Exploratory Investigation of Handwriting Disorders in School-Aged Children from First to Fifth Grade
Abstract
1. Introduction
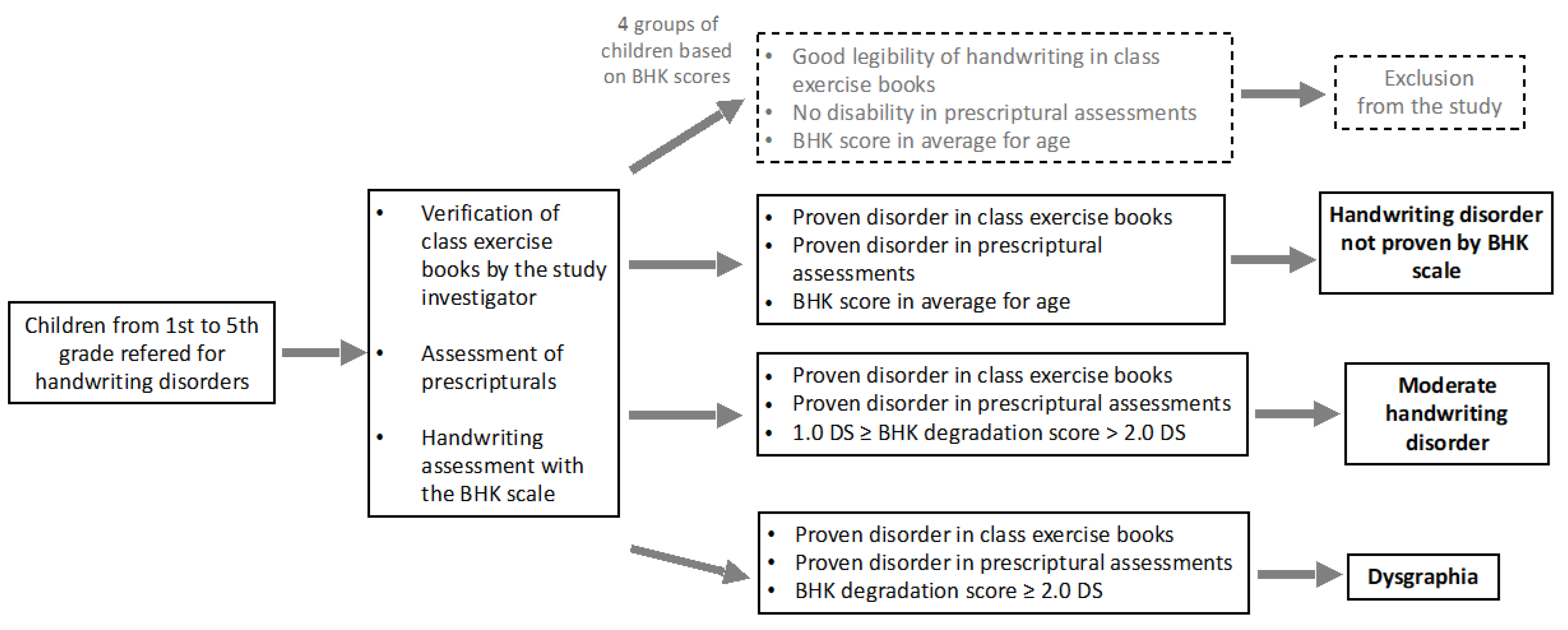
2. Material and Methods
2.1. Participants
2.2. Design and Measures
2.3. Handwriting Assessment
2.4. Clinical Assessments
2.4.1. Neuropsychomotor Assessment
2.4.2. Psychomotor Assessment
2.4.3. Neurovisual Assessment
2.4.4. Neuropsychological Assessment
2.4.5. Language Skills
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Sample
3.2. Results of the Handwriting Assessment
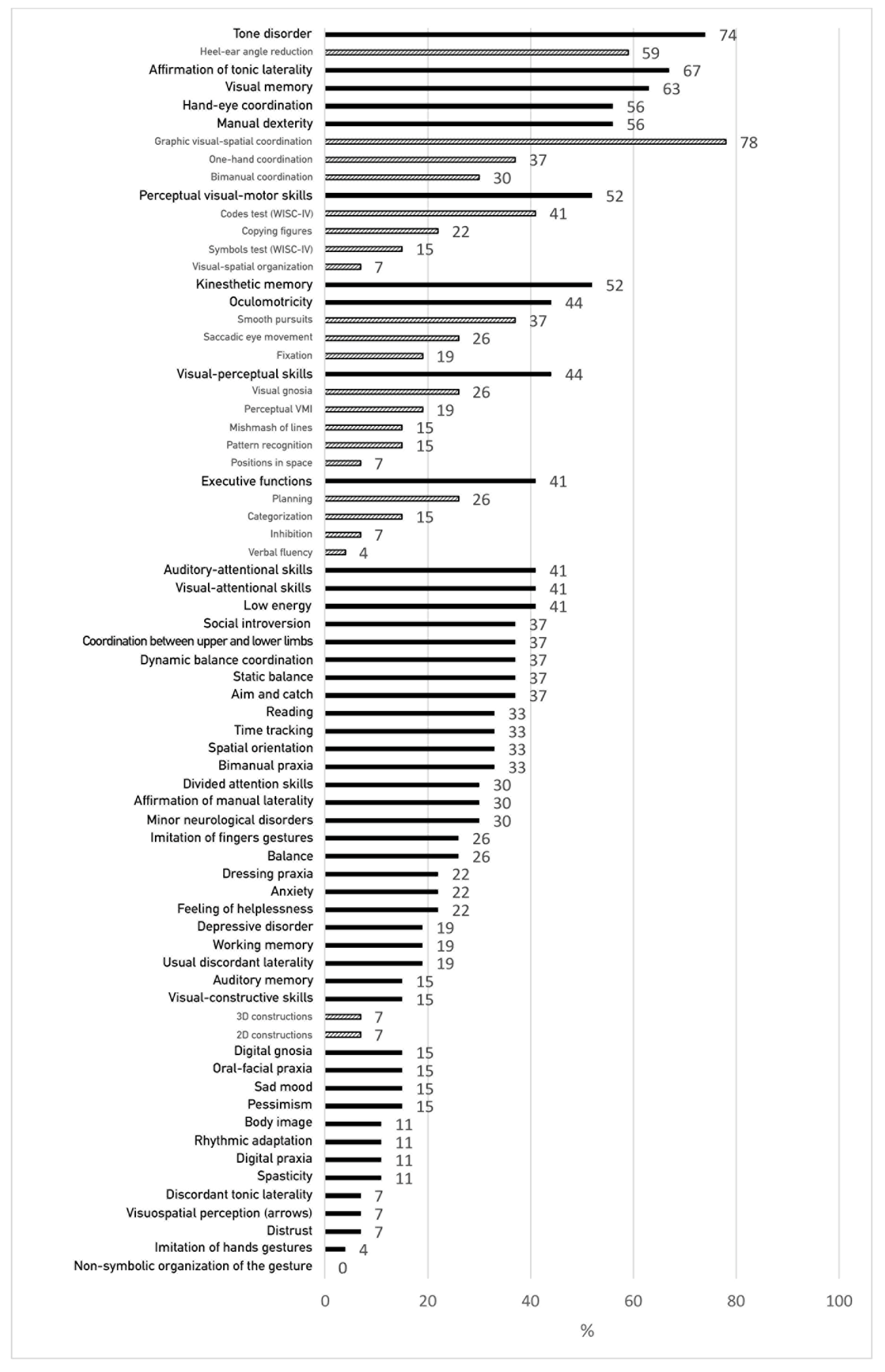
3.3. Percentage of Clinical Test Failures (Neuropsychomotor, Psychomotor, Neurovisual, Neuropsychological, Oculomotor and Language Assessments)
3.4. Frequency of Failures in Clinical Assessments between the Different Levels of Handwriting Disorder (HD Not Detected by BHK Scale, Moderate HD, Dysgraphia)
3.5. Failures Greater Than 40% in Each of the Three Groups Identified by the BHK Test (HD Not Detected by BHK, Moderate HD, Dysgraphia)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHale, K.; Cermak, S.A. Fine motor activities in elementary school: Preliminary findings and provisional implications for children with fine motor problems. Am. J. Occup. Ther. 1992, 46, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, R. Handwriting: A New Perspective; Stanley Thornes: Chelthenham, UK, 1990. [Google Scholar]
- Stewart, S.R. Developement of written language proficiency: Methods for teaching text structure. In Communication Skills and Classroom Success; Simon, C.C., Ed.; Taylor & Francis: Abingdon, UK, 1992. [Google Scholar]
- Chang, S.H.; Yu, N.Y. Handwriting movement analyses comparing first and second graders with normal or dysgraphic characteristics. Res. Dev. Disabil. 2013, 34, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Feder, K.P.; Majnemer, A. Handwriting development, competency, and intervention. Dev. Med. Child. Neurol. 2007, 49, 312–317. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders, DSM IV-TR, 4th ed.; American Psychiatric Publishing: Arlington, MA, USA, 2000. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, DSM-5, 5th ed.; American Psychiatric Publishing: Arlington, MA, USA, 2013. [Google Scholar]
