Trends in the Incidence of Bronchopulmonary Dysplasia after the Introduction of Neurally Adjusted Ventilatory Assist (NAVA)
Abstract
1. Introduction
2. Methods
2.1. Study Design and Groups
2.2. Use of NAVA
2.3. Inclusion and Exclusion Criteria
2.4. Measured Outcomes
3. Statistical Analysis
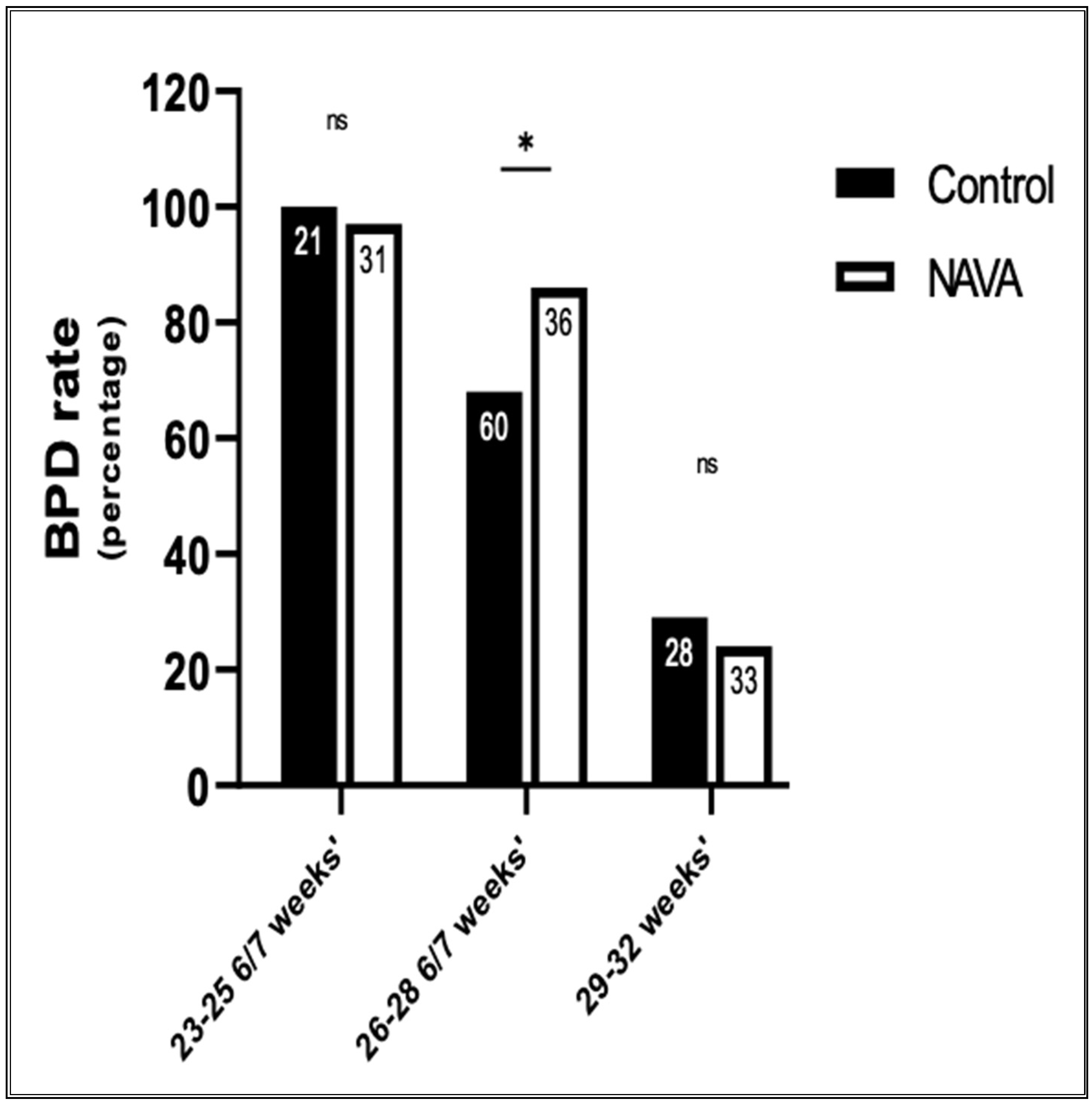
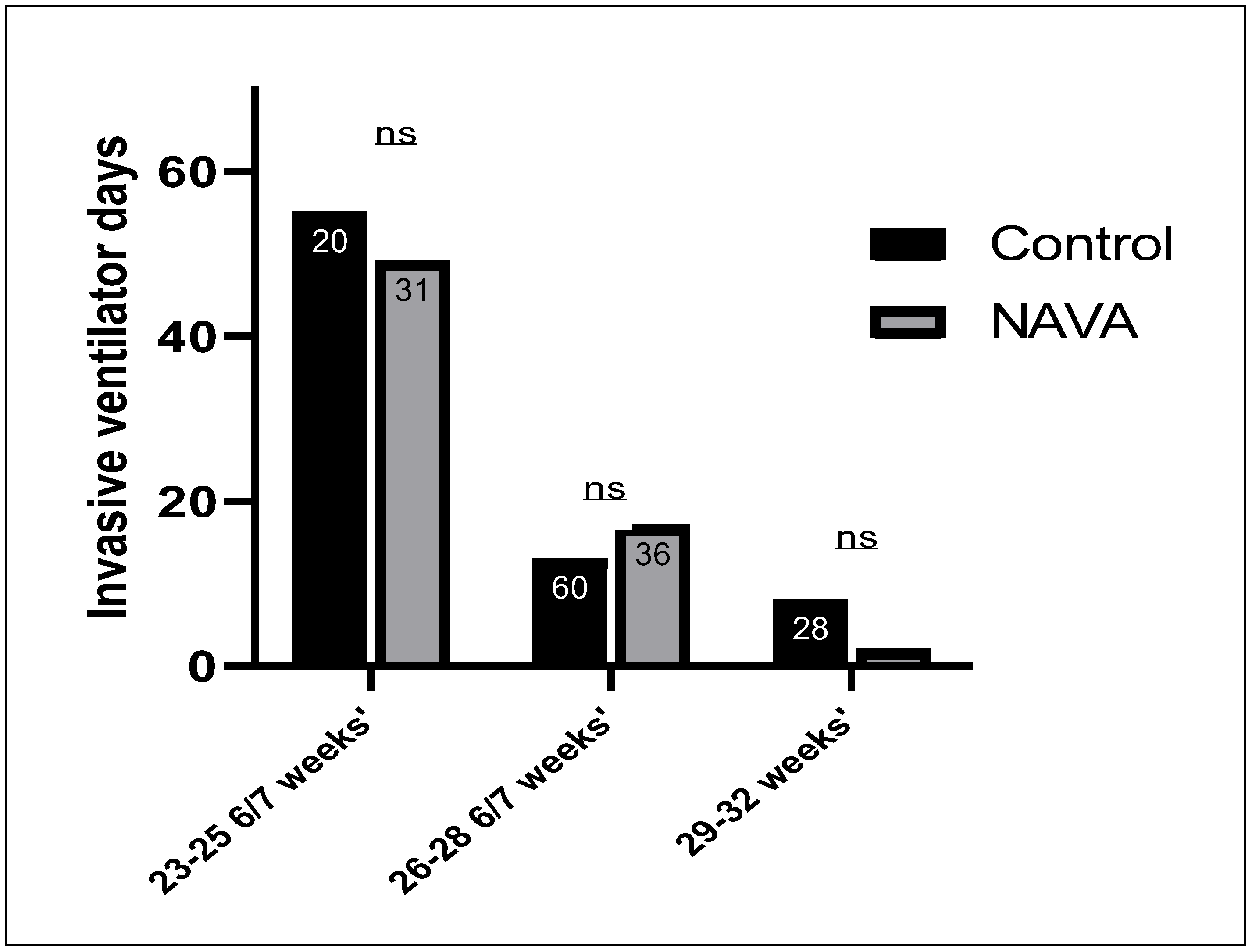
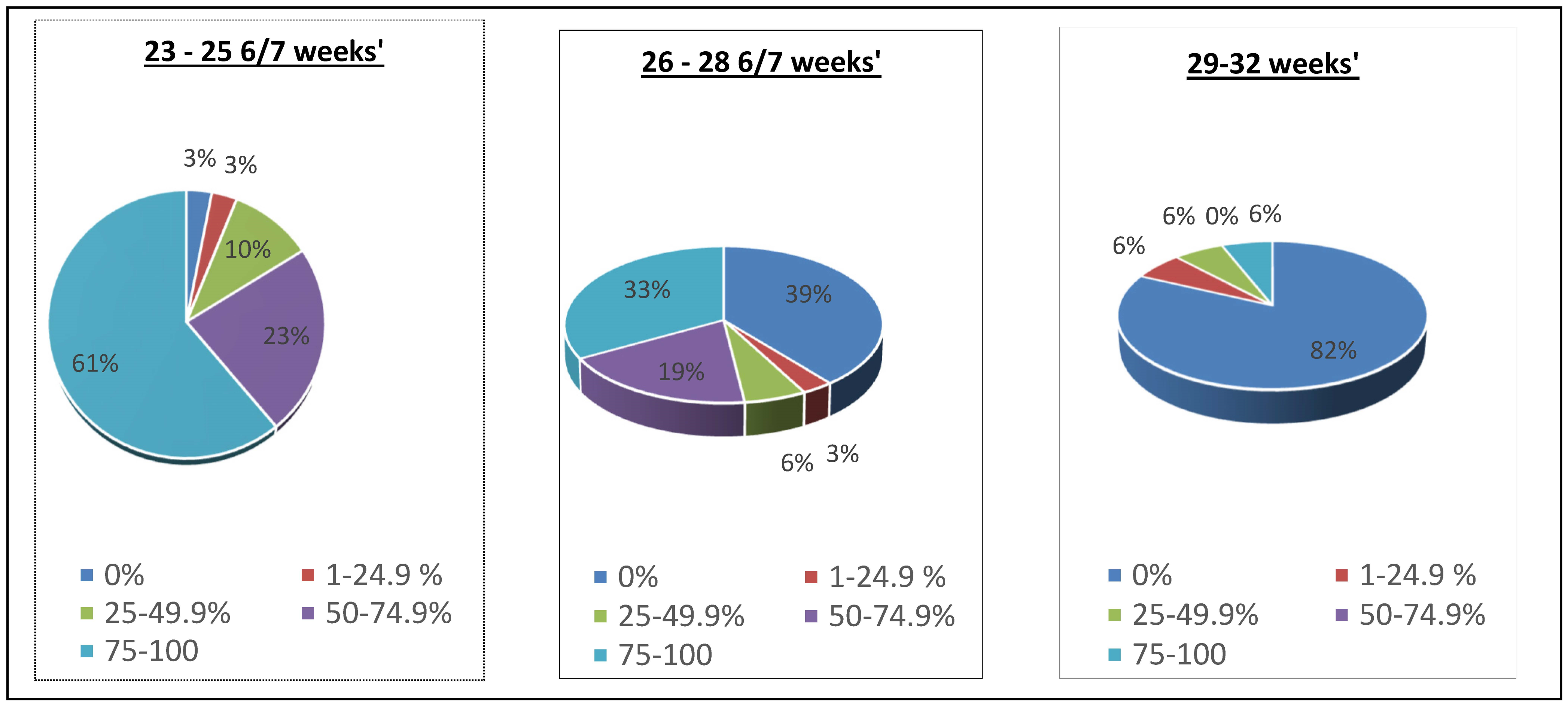
4. Results
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, M.C.; Bell, E.F.; Kandefer, S.; Saha, S.; Carlo, W.A.; D’Angio, C.T.; Laptook, A.R.; Sanchez, P.J.; Stoll, B.J.; Shankaran, S.; et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr. Res. 2017, 82, 297–304. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Ryan, R.M. Bronchopulmonary dysplasia: New becomes old again! Pediatr. Res. 2017, 81, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lapcharoensap, W.; Gage, S.C.; Kan, P.; Profit, J.; Shaw, G.M.; Gould, J.B.; Stevenson, D.K.; O’brodovich, H.; Lee, H.C. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015, 169, e143676. [Google Scholar] [CrossRef]
- Zysman-Colman, Z.; Tremblay, G.M.; Bandeali, S.; Landry, J.S. Bronchopulmonary dysplasia-trends over three decades. Paediatr. Child Health 2013, 18, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Bhandari, V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009, 123, 1562–1573. [Google Scholar] [CrossRef]
- Schmidt, B.; Asztalos, E.V.; Roberts, R.S.; Robertson, C.M.T.; Sauve, R.S.; Whitfield, M.F. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: Results from the trial of indomethacin prophylaxis in preterms. JAMA 2003, 289, 1124–1129. [Google Scholar] [CrossRef]
- Thompson, A.; Bhandari, V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights 2008, 3, 361–373. [Google Scholar] [CrossRef]
- Shah, J.; Jefferies, A.L.; Yoon, E.W.; Lee, S.K.; Shah, P.S. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at <32 Weeks’ Gestation. Am. J. Perinatol. 2015, 32, 675–682. [Google Scholar]
