Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography
Abstract
1. Introduction
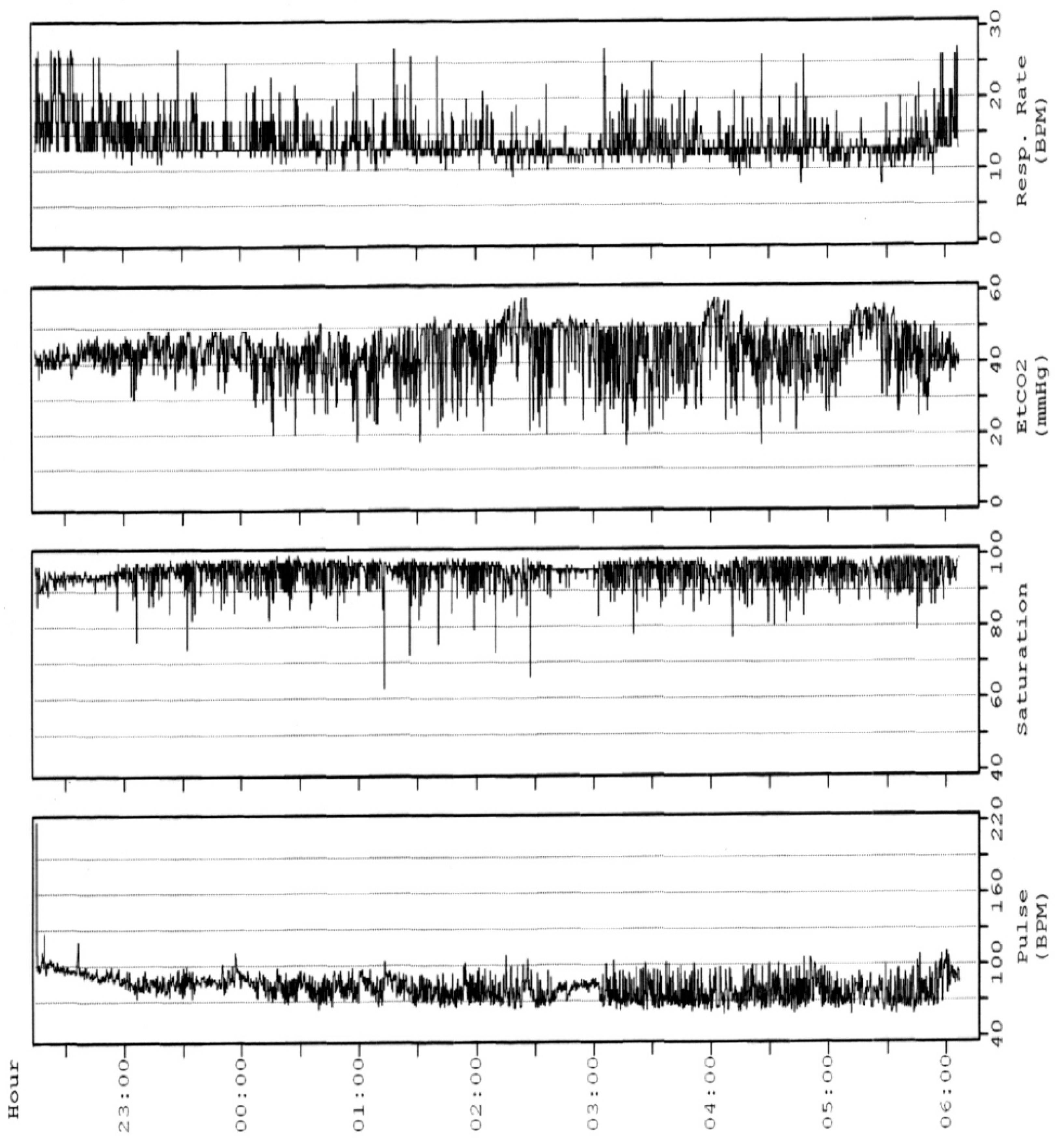
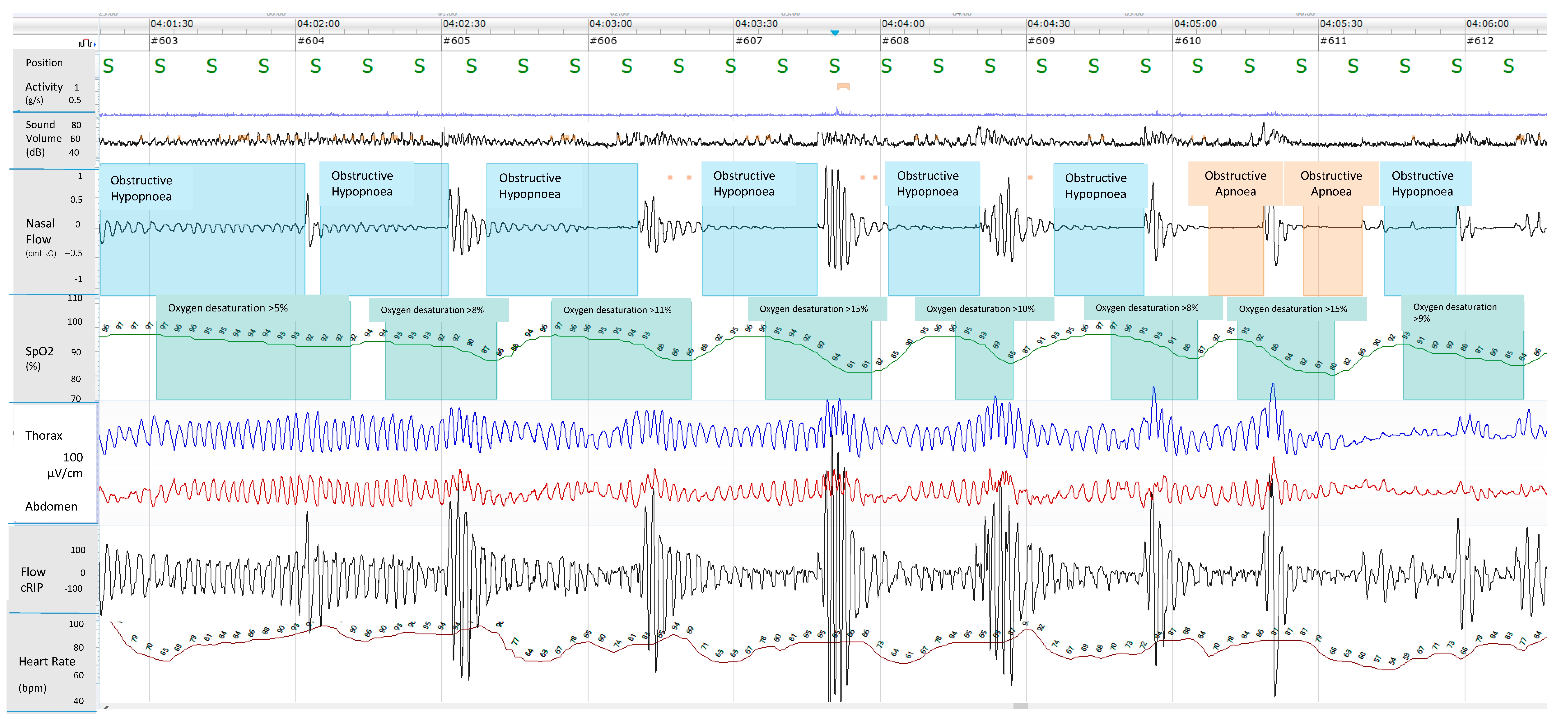
2. Polysomnography
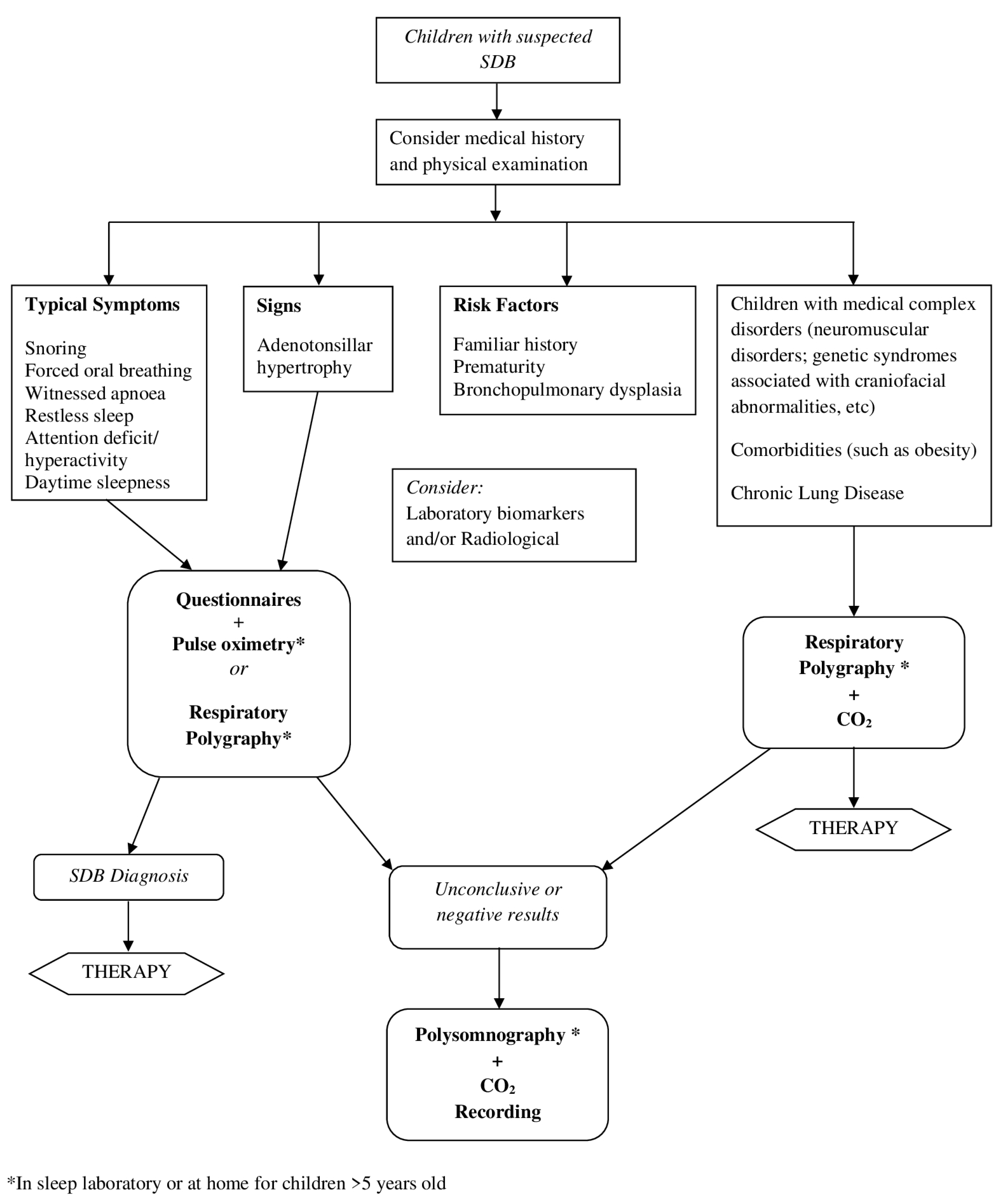
3. What Is the Role of Alternative Diagnostic Tools in the Diagnosis of SDB?
3.1. Medical History and Physical Examination
3.2. Questionnaires
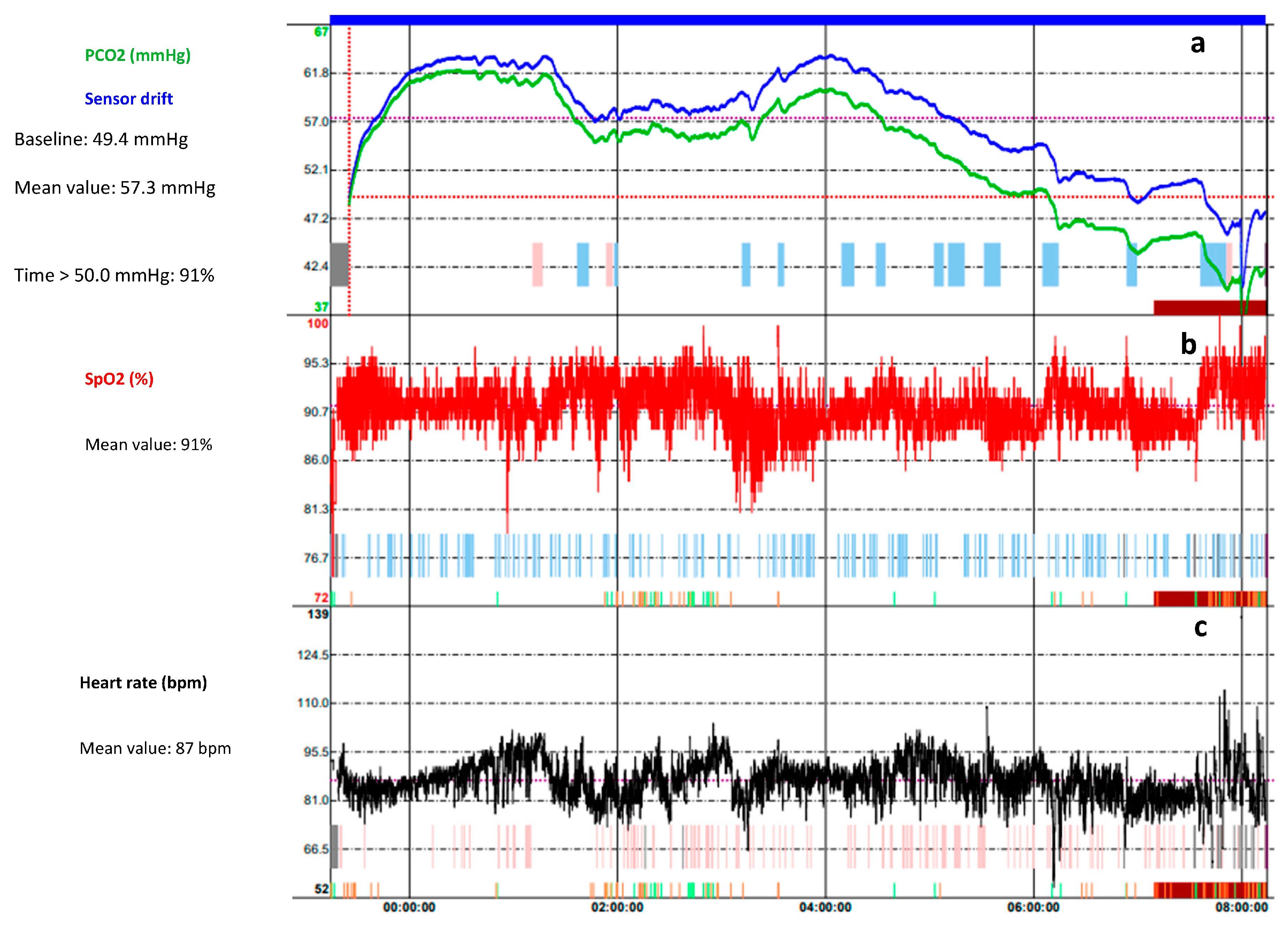
3.3. Nocturnal Oximetry
3.4. Respiratory Polygraphy
3.5. Nap Studies
3.6. Home Testing
3.6.1. Home Pulse Oximetry
3.6.2. Home RP
3.6.3. Home PSG
3.6.4. Video Recording
3.7. Sonography
3.8. Laboratory Biomarkers
3.9. Radiologic Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive Sleep Disordered Breathing in 2- to 18-Year-Old Children: Diagnosis and Management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef]
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-Adjusted 24-Hour Ambulatory Blood Pressure and Cardiac Remodeling in Children with Sleep Disordered Breathing. Hypertension 2008, 51, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Hwang, B.; Soong, W.-J.; Meng, C.C.L. The Specific Characteristics in Children with Obstructive Sleep Apnea and Cor Pulmonale. Sci. World J. 2012, 2012, 757283. [Google Scholar] [CrossRef] [PubMed]
- Bourke, R.; Anderson, V.; Yang, J.S.C.; Jackman, A.R.; Killedar, A.; Nixon, G.M.; Davey, M.J.; Walker, A.M.; Trinder, J.; Horne, R.S.C. Cognitive and Academic Functions Are Impaired in Children with All Severities of Sleep-Disordered Breathing. Sleep Med. 2011, 12, 489–496. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef]