- Karlsdottir, R.; Stefansson, T. Problems in developing functional handwriting. Percept. Mot. Sk. 2002, 94, 623–662. [Google Scholar] [CrossRef]
- Maeland, A.E. Handwriting and perceptual motor skills in clumsy, dysgraphic, and normal children. Percept. Mot. Sk. 1992, 75, 1207–1217. [Google Scholar]
- Naider-Steinhart, S.; Katz-Leurer, M. Analysis of proximal and distal muscle activity during handwriting tasks. Am. J. Occup. Ther. 2007, 61, 392–398. [Google Scholar] [CrossRef]
- De Ajuriaguerra, J.; Auzias, M.; Denner, A. The Child’s Writing. The Evolution of Writing and Its Difficulties, 4th ed.; Delachaux et Niestlé: Paris, France, 1964; Volume 1. [Google Scholar]
- Hamstra-Bletz, L.; Blöte, A.W. A Longitudinal Study on Dysgraphic Handwriting in Primary School. J. Learn. Disabil. 1993, 26, 689–699. [Google Scholar] [CrossRef]
- Van Dorn, R.R.A.; Keuss, P.J.G. Dysfluency in children’s handwriting. In The Development of Graphic Skills; Wann, J., Wing, A.M., Sovik, N., Eds.; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Vaivre-Douret, L.; Lopez, C.; Dutruel, A.; Vaivre, S. Phenotyping features in the genesis of pre-scriptural gestures in children to assess handwriting developmental levels. Sci. Rep. 2021, 11, 731. [Google Scholar] [CrossRef]
- Lopez, C.; Vaivre-Douret, L. Concurrent and predictive validity of a cycloid loops copy task to assess handwriting disorders in children. Children 2023, 10, 305. [Google Scholar] [CrossRef]
- Lopez, C.; Vaivre-Douret, L. Exploratory investigations of handwriting disorders in children from 1st to 5th grade. ANAE 2021, 170, 77–89. [Google Scholar]
- Asselborn, T.; Chapatte, M.; Dillenbourg, P. Extending the Spectrum of Dysgraphia: A Data Driven Strategy to Estimate Handwriting Quality. Sci. Rep. 2020, 10, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wann, J.P. Handwriting disturbance: Developmental trends. In Themes in Motor Development; Whiting, H.T.A., Wade, M.G., Eds.; Martinus Nijhoff Publishers: Leyde, The Netherlands, 1986. [Google Scholar]
