Parental Preferences for Expanded Newborn Screening: What Are the Limits?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Sample and Recruitment
2.2.1. Data Collection
2.2.2. Data Analysis
| Disease Category | Representative Condition | Details about Representative Condition |
|---|---|---|
| (i) Childhood-onset conditions which have an effective treatment and align with current NBS criteria | Riboflavin transporter deficiency (RTD; OMIM 211500, 211530, 614707) |
|
| (ii) Childhood-onset conditions which have no medication-related disease-modifying treatment but can benefit from behavioral interventions | Rett Syndrome (RETT; OMIM 312750) |
|
| (iii) Childhood-onset conditions which have no approved disease-modifying medication but have ongoing clinical trials/emerging treatments | Duchenne Muscular Dystrophy (DMD; OMIM 310200) |
|
| (iv) Adolescent–adult-onset conditions with no medication-related treatment, but can benefit from surveillance | Hypertrophic cardiomyopathy (HCM; OMIM 192600) |
|
3. Results
3.1. Awareness of NBS
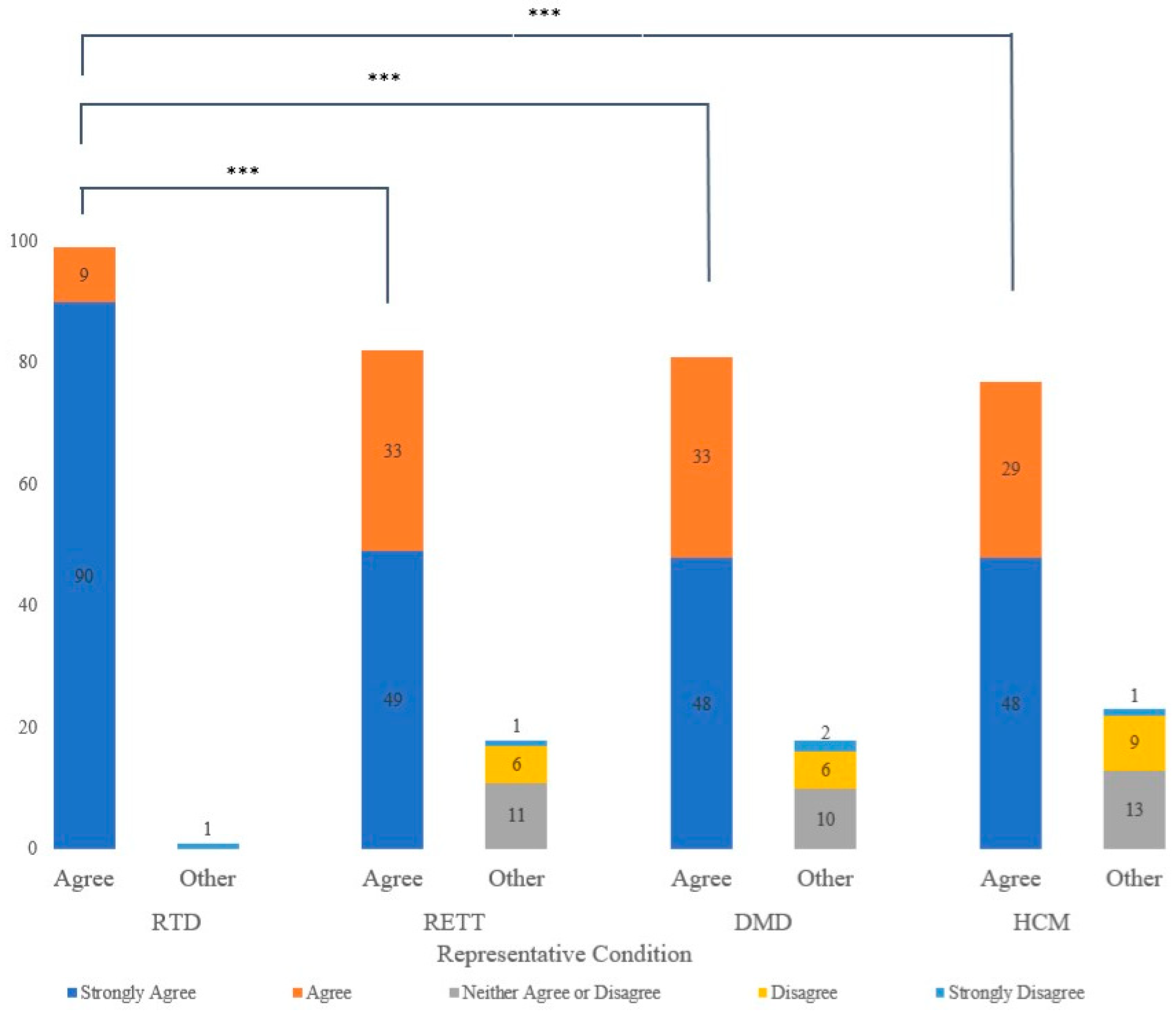
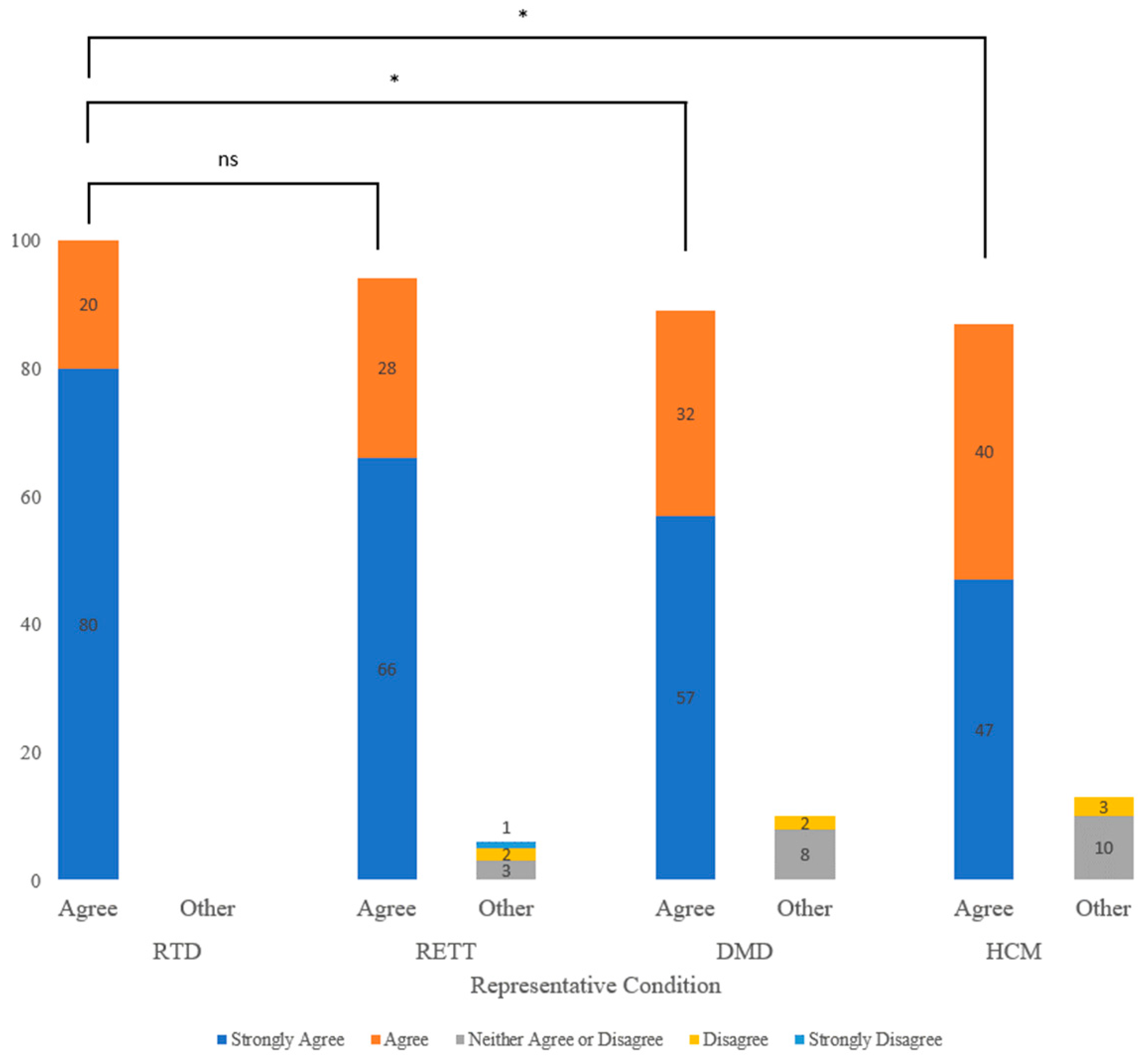
3.2. Willingness to Participate in NBS for Conditions with No Disease-Modifying Treatment
3.3. Perceived Benefits and Risks toward NBS for Different Conditions
3.3.1. Perceived Benefit to the Child and Better Support for Families
3.3.2. Interference with Parent–Child Bonding, Parental Anxiety, and Stigma
3.3.3. Uncertain Results
3.4. Consent Preferences
3.5. Trust in the Healthcare System
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pluscauskas, M.; Henderson, M.; Milburn, J.; Chakraborty, P. Building a newborn screening information management system from theory to practice. Int. J. Neonatal Screen. 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Bodian, D.L.; Klein, E.; Iyer, R.K.; Wong, W.S.W.; Kothiyal, P.; Stauffer, D.; Huddleston, K.C.; Gaither, A.D.; Remsburg, I.; Khromykh, A.; et al. Utility of whole-genome sequencing for detection of newborn screening disorders in a population cohort of 1,696 neonates. Genet. Med. 2016, 18, 221–230. [Google Scholar] [CrossRef]
- Boemer, F.; Fasquelle, C.; d’Otreppe, S.; Josse, C.; Dideberg, V.; Segers, K.; Guissard, V.; Capraro, V.; Debray, F.G.; Bours, V. A next-generation newborn screening pilot study: NGS on dried blood spots detects causal mutations in patients with inherited metabolic diseases. Sci. Rep. 2017, 7, 17641. [Google Scholar] [CrossRef]