- Fund, N.; Green, A.; Chodick, G.; Orin, M.; Koren, G.; Shalev, V.; Dagan, Y. The Epidemiology of Sleep Disorders in Israel: Results from a Population-Wide Study. Sleep Med. 2020, 67, 120–127. [Google Scholar] [CrossRef]
- Kara, C.O.; Ergin, H.; Koçak, G.; Kiliç, I.; Yurdakul, M. Prevalence of Tonsillar Hypertrophy and Associated Oropharyngeal Symptoms in Primary School Children in Denizli, Turkey. Int. J. Pediatr. Otorhinolaryngol. 2002, 66, 175–179. [Google Scholar] [CrossRef]
- Castronovo, V.; Zucconi, M.; Nosetti, L.; Marazzini, C.; Hensley, M.; Veglia, F.; Nespoli, L.; Ferini-Strambi, L. Prevalence of Habitual Snoring and Sleep-Disordered Breathing in Preschool-Aged Children in an Italian Community. J. Pediatr. 2003, 142, 377–382. [Google Scholar] [CrossRef]
- Borrelli, M.; Corcione, A.; Rongo, R.; Cantone, E.; Scala, I.; Bruzzese, D.; Martina, S.; Strisciuglio, P.; Michelotti, A.; Santamaria, F. Obstructive Sleep Apnoea in Children with Down Syndrome: A Multidisciplinary Approach. J. Pers. Med. 2022, 13, 71. [Google Scholar] [CrossRef]
- Canora, A.; Franzese, A.; Mozzillo, E.; Fattorusso, V.; Bocchino, M.; Sanduzzi, A. Severe Obstructive Sleep Disorders in Prader-Willi Syndrome Patients in Southern Italy. Eur. J. Pediatr. 2018, 177, 1367–1370. [Google Scholar] [CrossRef]
- Koyuncu, E.; Türkkani, M.H.; Sarikaya, F.G.; Özgirgin, N. Sleep Disordered Breathing in Children with Cerebral Palsy. Sleep Med. 2017, 30, 146–150. [Google Scholar] [CrossRef]
- Ramezani, R.J.; Stacpoole, P.W. Sleep Disorders Associated with Primary Mitochondrial Diseases. J. Clin. Sleep Med. 2014, 10, 1233–1239. [Google Scholar] [CrossRef]
