0.8 | 1.1 (0.6–2.0) | 0.8 | 1.1 (0.6–2.0) | 0.8 |
| Low */high | 1.7 (0.8–3.7) | 0.1 | 1.8 (0.8–3.9) | 0.1 | 1.7 (0.8–3.7) | 0.2 |
| Father’s education | ||||||
| Low */middle | 1.3 (0.8–2.0) | 0.2 | 1.2 (0.8–1.9) | 0.3 | 1.3 (0.8–2.0) | 0.7 |
| Middle */high | 1.3 (0.9–1.6) | 0.2 | 1.5 (0.8–2.5) | 0.2 | 1.4 (0.8–2.5) | 0.8 |
| Low */high | 1.7 (1.0–2.8) | 0.06 | 1.7 (1.0–2.9) | 0.05 | 1.7 (1.0–2.8) | 0.06 |
| Presence of mold or moisture in the apartment | ||||||
| None */removed | 1.1 (0.7–1.9) | 0.6 | 1.2 (0.7–2.0) | 0.5 | 1.2 (0.7–1.9) | 0.6 |
| Removed */present | 1.6 (0.9–2.2) | 0.1 | 1.5 (0.9–2.6) | 0.1 | 1.5 (0.9–2.6) | 0.1 |
| None */present | 2.8 (1.5–4.9) | <0.001 | 2.8 (1.7–5.0) | <0.001 | 1.8 (1.2–3.7) | <0.001 |
| Disease/symptom | ||||||
| No/yes * | 1.5 (1.2–2.0) | <0.001 | 1.6 (0.9–2.8) | 0.1 | 1.1 (0.6–2.0) | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wypych-Ślusarska, A.; Krupa-Kotara, K.; Oleksiuk, K.; Głogowska-Ligus, J.; Słowiński, J.; Niewiadomska, E. Socioeconomic and Health Determinants of the Prevalence of COVID-19 in a Population of Children with Respiratory Diseases and Symptoms. Children 2024, 11, 88. https://doi.org/10.3390/children11010088
Wypych-Ślusarska A, Krupa-Kotara K, Oleksiuk K, Głogowska-Ligus J, Słowiński J, Niewiadomska E. Socioeconomic and Health Determinants of the Prevalence of COVID-19 in a Population of Children with Respiratory Diseases and Symptoms. Children. 2024; 11(1):88. https://doi.org/10.3390/children11010088
Chicago/Turabian StyleWypych-Ślusarska, Agata, Karolina Krupa-Kotara, Klaudia Oleksiuk, Joanna Głogowska-Ligus, Jerzy Słowiński, and Ewa Niewiadomska. 2024. "Socioeconomic and Health Determinants of the Prevalence of COVID-19 in a Population of Children with Respiratory Diseases and Symptoms" Children 11, no. 1: 88. https://doi.org/10.3390/children11010088
APA StyleWypych-Ślusarska, A., Krupa-Kotara, K., Oleksiuk, K., Głogowska-Ligus, J., Słowiński, J., & Niewiadomska, E. (2024). Socioeconomic and Health Determinants of the Prevalence of COVID-19 in a Population of Children with Respiratory Diseases and Symptoms. Children, 11(1), 88. https://doi.org/10.3390/children11010088