- Bhandari, V.; Gruen, J.R. The genetics of bronchopulmonary dysplasia. Semin. Perinatol. 2006, 30, 185–191. [Google Scholar] [CrossRef]
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H. Animal Models, Learning Lessons to Prevent and Treat Neonatal Chronic Lung Disease. Front. Med. 2015, 2, 49. [Google Scholar] [CrossRef]
- Allison, B.J.; Crossley, K.J.; Flecknoe, S.J.; Davis, P.G.; Morley, C.J.; Harding, R.; Hooper, S.B. Ventilation of the very immature lung in utero induces injury and BPD-like changes in lung structure in fetal sheep. Pediatr. Res. 2008, 64, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S. Ventilation-induced lung injury and mechanotransduction: Stretching it too far? Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L892–L896. [Google Scholar] [CrossRef][Green Version]
- Whitehead, T.; Slutsky, A.S. The pulmonary physician in critical care * 7: Ventilator induced lung injury. Thorax 2002, 57, 635–642. [Google Scholar] [CrossRef]
- Carlton, D.P.; Cummings, J.J.; Scheerer, R.G.; Poulain, F.R.; Bland, R.D.; Adir, Y.; Welch, L.C.; Dumasius, V.; Factor, P.; Sznajder, J.I.; et al. Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J. Appl. Physiol. 1990, 69, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.A.; Peevy, K.J.; Moise, A.A.; Hillman, N.H.; Kemp, M.W.; Noble, P.B.; Kallapur, S.G.; Jobe, A.H.; Yoshikawa, S.; King, J.A.; et al. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J. Appl. Physiol. 1989, 66, 2364–2368. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Jobe, A.H.; Ikegami, M. Lung development and function in preterm infants in the surfactant treatment era. Annu. Rev. Physiol. 2000, 62, 825–846. [Google Scholar] [CrossRef]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Graham, P.J.; Rojas-Reyes, M.X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 2016, 2016, CD000501. [Google Scholar] [CrossRef]