- Lopez, C.; Vaivre-Douret, L. Influence of visual control on the quality of graphic gesture in children with handwriting disorders. Sci. Rep. 2021, 11, 23537. [Google Scholar] [CrossRef] [PubMed]
- Smits-Engelsman, B.C.; van Galen, G.P. Dysgraphia in Children: Lasting Psychomotor Deficiency or Transient Developmental Delay? J. Exp. Child Psychol. 1997, 67, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, G.P.; Portier, S.J.; Smits-Engelsman, B.C.; Schomaker, L.R. Neuromotor noise and poor handwriting in children. Acta Psychol. 1993, 82, 161–178. [Google Scholar] [CrossRef]
- Wann, J.P.; Kardirkamanathan, M. Variability in children’s handwriting: Computer diagnosis of writing difficulties. In The Development of Graphic Skills; Wann, J., Wing, A.M., Sovik, N., Eds.; Academic Press: Cambrige, MA, USA, 1991. [Google Scholar]
- Zesiger, P. Learning to write and hardwriting disorders. Enfance 2003, 55, 56–64. [Google Scholar] [CrossRef]
- Lopez, C.; Hemimou, C.; Golse, B.; Vaivre-Douret, L. Developmental dysgraphia is often associated with minor neurological dysfunction in children with developmental coordination disorder (DCD). Clin. Neurophysiol. 2018, 48, 207–217. [Google Scholar] [CrossRef]
- Charles, M.; Soppelsa, R.; Albaret, J.M. Concise Evaluation Scale for Children’s Handwriting (BHK); ECPA-Pearson: Paris, France, 2004. [Google Scholar]
- Hamstra-Bletz, L.; DeBie, J.; Den Brinker, B. Concise Evaluation Scale for Children’s Handwriting; Swets Zeitlinger: Lisse, The Netherlands, 1987. [Google Scholar]
- Vaivre-Douret, L. Neuro-Psychomotor Functions Assessment Battery; ECPA-Pearson: Paris, France, 2006. [Google Scholar]
- Bruininks, R.H. Bruininks Oseretsky Test of Motor Proficiency; American Guidance Service: Circle Pines, MN, USA, 1978. [Google Scholar]
- Vaivre-Douret, L. Digital scoring tool and standardized scoring of the developmental standardized assessment of the neuro-psychomotor functions of the child, NP-MOT up to 12 years and older. In Battery of Child Neuro-Psychomotor Functions; Vaivre-Douret, L., Ed.; Editions Neuralix: Paris, France, 2021. [Google Scholar]