- Bick, D.; Ahmed, A.; Deen, D.; Ferlini, A.; Garnier, N.; Kasperaviciute, D.; Leblond, M.; Pichini, A.; Rendon, A.; Satija, A.; et al. Newborn Screening by Genomic Sequencing: Opportunities and Challenges. Int. J. Neonatal Screen. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- van Campen, J.C.; Sollars, E.S.A.; Thomas, R.C.; Bartlett, C.M.; Milano, A.; Parker, M.D.; Dawe, J.; Winship, P.R.; Peck, G.; Grafham, D.; et al. Next generation sequencing in newborn screening in the United Kingdom National Health Service. Int. J. Neonatal Screen. 2019, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Cornel, M.C.; Goldenberg, A.J.; Lister, K.J.; Sénécal, K.; Vears, D.F. Genomic newborn screening: Public health policy considerations and recommendations. BMC Med. Genom. 2017, 10, 9. [Google Scholar] [CrossRef]
- Narravula, A.; Garber, K.B.; Askree, S.H.; Hegde, M.; Hall, P.L. Variants of uncertain significance in newborn screening disorders: Implications for large-scale genomic sequencing. Genet. Med. 2017, 19, 77–82. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Almannai, M.; Sutton, V.R. Newborn Screening: History, Current Status, and Future Directions. Pediatr. Clin. N. Am. 2018, 65, 389–405. [Google Scholar] [CrossRef]
- King, J.R.; Grill, K.; Hammarström, L. Genomic-Based Newborn Screening for Inborn Errors of Immunity: Practical and Ethical Considerations. Int. J. Neonatal Screen. 2023, 9, 22. [Google Scholar] [CrossRef]
- Milko, L.V.; Rini, C.; Lewis, M.A.; Butterfield, R.M.; Lin, F.-C.; Paquin, R.S.; Powell, B.C.; Roche, M.I.; Souris, K.J.; Bailey, D.B., Jr.; et al. Evaluating parents’ decisions about next-generation sequencing for their child in the NC NEXUS (North Carolina Newborn Exome Sequencing for Universal Screening) study: A randomized controlled trial protocol. Trials 2018, 19, 344. [Google Scholar] [CrossRef] [PubMed]
- DeCristo, D.M.; Milko, L.V.; O’Daniel, J.M.; Foreman, A.K.M.; Mollison, L.F.; Powell, B.C.; Powell, C.M.; Berg, J.S. Actionability of commercial laboratory sequencing panels for newborn screening and the importance of transparency for parental decision-making. Genome Med. 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.S.; Agrawal, P.B.; Bailey, D.B., Jr.; Beggs, A.H.; Brenner, S.E.; Brower, A.M.; Cakici, J.A.; Ceyhan-Birsoy, O.; Chan, K.; Chen, F.; et al. Newborn Sequencing in Genomic Medicine and Public Health. Pediatrics 2017, 139, e20162252. [Google Scholar] [CrossRef]