- Verhulst, S.L.; Van Gaal, L.; De Backer, W.; Desager, K. The Prevalence, Anatomical Correlates and Treatment of Sleep-Disordered Breathing in Obese Children and Adolescents. Sleep Med. Rev. 2008, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, Y.; Tai, J.; Feng, G.; Ge, W.; Zheng, L.; Zhou, Z.; Ni, X. Risk Factors of Obstructive Sleep Apnea Syndrome in Children. J. Otolaryngol. Head Neck Surg. 2020, 49, 11. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.; Gozal, D.; Marcus, C.L.; Quan, S.F.; Rosen, C.L.; Chervin, R.D.; Wise, M.; Picchietti, D.L.; Sheldon, S.H.; Iber, C. The Visual Scoring of Sleep and Arousal in Infants and Children. J. Clin. Sleep Med. 2007, 3, 201–240. [Google Scholar] [CrossRef] [PubMed]
- Scholle, S.; Zwacka, G. Arousals and Obstructive Sleep Apnea Syndrome in Children. Clin. Neurophysiol. 2001, 112, 984–991. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Guilleminault, C.; Poyares, D.; Palombini, L.; Koester, U.; Pelin, Z.; Black, J. Variability of Respiratory Effort in Relation to Sleep Stages in Normal Controls and Upper Airway Resistance Syndrome Patients. Sleep Med. 2001, 2, 397–405. [Google Scholar] [CrossRef]
- Amaddeo, A.; Fauroux, B. Oxygen and Carbon Dioxide Monitoring during Sleep. Paediatr. Respir. Rev. 2016, 20, 42–44. [Google Scholar] [CrossRef]
- Brietzke, S.E.; Katz, E.S.; Roberson, D.W. Can History and Physical Examination Reliably Diagnose Pediatric Obstructive Sleep Apnea/Hypopnea Syndrome? A Systematic Review of the Literature. Otolaryngol. Neck Surg. 2004, 131, 827–832. [Google Scholar] [CrossRef]