- Marquet-Doléac, J.; Soppelsa, R.; Albaret, J.M. MABC-2 Movement Assessment Battery for Children, 2nd ed.; ECPA-Pearson: Paris, France, 2016. [Google Scholar]
- Henderson, S.E.; Sugden, D.A.; Barnett, A. Movement Assessment Battery for Children-2: Movement ABC-2; Pearson: London, UK, 2007. [Google Scholar]
- Vaivre-Douret, L. Assessment of Distal Gnosopraxic Motor Skills EMG; ECPA-Pearson: Paris, France, 1997. [Google Scholar]
- Vaivre-Douret, L. Normes 8-12 ans pour la notation automatisée de l’évaluation de la motricité gnosopraxique (EMG). In Evaluation de la Motricité Gnosopraxique Distale: Révision et Adaptation du Test de Bergès-Lézine; Vaivre-Douret, L., Ed.; ECPA-Pearson: Paris, France, 2021. [Google Scholar]
- Vaivre-Douret, L. Evaluation of the distal motor gnosopraxia. An adaptation of Berges and Lezine’s Imitation of Gestures test. ANAE 1999, 51, 13–20. [Google Scholar]
- Meljac, C.; Fauconnier, E.; Scalabrini, J. Body Schema-R. Body Schema Test—Revised; ECPA-Pearson: Paris, France, 2010. [Google Scholar]
- Frostig, M. Visual Perception Development Test: Manual; ECPA-Pearson: Paris, France, 1973. [Google Scholar]
- Kaufman, A.S.; Kaufman, N.L. KABC-II—Battery for the Psychological Examination of the Child, 2nd ed.; ECPA-Pearson: Paris, France, 2008. [Google Scholar]
- Beery, K.E.; Buktenica, N.A.; Beery, N.A. The Beery–Buktenica Developmental Test of Visual–Motor Integratio: Administration, Scoring, and Teaching Manual, 6th ed.; Pearson: London, UK, 2010. [Google Scholar]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY-II—Neuropsychological Assessment of the Child, 2nd ed.; ECPA-Pearson: Paris, France, 2012. [Google Scholar]
- Wallon, P.; Mesmin, C. CFR—Test of the Complex Figure of Rey; ECPA-Pearson: Paris, France, 2009. [Google Scholar]
- Wechsler, D. WISC-IV—Wechsler Intelligence Scale for Children and Adolescents, 4th ed.; ECPA-Pearson: Paris, France, 2005. [Google Scholar]
- Kohs, S. Kohs Cube Test: Manual; ECPA-Pearson: Paris, France, 1972. [Google Scholar]
- Jazz Novo Ober Consulting. Ober Consulting Homepage®. 2018. Available online: http://www.ober-consulting.com/9/lang/1/ (accessed on 17 June 2021).