- Holm, I.A.; Agrawal, P.B.; Ceyhan-Birsoy, O.; Christensen, K.D.; Fayer, S.; Frankel, L.A.; Genetti, C.A.; Krier, J.B.; LaMay, R.C.; Levy, H.L.; et al. The BabySeq project: Implementing genomic sequencing in newborns. BMC Pediatr. 2018, 18, 225. [Google Scholar] [CrossRef]
- van der Burg, S.; Oerlemans, A. Fostering caring relationships: Suggestions to rethink liberal perspectives on the ethics of newborn screening. Bioethics 2018, 32, 171–183. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G.; WHO. Principles and practice of screening for disease. Public Health Pap. 1968, 123, 349. [Google Scholar] [CrossRef]
- DeLuca, J.M. Public Attitudes toward Expanded Newborn Screening. J. Pediatr. Nurs. 2018, 38, e19–e23. [Google Scholar] [CrossRef]
- Moultrie, R.R.; Paquin, R.; Rini, C.; Roche, M.I.; Berg, J.S.; Powell, C.M.; Lewis, M.A. Parental Views on Newborn Next Generation Sequencing: Implications for Decision Support. Matern. Child Health J. 2020, 24, 856–864. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Bombard, Y.; Avard, D.; Carroll, J.; Wilson, B.; Little, J.; Chakraboty, P.; Bytautas, J.; Giguere, Y.; et al. Expectations and values about expanded newborn screening: A public engagement study. Health Expect. 2015, 18, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Grob, R.; Roberts, S.; Timmermans, S. Families’ Experiences with Newborn Screening: A Critical Source of Evidence. Hastings Cent. Rep. 2018, 48, S29–S31. [Google Scholar] [CrossRef]
- White, A.L.; Boardman, F.; McNiven, A.; Locock, L.; Hinton, L. Absorbing it all: A meta-ethnography of parents’ unfolding experiences of newborn screening. Soc. Sci. Med. 2021, 287, 114367. [Google Scholar] [CrossRef]
- Milko, L.V.; O’Daniel, J.; DeCristo, D.M.; Crowley, S.B.; Foreman, A.K.M.; Wallace, K.E.; Mollison, L.F.; Strande, N.T.; Girnary, Z.S.; Boshe, L.J.; et al. An Age-Based Framework for Evaluating Genome-Scale Sequencing Results in Newborn Screening. J. Pediatr. 2019, 209, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Dillman, D.A. Mail and Internet Surveys: The Tailored Design Method, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bombard, Y.; Miller, F.A.; Hayeems, R.Z.; Barg, C.; Cressman, C.; Carroll, J.C.; Wilson, B.J.; Little, J.; Avard, D.; Painter-Main, M.; et al. Public views on participating in newborn screening using genome sequencing. Eur. J. Hum. Genet. 2018, 22, 1248–1254. [Google Scholar] [CrossRef]