- Beck, J.; Reilly, M.; Grasselli, G.; Mirabella, L.; Slutsky, A.S.; Dunn, M.S.; Sinderby, C. Patient-ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatr. Res. 2009, 65, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Donn, S.M.; Sinha, S.K. Can mechanical ventilation strategies reduce chronic lung disease? Semin. Neonatol. 2003, 8, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.C.; Thurman, T.L.; Holt, S.J.; Heulitt, M.J. Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med. 2011, 37, 1826. [Google Scholar] [CrossRef]
- Rossor, T.E.; Hunt, K.A.; Shetty, S.; Greenough, A. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database Syst. Rev. 2017, 10, CD012251. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Campoccia, F.; Allo, J.-C.; Brander, L.; Brunet, F.; Slutsky, A.S.; Sinderby, C. Improved synchrony and respiratory unloading by neurally adjusted ventilatory assist (NAVA) in lung-injured rabbits. Pediatr. Res. 2007, 61, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Piquilloud, L.; Vignaux, L.; Bialais, E.; Roeseler, J.; Sottiaux, T.; Laterre, P.-F.; Jolliet, P.; Tassaux, D. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med. 2011, 37, 263–271. [Google Scholar] [CrossRef]
- Terzi, N.; Pelieu, I.; Guittet, L.; Ramakers, M.; Seguin, A.; Daubin, C.; Charbonneau, P.; du Cheyron, D.; Lofaso, F. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: Physiological evaluation. Crit. Care Med. 2010, 38, 1830–1837. [Google Scholar] [CrossRef]
- Spahija, J.; de Marchie, M.; Albert, M.; Bellemare, P.; Delisle, S.; Beck, J.; Sinderby, C. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit. Care Med. 2010, 38, 518–526. [Google Scholar] [CrossRef]
- Stein, H.; Firestone, K. Application of neurally adjusted ventilatory assist in neonates. Semin. Fetal. Neonatal. Med. 2014, 19, 60–69. [Google Scholar] [CrossRef]
- Beck, J.; Brander, L.; Slutsky, A.S.; Reilly, M.C.; Dunn, M.S.; Sinderby, C. Non-invasive neurally adjusted ventilatory assist in rabbits with acute lung injury. Intensive Care Med. 2008, 34, 316–323. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.S.; Sohn, J.A.; Lee, J.A.; Choi, C.W.; Kim, E.K.; Kim, B.I.; Choi, J.H. Randomized crossover study of neurally adjusted ventilatory assist in preterm infants. J. Pediatr. 