- Manly, T.; Robertson, I.H.; Anderson, V.; Mimmo-Smith, I. TEAC-Ch—Childhood Attention Assessment Test; ECPA-Pearson: Paris, France, 2006. [Google Scholar]
- Marquet-Doléac, J.; Soppelsa, R.; Albaret, J.M. Laby 5–12: Labyrinths Test for Children Aged 5 to 12; Hogrefe: Paris, France, 2010. [Google Scholar]
- Albaret, J.M.; Migliore, L. Stroop—Stroop Selective Attention Test; ECPA-Pearson: Paris, France, 1999. [Google Scholar]
- Jacquier-Roux, M.; Valdois, S.; Zorman, M.; Lequette, C.; Pouget, G. ODEDYS—Dyslexia Screening Tool, 2nd ed.; Cogni-Sciences: Grenoble, France, 2005. [Google Scholar]
- Berndt, D.J.; Kaiser, C.F. MDI-C—Children’s Composite Depression Scale; ECPA-Pearson: Paris, France, 1999. [Google Scholar]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
- Wilson, P.H.; Ruddock, S.; Smits-Engelsman, B.C.M.; Polatajko, H.; Blank, R. Understanding performance deficits in developmental coordination disorder: A meta-analysis of recent research. Dev. Med. Child Neurol. 2013, 55, 217–228. [Google Scholar] [CrossRef]
- Longcamp, M.; Lagarrigue, A.; Nazarian, B.; Roth, M.; Anton, J.L.; Alario, F.X.; Velay, J.L. Functional specificity in the motor system: Evidence from coupled fMRI and kinematic recordings during letter and digit writing: Functional Specificity during Letter and Digit Writing. Hum. Brain Mapp. 2014, 35, 6077–6087. [Google Scholar] [CrossRef]
- Richards, T.L.; Berninger, V.W.; Stock, P.; Altemeier, L.; Trivedi, P.; Maravilla, K.R. Differences between good and poor child writers on fMRI contrasts for writing newly taught and highly practiced letter forms. Read. Writ. 2011, 24, 493–516. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.I.; Fawcett, A.J.; Dean, P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001, 24, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Missiuna, C.; Boyd, L.A. Neural Correlates of Developmental Coordination Disorder: A Review of Hypotheses. J. Child Neurol. 2009, 24, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.I.; Fawcett, A.J.; Berry, E.L.; Jenkins, I.H.; Dean, P.; Brooks, D.J. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet 1999, 353, 1662–1667. [Google Scholar] [CrossRef]
- Fawcett, A.J.; Nicolson, R.I. Performance of Dyslexic Children on Cerebellar and Cognitive Tests. J. Mot. Behav. 1999, 31, 68–78. [Google Scholar] [CrossRef]
- Velay, J.L. Interhemispheric sensorimotor integration in pointing movements: A study on dyslexic adults. Neuropsychologia 2002, 40, 827–834. [Google Scholar] [CrossRef]
- Lee, D.N.; Lishman, J.R. Visual proprioceptive control of stance. J. Hum. Mov. Stud. 1975, 1, 87–95. [Google Scholar]
- Berthoz, A.; Lacour, M.; Soechting, J.F.; Vidal, P.P. The role of vision during linear motion. In Reflex Control of Posture and Movement. Progress in Brain Research; Granit, R., Pompeiano, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1979; Volume 50. [Google Scholar]
- Gaymard, B.; Giannitelli, M.; Challes, G.; Rivaud-Péchoux, S.; Bonnot, O.; Cohen, D.; Xavier, J. Oculomotor Impairments in Developmental Dyspraxia. Cerebellum 2017, 16, 411–420. [Google Scholar] [CrossRef][Green Version]