- Araia, M.H.; Wilson, B.J.; Chakraborty, P.; Gall, K.; Honeywell, C.; Milburn, J.; Ramsay, T.; Potter, B.K. Factors associated with knowledge of and satisfaction with newborn screening education: A survey of mothers. Genet. Med. 2012, 14, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Erfanian, F.; Roudsari, R.L.; Heydari, A.; Bahmani, M.N.D. A Narrative on Using Vignettes: Advantages and Drawbacks. J. Midwifery Reprod. Health 2020, 8, 2134–2145. [Google Scholar] [CrossRef]
- Straten, G.F.M.; Friele, R.D.; Groenewegen, P.P. Public trust in Dutch health care. Soc. Sci. Med. 2002, 55, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin deficiency—Implications for general human health and inborn errors of metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Cosentino, L.; Vigli, D.; Franchi, F.; Laviola, G.; De Filippis, B. Rett syndrome before regression: A time window of overlooked opportunities for diagnosis and intervention. Neurosci. Biobehav. Rev. 2019, 107, 115–135. [Google Scholar] [CrossRef]
- Ke, Q.; Zhao, Z.-Y.; Mendell, J.R.; Baker, M.; Wiley, V.; Kwon, J.M.; Alfano, L.N.; Connolly, A.M.; Jay, C.; Polari, H.; et al. Progress in treatment and newborn screening for Duchenne muscular dystrophy and spinal muscular atrophy. World J. Pediatr. 2019, 15, 219–225. [Google Scholar] [CrossRef]
- Christian, S.; Atallah, J.; Clegg, R.; Giuffre, M.; Huculak, C.; Dzwiniel, T.; Parboosingh, J.; Taylor, S.; Somerville, M. Uptake of Predictive Genetic Testing and Cardiac Evaluation for Children at Risk for an Inherited Arrhythmia or Cardiomyopathy. J. Genet. Couns. 2018, 27, 124–130. [Google Scholar] [CrossRef]
- Genetti, C.A.; Schwartz, T.S.; Robinson, J.O.; VanNoy, G.E.; Petersen, D.; Pereira, S.; Fayer, S.; Peoples, H.A.; Agrawal, P.B.; Betting, W.N.; et al. Parental interest in genomic sequencing of newborns: Enrollment experience from the BabySeq Project. Genet. Med. 2019, 21, 622–630. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Bäck, D.K.; Liu, C.; Kalia, S.S.; Ringer, S.A.; Holm, I.A.; Green, R.C. Parents are interested in newborn genomic testing during the early postpartum period. Genet. Med. 2015, 17, 501–504. [Google Scholar] [CrossRef]
- Downie, L.; Halliday, J.; Lewis, S.; Lunke, S.; Lynch, E.; Martyn, M.; Gaff, C.; Jarmolowicz, A.; Amor, D.J. Exome sequencing in newborns with congenital deafness as a model for genomic newborn screening: The Baby Beyond Hearing project. Genet. Med. 2020, 22, 937–944. [Google Scholar] [CrossRef]
- Lindor, N.M.; Johnson, K.J.; McCormick, J.B.; Klee, E.W.; Ferber, M.J.; Farrugia, G. Preserving personal autonomy in a genomic testing era. Genet. Med. 2013, 15, 408–409. [Google Scholar] [CrossRef]