- Rosen, C.L.; Larkin, E.K.; Kirchner, H.L.; Emancipator, J.L.; Bivins, S.F.; Surovec, S.A.; Martin, R.J.; Redline, S. Prevalence and Risk Factors for Sleep-Disordered Breathing in 8- to 11-Year-Old Children: Association with Race and Prematurity. J. Pediatr. 2003, 142, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.M.D.; Collins, J.; Hicks, D. Collagen Type VI Myopathies. Adv. Exp. Med. Biol. 2014, 802, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Skotko, B.G.; Macklin, E.A.; Muselli, M.; Voelz, L.; McDonough, M.E.; Davidson, E.; Allareddy, V.; Jayaratne, Y.S.N.; Bruun, R.; Ching, N.; et al. A Predictive Model for Obstructive Sleep Apnea and Down Syndrome. Am. J. Med. Genet. A 2017, 173, 889–896. [Google Scholar] [CrossRef]
- Cielo, C.M.; Marcus, C.L. Obstructive Sleep Apnoea in Children with Craniofacial Syndromes. Paediatr. Respir. Rev. 2015, 16, 189–196. [Google Scholar] [CrossRef]
- Maroda, A.J.; Spence, M.N.; Larson, S.R.; Estepp, J.H.; Gillespie, M.B.; Harris, A.J.; Mamidala, M.P.; Sheyn, A.M. Screening for Obstructive Sleep Apnea in Children with Sickle Cell Disease: A Pilot Study. Laryngoscope 2021, 131, E1022–E1028. [Google Scholar] [CrossRef]
- Kaleyias, J.; Mostofi, N.; Grant, M.; Coleman, C.; Luck, L.; Dampier, C.; Kothare, S.V. Severity of Obstructive Sleep Apnea in Children with Sickle Cell Disease. J. Pediatr. Hematol. Oncol. 2008, 30, 659–665. [Google Scholar] [CrossRef]
- Gipson, K.; Lu, M.; Kinane, T.B. Sleep-Disordered Breathing in Children. Pediatr. Rev. 2019, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.L.; McColley, S.A.; Marcus, C.L.; Curtis, S.; Loughlin, G.M. Inability of Clinical History to Distinguish Primary Snoring from Obstructive Sleep Apnea Syndrome in Children. Chest 1995, 108, 610–618. [Google Scholar] [CrossRef]