2012, 161, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Koskela, U.; Peltoniemi, O.; Kontiokari, T.; Pokka, T.; Suo-Palosaari, M.; Saarela, T. Neurally adjusted ventilatory assist (NAVA) in preterm newborn infants with respiratory distress syndrome-a randomized controlled trial. Eur. J. Pediatr. 2016, 175, 1175–1183. [Google Scholar] [CrossRef]
- Di Mussi, R.; Spadaro, S.; Mirabella, L.; Volta, C.A.; Serio, G.; Staffieri, F.; Dambrosio, M.; Cinnella, G.; Bruno, F.; Grasso, S. Impact of prolonged assisted ventilation on diaphragmatic efficiency: NAVA versus PSV. Crit. Care 2016, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Roze, H.; Lafrikh, A.; Perrier, V.; Germain, A.; Dewitte, A.; Gomez, F.; Janvier, G.; Ouattara, A. Daily titration of neurally adjusted ventilatory assist using the diaphragm electrical activity. Intensive Care Med. 2011, 37, 1087–1094. [Google Scholar] [CrossRef]
- Lagatta, J.M.; Clark, R.H.; Brousseau, D.C.; Hoffmann, R.G.; Spitzer, A.R. Varying patterns of home oxygen use in infants at 23–43 weeks’ gestation discharged from United States neonatal intensive care units. J. Pediatr. 2013, 163, 976–982.e2. [Google Scholar] [CrossRef] [PubMed]
- Lagatta, J.; Clark, R.; Spitzer, A. Clinical predictors and institutional variation in home oxygen use in preterm infants. J. Pediatr. 2012, 160, 232–238. [Google Scholar] [CrossRef]
- Seaton, S.E.; Barker, L.; Draper, E.S.; Abrams, K.R.; Modi, N.; Manktelow, B.N. Estimating neonatal length of stay for babies born very preterm. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F182–F186. [Google Scholar] [CrossRef]
- Piastra, M.; De Luca, D.; Costa, R.; Pizza, A.; De Sanctis, R.; Marzano, L.; Biasucci, D.; Visconti, F.; Conti, G. Neurally adjusted ventilatory assist vs pressure support ventilation in infants recovering from severe acute respiratory distress syndrome: Nested study. J. Crit. Care 2014, 29, 312.e1–312.e5. [Google Scholar] [CrossRef]