- Robert, M.P.; Ingster-Moati, I.; Albuisson, E.; Cabrol, D.; Golse, B.; Vaivre-Douret, L. Vertical and horizontal smooth pursuit eye movements in children with developmental coordination disorder. Dev. Med. Child Neurol. 2014, 56, 595–600. [Google Scholar] [CrossRef]
- Planton, S.; Longcamp, M.; Péran, P.; Démonet, J.F.; Jucla, M. How specialized are writing-specific brain regions? An fMRI study of writing, drawing and oral spelling. Cortex 2017, 88, 66–80. [Google Scholar] [CrossRef]
- Gheysen, F.; Van Waelvelde, H.; Fias, W. Impaired visuo-motor sequence learning in Developmental Coordination Disorder. Res. Dev. Disabil. 2011, 32, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ben-Pazi, H.; Kukke, S.; Sanger, T.D. Poor Penmanship in Children Correlates with Abnormal Rhythmic Tapping: A Broad Functional Temporal Impairment. J. Child Neurol. 2007, 22, 543–549. [Google Scholar] [CrossRef]
- Collewijn, H.; Kowler, E. The significance of microsaccades for vision and oculomotor control. J. Vis. 2008, 8, 20–21. [Google Scholar] [CrossRef]
- Paillard, J. The Body and Its Languages of Space. In Le Corps en Psychiatrie [The Body in Psychiatry]; Jeddi, E., Ed.; Masson: Paris, France, 1982. [Google Scholar]
- Pfeiffer, B.; Moskowitz, B.; Paoletti, A.; Brusilovskiy, E.; Zylstra, S.E.; Murray, T. Developmental Test of Visual–Motor Integration (VMI): An Effective Outcome Measure for Handwriting Interventions for Kindergarten, First-Grade, and Second-Grade Students? Am. J. Occup. Ther. 2015, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Auzias, M.; de Ajuriaguerra, J. The cultural functions of writing and the conditions for its development in children. Enfance 1986, 39, 145–167. [Google Scholar] [CrossRef]
- Assaiante, C. Action and representation of action during childhood and adolescence: A functional approach. Clin. Neurophysiol. 2012, 42, 43–51. [Google Scholar] [CrossRef]
- Assaiante, C.; Schmitz, C. Construction of representations of action in children: What impairment in autism? Enfance 2009, 1, 111–120. [Google Scholar]
- Bara, F.; Gentaz, E. Haptics in teaching handwriting: The role of perceptual and visuo-motor skills. Hum. Mov. Sci. 2011, 30, 745–759. [Google Scholar] [CrossRef]
- Cornhill, H.; Case-Smith, J. Factors that relate to good and poor handwriting. Am. J. Occup. Ther. 1996, 50, 732–739. [Google Scholar] [CrossRef]
- Tseng, M.H.; Chow, S.M. Perceptual-motor function of school-age children with slow handwriting speed. Am. J. Occup. Ther. 2000, 54, 83–88. [Google Scholar] [CrossRef]
- Weintraub, N.; Graham, S. The contribution of gender, orthographic, finger function, and visual–motor processes to the prediction of handwriting status. Occup. Ther. J. Res. 2000, 20, 121–141. [Google Scholar] [CrossRef]
- Williams, J.; Zolten, A.J.; Rickert, V.I.; Spence, G.T.; Ashcraft, E.W. Use of non verbal tests to screen for writing dysfluency in school-age children. Percept. Mot. Sk. 1993, 76, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Yochman, A.; Parush, S. Differences in Hebrew handwriting skills between Israeli children in second and third grade. Phys. Occup. Ther. Ped. 1998, 18, 53–65. [Google Scholar] [CrossRef]