- El-Mitwalli, A.; Bediwy, A.S.; Zaher, A.A.; Belal, T.; Saleh, A.B.M. Sleep Apnea in Children with Refractory Monosymptomatic Nocturnal Enuresis. Nat. Sci. Sleep 2014, 6, 37–42. [Google Scholar] [CrossRef]
- Guilleminault, C.; Palombini, L.; Pelayo, R.; Chervin, R.D. Sleepwalking and Sleep Terrors in Prepubertal Children: What Triggers Them? Pediatrics 2003, 111, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef]
- Andersen, I.G.; Holm, J.-C.; Homøe, P. Obstructive Sleep Apnea in Obese Children and Adolescents, Treatment Methods and Outcome of Treatment—A Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 190–197. [Google Scholar] [CrossRef]
- Kang, J.-M.; Auo, H.-J.; Yoo, Y.-H.; Cho, J.-H.; Kim, B.-G. Changes in Serum Levels of IGF-1 and in Growth following Adenotonsillectomy in Children. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Savini, S.; Ciorba, A.; Bianchini, C.; Stomeo, F.; Corazzi, V.; Vicini, C.; Pelucchi, S. Assessment of Obstructive Sleep Apnoea (OSA) in Children: An Update. Acta Otorhinolaryngol. Ital. 2019, 39, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Øverland, B.; Berdal, H.; Akre, H. Obstructive Sleep Apnea in 2–6 Year Old Children Referred for Adenotonsillectomy. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2097–2104. [Google Scholar] [CrossRef]
- Lund, V.J.; Kennedy, D.W. Quantification for Staging Sinusitis. The Staging and Therapy Group. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 167, 17–21. [Google Scholar] [CrossRef]
- Friedman, M.; Ibrahim, H.; Joseph, N.J. Staging of Obstructive Sleep Apnea/Hypopnea Syndrome: A Guide to Appropriate Treatment. Laryngoscope 2004, 114, 454–459. [Google Scholar] [CrossRef]