- Boardman, F.K. Attitudes toward population screening among people living with fragile X syndrome in the UK: ‘I wouldn’t wish him away, I’d just wish his fragile X syndrome away. J. Genet. Couns. 2021, 30, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Winarni, T.I.; Schneider, A.; Borodyanskara, M.; Hagerman, R.J. Early Intervention Combined with Targeted Treatment Promotes Cognitive and Behavioral Improvements in Young Children with Fragile X Syndrome. Case Rep. Genet. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Bullard, L.; McDuffie, A.; Abbeduto, L. Distance delivery of a parent-implemented language intervention for young boys with fragile X syndrome. Autism Dev. Lang. Impair. 2017, 2, 239694151772869. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.C.; Gwaltney, A.; Raspa, M.; Okoniewski, K.C.; Berry-Kravis, E.; Botteron, K.N.; Budimirovic, D.; Hazlett, H.C.; Hessl, D.; Losh, M.; et al. Emergence of developmental delay in infants and toddlers with an fmr1 mutation. Pediatrics 2021, 147, e2020011528. [Google Scholar] [CrossRef]
- Sawyer, S.L.; Hartley, T.; Dyment, D.A.; Beaulieu, C.L.; Schwartzentruber, J.; Smith, A.; Bedford, H.M.; Bernard, G.; Bernier, F.P.; Brais, B.; et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: Time to address gaps in care. Clin. Genet. 2016, 89, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Thevenon, J.; Duffourd, Y.; Masurel-Paulet, A.; Lefebvre, M.; Feillet, F.; El Chehadeh-Djebbar, S.; St-Onge, J.; Steinmetz, A.; Huet, F.; Chouchane, M.; et al. Diagnostic odyssey in severe neurodevelopmental disorders: Toward clinical whole-exome sequencing as a first-line diagnostic test. Clin. Genet. 2016, 89, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Okoniewski, K.C.; Wheeler, A.C.; Lee, S.; Boyea, B.; Raspa, M.; Taylor, J.L.; Bailey, D.B., Jr. Early identification of fragile X syndrome through expanded newborn screening. Brain Sci. 2019, 9, 4. [Google Scholar] [CrossRef]
- Pereira, S.; Smith, H.S.; Frankel, L.A.; Christensen, K.D.; Islam, R.; Robinson, O.; Genetti, C.A.; Zawatsky, C.L.B.; Zettler, B.; Parad, R.B.; et al. Psychosocial Effect of Newborn Genomic Sequencing on Families in the BabySeq Project: A Randomized Clinical Trial. JAMA Pediatr. 2021, 176, 1132–1141. [Google Scholar] [CrossRef]