- Levine, M.D. Developmental Variation and Learning Disorders; Educators Publishing Service: Cambridge, MA, USA, 1987. [Google Scholar]
- Schneck, C.M. Comparison of pencil-grip patterns in first graders with good and poor writing skills. Am. J. Occup. Ther. 1991, 45, 701–706. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jung, N.H.; Kim, K.M. The correlation between proprioception and handwriting legibility in children. J. Phys. Ther. Sci. 2016, 28, 2849–2851. [Google Scholar] [CrossRef]
- Richards, T.L.; Berninger, V.W.; Fayol, M. FMRI activation differences between 11-years-old good and poor spellers’ access in working memory to temporary and long-term orthographic representations. J. Neurolinguist. 2009, 22, 327–353. [Google Scholar] [CrossRef]
- Van Hoorn, J.F.; Maathuis, C.G.B.; Hadders-Algra, M. Neural correlates of paediatric dysgraphia. Dev. Med. Child Neurol. 2013, 55, 65–68. [Google Scholar] [CrossRef]
| Handwriting Disorder Not Identified by the BHK Test (n = 7) | Moderate Handwriting Disorder (n = 9) | Dysgraphia (n = 11) | Total (n = 27) | |
|---|---|---|---|---|
| Age (years) [m (SD)] | 8.30 (0.81) | 7.79 (1.53) | 8.36 (1.87) | 8.15 (1.51) |
| Gender [n(F/M)] | 0/7 | 1/8 | 3/8 | 4/23 |
| Functions | Whole HD Group (n = 27) | HD Not Detected by BHK Scale (n = 7) | Moderate HD on BHK (n = 9) | Dysgraphia on BHK (n = 11) | p-Value |
|---|---|---|---|---|---|
| TONE DISORDER (NP-MOT) | 74 | 71 | 78 | 73 | 0.95 |
| Heel–ear angle reduction | 59 | 71 | 56 | 55 | 0.76 |
| AFFIRMATION OF TONIC LATERALITY (NP-MOT) | 67 | 43 | 67 | 82 | 0.24 |
| VISUAL MEMORY (REY, NEPSY-II) | 63 | 43 | 78 | 64 | 0.86 |
| HAND-EYE COORDINATION (NP-MOT, MABC-2) | 56 | 43 | 67 | 55 | 0.64 |
| MANUAL DEXTERITY (NP-MOT, MABC-2) | 56 | 43 | 67 | 55 | 0.64 |
| Graphic visual-spatial coordination | 78 | 71 | 78 | 82 | 0.88 |
| One-hand coordination | 37 | 29 | 33 | 45 | 0.75 |
| Bimanual coordination | 30 | 14 | 44 | 27 | 0.43 |
| PERCEPTUAL VISUAL-MOTOR SKILLS | 52 | 14 | 67 | 64 | 0.076 |
| Codes test (WISC-IV) | 41 | 14 | 44 | 55 | 0.24 |
| Copying figures (VMI) | 22 | 0 | 22 | 36 | 0.21 |
| Symbols test (WISC-IV) | 15 | 0 | 11 | 27 | 0.28 |
| Visual-spatial organization (Rey) | 7 | 0 | 11 | 9 | 0.69 |
| KINESTHETIC MEMORY | 52 | 29 | 67 | 55 | 0.32 |
| OCULOMOTRICITY | 44 | 29 | 44 | 55 | 0.26 |
| Smooth pursuits | 37 | 14 | 33 | 55 | 0.16 |
| Saccadic eye movements | 26 | 29 | 11 | 36 | 0.25 |
| Fixation | 19 | 0 | 33 | 18 | 0.22 |
| VISUAL-PERCEPTUAL SKILLS | 44 | 14 | 33 | 73 | 0.016 * |
| Visual gnosis | 26 | 14 | 33 | 27 | 0.69 |
| Perceptual (VMI) | 19 | 0 | 22 | 27 | 0.34 |
| Mishmash of lines | 15 | 0 | 11 | 27 | 0.28 |
| Pattern recognition | 15 | 0 | 22 | 18 | 0.44 |
| Positions in space (Frostig) | 7 | 0 | 0 | 18 | 0.22 |
| EXECUTIVE FUNCTIONS | 41 | 29 | 33 | 55 | 0.49 |
| Planning | 26 | 29 | 22 | 27 | 0.95 |
| Categorization (Nepsy-II) | 15 | 14 | 0 | 27 | 0.25 |
| Inhibition (Laby 5–12, Stroop) | 7 | 14 | 11 | 0 | 0.48 |
| Verbal fluency (Nepsy-II) | 4 | 0 | 0 | 9 | 0.48 |
| AUDITORY-ATTENTIONAL SKILLS (TEA-CH) | 41 | 29 | 33 | 55 | 0.49 |
| VISUAL-ATTENTIONAL SKILLS (TEA-CH) | 41 | 43 | 56 | 27 | 0.45 |