- Cassano, P.; Gelardi, M.; Cassano, M.; Fiorella, M.L.; Fiorella, R. Adenoid Tissue Rhinopharyngeal Obstruction Grading Based on Fiberendoscopic Findings: A Novel Approach to Therapeutic Management. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 1303–1309. [Google Scholar] [CrossRef]
- Ciavarella, D.; Campobasso, A.; Conte, E.; Burlon, G.; Guida, L.; Montaruli, G.; Cassano, M.; Laurenziello, M.; Illuzzi, G.; Tepedino, M. Correlation between Dental Arch Form and OSA Severity in Adult Patients: An Observational Study. Prog. Orthod. 2023, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, I.Q. Lingual Frenulum Protocol. Int. J. Orofac. Myol. 2012, 38, 89–103. [Google Scholar] [CrossRef]
- Ingram, D.G.; Singh, A.V.; Ehsan, Z.; Birnbaum, B.F. Obstructive Sleep Apnea and Pulmonary Hypertension in Children. Paediatr. Respir. Rev. 2017, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric Sleep Questionnaire (PSQ): Validity and Reliability of Scales for Sleep-Disordered Breathing, Snoring, Sleepiness, and Behavioral Problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef] [PubMed]
- De Luca Canto, G.; Singh, V.; Major, M.P.; Witmans, M.; El-Hakim, H.; Major, P.W.; Flores-Mir, C. Diagnostic Capability of Questionnaires and Clinical Examinations to Assess Sleep-Disordered Breathing in Children: A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2014, 145, 165–178. [Google Scholar] [CrossRef]
- Pabary, R.; Goubau, C.; Russo, K.; Laverty, A.; Abel, F.; Samuels, M. Screening for Sleep-Disordered Breathing with Pediatric Sleep Questionnaire in Children with Underlying Conditions. J. Sleep Res. 2019, 28, e12826. [Google Scholar] [CrossRef]