- Kallio, M.; Peltoniemi, O.; Anttila, E.; Pokka, T.; Kontiokari, T. Neurally adjusted ventilatory assist (NAVA) in pediatric intensive care—A randomized controlled trial. Pediatr. Pulmonol. 2015, 50, 55–62. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Control (n = 109) | NAVA (n = 97) | p Value |
|---|---|---|---|
| 1. Gestational age in weeks (mean ± SD) | 27.3 ± 2.1 | 26.8 ± 2.2 | 0.15 |
| 2. Sex | |||
| Males (%) | 54% | 56% | 0.82 |
| Females (%) | 46% | 44% | |
| 3. Birth weight in grams (mean ± SD) | 1007 ± 300 | 955 ± 298 | 0.21 |
| 4. Antenatal steroids (%) | 80% | 89% | 0.04 |
| 5. APGAR 1 min (median) | 4 | 5 | 0.2 |
| 6. APGAR 5 min (median) | 7 | 7 | 0.18 |
| 7. Chorioamnionitis (%) | 6% | 3% | 0.4 |
| 8. Preterm labor (%) | 42% | 54% | 0.1 |
| 9. Premature rupture of membranes (%) | 6% | 12% | 0.08 |
| 10. Preeclampsia (%) | 25% | 24% | 0.9 |
| 11. Placental abruption (%) | 8% | 12% | 0.3 |
| Variable | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Gestational age | 27 | 2.3–307.3 | 0.0013 * |
| Infection | 4 | 0.98–13.8 | 0.05 |
| Vitamin A | 0.9 | 0.28–2.7 | 0.8 |
| Caffeine | 0.3 | 0.08–0.98 | 0.05 |
| PDA | 1 | 0.28–3.64 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehra, K.; Kresch, M. Trends in the Incidence of Bronchopulmonary Dysplasia after the Introduction of Neurally Adjusted Ventilatory Assist (NAVA). Children 2024, 11, 113. https://doi.org/10.3390/children11010113
Mehra K, Kresch M. Trends in the Incidence of Bronchopulmonary Dysplasia after the Introduction of Neurally Adjusted Ventilatory Assist (NAVA). Children. 2024; 11(1):113. https://doi.org/10.3390/children11010113
Chicago/Turabian StyleMehra, Kashish, and Mitchell Kresch. 2024. "Trends in the Incidence of Bronchopulmonary Dysplasia after the Introduction of Neurally Adjusted Ventilatory Assist (NAVA)" Children 11, no. 1: 113. https://doi.org/10.3390/children11010113
APA StyleMehra, K., & Kresch, M. (2024). Trends in the Incidence of Bronchopulmonary Dysplasia after the Introduction of Neurally Adjusted Ventilatory Assist (NAVA). Children, 11(1), 113. https://doi.org/10.3390/children11010113