| LOW ENERGY (MDI-C) | 41 | 43 | 44 | 36 | 0.93 |
| SOCIAL INTROVERSION (MDI-C) | 37 | 14 | 22 | 64 | 0.06 |
| COORDINATION BETWEEN UPPER AND LOWER LIMBS (NP-MOT) | 37 | 43 | 33 | 36 | 0.93 |
| DYNAMIC BALANCE COORDINATION (NP-MOT) | 37 | 43 | 33 | 36 | 0.93 |
| STATIC BALANCE (NP-MOT) | 37 | 29 | 11 | 64 | 0.052 |
| AIM AND CATCH (MABC-2) | 37 | 14 | 22 | 64 | 0.06 |
| READING (ODEDYS) | 33 | 43 | 33 | 27 | 0.80 |
| TIME TRACKING (NP-MOT) | 33 | 0 | 33 | 55 | 0.06 |
| BODILY SPATIAL INTEGRATION (NP-MOT) | 33 | 0 | 67 | 27 | 0.019 * |
| BIMANUAL PRAXIS(NP-MOT) | 33 | 0 | 44 | 45 | 0.10 |
| DIVIDED ATTENTION SKILLS (TEA-CH) | 30 | 29 | 22 | 36 | 0.79 |
| AFFIRMATION OF MANUAL LATERALITY (NP-MOT) | 30 | 0 | 56 | 27 | 0.053 |
| NEUROLOGICAL SOFT SIGNS (NP-MOT) | 30 | 0 | 33 | 45 | 0.12 |
| IMITATION OF FINGER GESTURES (EMG) | 26 | 14 | 22 | 36 | 0.57 |
| BALANCE (MABC-2) | 26 | 0 | 11 | 55 | 0.016 * |
| DRESSING PRAXIS | 22 | 14 | 11 | 36 | 0.35 |
| ANXIETY (MDI-C) | 22 | 14 | 33 | 18 | 0.62 |
| FEELING OF HELPLESSNESS (MDI-C) | 22 | 14 | 11 | 36 | 0.35 |
| DEPRESSIVE DISORDER (MDI-C) | 19 | 14 | 22 | 18 | 0.92 |
| WORKING MEMORY (ODEDYS) | 19 | 14 | 22 | 18 | 0.92 |
| USUAL DISCORDANT LATERALITY (NP-MOT) | 19 | 0 | 33 | 18 | 0.25 |
| AUDITORY MEMORY(ODEDYS) | 15 | 0 | 11 | 27 | 0.28 |
| VISUAL-CONSTRUCTIVE SKILLS | 15 | 0 | 11 | 27 | 0.28 |
| 3D constructions (Nepsy-II) | 7 | 0 | 0 | 18 | 0.22 |
| 2D constructions (Kohs) | 7 | 0 | 11 | 9 | 0.69 |
| DIGITAL GNOSIS (NP-MOT) | 15 | 0 | 22 | 18 | 0.44 |
| ORAL-FACIAL PRAXIS | 15 | 0 | 11 | 27 | 0.29 |
| SAD MOOD(MDI-C) | 15 | 29 | 22 | 0 | 0.20 |
| PESSIMISM (MDI-C) | 15 | 43 | 11 | 0 | 0.046 * |
| BODY IMAGE(CORP-R) | 11 | 0 | 0 | 27 | 0.09 |
| RHYTHMIC ADAPTATION (NP-MOT) | 11 | 0 | 0 | 27 | 0.09 |
| DIGITAL PRAXIS (NP-MOT) | 11 | 0 | 11 | 18 | 0.50 |
| SPASTICITY (NP-MOT) | 11 | 0 | 22 | 9 | 0.37 |
| DISCORDANT TONIC LATERALITY (NP-MOT) | 7 | 0 | 11 | 9 | 0.69 |
| VISUAL-SPATIAL PERCEPTION (NEPSY-II) | 7 | 14 | 11 | 0 | 0.48 |
| DISTRUST (MDI-C) | 7 | 0 | 11 | 9 | 0.69 |
| IMITATION OF HAND GESTURES (EMG) | 4 | 0 | 0 | 9 | 0.48 |
| SELF-ESTEEM (MDI-C) | 4 | 14 | 0 | 0 | 0.24 |
| NON-SYMBOLIC ORGANIZATION OF THE GESTURE (NP-MOT) | 0 | 0 | 0 | 0 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, C.; Vaivre-Douret, L. Exploratory Investigation of Handwriting Disorders in School-Aged Children from First to Fifth Grade. Children 2023, 10, 1512. https://doi.org/10.3390/children10091512
Lopez C, Vaivre-Douret L. Exploratory Investigation of Handwriting Disorders in School-Aged Children from First to Fifth Grade. Children. 2023; 10(9):1512. https://doi.org/10.3390/children10091512
Chicago/Turabian StyleLopez, Clémence, and Laurence Vaivre-Douret. 2023. "Exploratory Investigation of Handwriting Disorders in School-Aged Children from First to Fifth Grade" Children 10, no. 9: 1512. https://doi.org/10.3390/children10091512
APA StyleLopez, C., & Vaivre-Douret, L. (2023). Exploratory Investigation of Handwriting Disorders in School-Aged Children from First to Fifth Grade. Children, 10(9), 1512. https://doi.org/10.3390/children10091512