- Lewis, M.A.; Stine, A.; Paquin, R.S.; Mansfield, C.; Wood, D.; Rini, C.; Roche, M.I.; Powell, C.M.; Berg, J.S.; Bailey, D.B. Parental preferences toward genomic sequencing for non-medically actionable conditions in children: A discrete-choice experiment. Genet. Med. 2018, 20, 181–189. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Carroll, J.C.; Tam, K.; Kerr, E.; Chakraborty, P.; Potter, B.K.; Patton, S.; et al. Psychosocial Response to Uncertain Newborn Screening Results for Cystic Fibrosis. J. Pediatr. 2017, 184, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, P.J.; Upshur, R.E.G.; Luca, S.; Venkataramanan, V.; Potter, B.K.; Chakraborty, P.K.; Hayeems, R.Z. Health-care providers’ perspectives on uncertainty generated by variant forms of newborn screening targets. Genet. Med. 2020, 22, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gonska, T.; Keenan, K.; Au, J.; Dupuis, A.; Chilvers, M.A.; Burgess, C. Outcomes of Cystic Fibrosis Screening—Positive Infants with Inconclusive Diagnosis at School Age. Pediatrics 2021, 148, e2021051740. [Google Scholar] [CrossRef]
- Pereira, S.; Robinson, J.O.; Gutierrez, A.; Petersen, D.K.; Hsu, R.; Lee, C.H. Perceived Benefits, Risks and Utility of Newborn Genomic Sequencing in the BabySeq Project. Pediatrics 2019, 143 (Suppl. S1), S6–S13. [Google Scholar] [CrossRef]
- Beckers, P.; Caberg, J.-H.; Dideberg, V.; Dangouloff, T.; Dunnen, J.T.D.; Bours, V.; Servais, L.; Boemer, F. Newborn screening of duchenne muscular dystrophy specifically targeting deletions amenable to exon-skipping therapy. Sci. Rep. 2021, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.; Makarem, D.C.; Wasserstein, M.P. Screening of newborns for disorders with high benefit- risk ratios should be mandatory. J. Law Med. Ethics 2016, 44, 231–240. [Google Scholar] [CrossRef]
- Howard, H.C.; Knoppers, B.M.; Cornel, M.C.; Clayton, E.W.; Sénécal, K.; Borry, P. Whole-genome sequencing in newborn screening? A statement on the continued importance of targeted approaches in newborn screening programmes. Eur. J. Hum. Genet. 2015, 23, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B.; Gehtland, L. Newborn screening evolving challenges in an era of rapid discovery. JAMA—J. Am. Med. Assoc. 2015, 313, 1511–1512. [Google Scholar] [CrossRef]
- Tarini, B.A.; Goldenberg, A.J. Ethical Issues with Newborn Screening in the Genomics Era. Annu. Rev. Genom. Hum. Genet. 2012, 13, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Kühberger, A.; Schulte-Mecklenbeck, M.; Perner, J. Framing decisions: Hypothetical and real. Organ. Behav. Hum. Decis. Process. 2002, 89, 1162–1175. [Google Scholar] [CrossRef]
| Characteristic | Total Participants (n = 100) |
|---|---|
| Gender | n (%) |
| Female | 98 (98%) |
| Male | 2 (2%) |
| Age | |
| 20–29 | 8 (8%) |
| 30–39 | 74 (74%) |
| 40–49 | 18 (18%) |
| Marital status | |
| Married or living common-law | 94 (94%) |
| Other | 6 (6%) |
| Highest level of education | |
| High school or less | 4 (4%) |
| College/Trade diploma | 20 (20%) |
| University degree or higher | 76 (76%) |
| Population of city or town | |
| Rural area | 9 (9%) |
| Small city/town (less than 100,000 people) | 12 (12%) |
| Medium-sized city (100,000–499,999 people) | 12 (12%) |
| Large city (500,000 or more people) | 66 (66%) |
| Unknown | 1 (1%) |
| Number of children | |
| One | 40 (40%) |
| Two | 39 (39%) |
| Three or more | 21 (21%) |
| Experience with positive NBS results | |
| Yes | 2 (2%) |
| No | 94 (94%) |
| Do not recall | 4 (4%) |
| Experience with genetic testing in a family member | |
| Yes | 10 (10%) |
| No | 86 (86%) |
| I do not know | 4 (4%) |
| Q: “For This Condition, Parents Should Be …” | RTD | RETT | DMD | HCM |
|---|---|---|---|---|
| … required to have their baby screened. | ||||
| Yes | 29 (29%) | 22 (22%) | 23 (24%) | 21 (21%) |
| No | 71 (71%) | 76 (78%) | 75 (76%) | 79 (79%) |
| …strongly encouraged to have their baby screened, but parents can still decline. | ||||
| Yes | 91 (91%) | 76 (76%) | 71 (72%) | 74 (74%) |
| No | 9 (9%) | 24 (24%) | 28 (28%) | 26 (26%) |
| …able to choose whether they want their baby screened. | ||||
| Yes | 66 (66%) | 76 (76%) | 76 (77%) | 76 (76%) |
| No | 34 (34%) | 24 (24%) | 23 (23%) | 24 (24%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, N.S.Y.; Watts-Dickens, A.; Chitayat, D.; Babul-Hirji, R.; Chakraborty, P.; Hayeems, R.Z. Parental Preferences for Expanded Newborn Screening: What Are the Limits? Children 2023, 10, 1362. https://doi.org/10.3390/children10081362
Liang NSY, Watts-Dickens A, Chitayat D, Babul-Hirji R, Chakraborty P, Hayeems RZ. Parental Preferences for Expanded Newborn Screening: What Are the Limits? Children. 2023; 10(8):1362. https://doi.org/10.3390/children10081362
Chicago/Turabian StyleLiang, Nicole S. Y., Abby Watts-Dickens, David Chitayat, Riyana Babul-Hirji, Pranesh Chakraborty, and Robin Z. Hayeems. 2023. "Parental Preferences for Expanded Newborn Screening: What Are the Limits?" Children 10, no. 8: 1362. https://doi.org/10.3390/children10081362
APA StyleLiang, N. S. Y., Watts-Dickens, A., Chitayat, D., Babul-Hirji, R., Chakraborty, P., & Hayeems, R. Z. (2023). Parental Preferences for Expanded Newborn Screening: What Are the Limits? Children, 10(8), 1362. https://doi.org/10.3390/children10081362