- Brouilette, R.; Hanson, D.; David, R.; Klemka, L.; Szatkowski, A.; Fernbach, S.; Hunt, C. A Diagnostic Approach to Suspected Obstructive Sleep Apnea in Children. J. Pediatr. 1984, 105, 10–14. [Google Scholar] [CrossRef]
- Franco, R.A.J.; Rosenfeld, R.M.; Rao, M. First Place–Resident Clinical Science Award 1999. Quality of Life for Children with Obstructive Sleep Apnea. Otolaryngol. Neck Surg. 2000, 123, 9–16. [Google Scholar] [CrossRef]
- Borrelli, M.; Scala, I.; Festa, P.; Bruzzese, D.; Michelotti, A.; Cantone, E.; Corcione, A.; Fragnito, M.; Miranda, V.; Santamaria, F. Linguistic Adaptation and Psychometric Evaluation of Italian Version of Children’s Sleep Habits Questionnaire. Ital. J. Pediatr. 2021, 47, 170. [Google Scholar] [CrossRef]
- Combs, D.; Goodwin, J.L.; Quan, S.F.; Morgan, W.J.; Parthasarathy, S. Modified STOP-Bang Tool for Stratifying Obstructive Sleep Apnea Risk in Adolescent Children. PLoS ONE 2015, 10, e0142242. [Google Scholar] [CrossRef]
- Villa, M.P.; Paolino, M.C.; Castaldo, R.; Vanacore, N.; Rizzoli, A.; Miano, S.; Del Pozzo, M.; Montesano, M. Sleep Clinical Record: An Aid to Rapid and Accurate Diagnosis of Paediatric Sleep Disordered Breathing. Eur. Respir. J. 2013, 41, 1355–1361. [Google Scholar] [CrossRef]
- Brouillette, R.T.; Morielli, A.; Leimanis, A.; Waters, K.A.; Luciano, R.; Ducharme, F.M. Nocturnal Pulse Oximetry as an Abbreviated Testing Modality for Pediatric Obstructive Sleep Apnea. Pediatrics 2000, 105, 405–412. [Google Scholar] [CrossRef]
- Nixon, G.M.; Kermack, A.S.; Davis, G.M.; Manoukian, J.J.; Brown, K.A.; Brouillette, R.T. Planning Adenotonsillectomy in Children with Obstructive Sleep Apnea: The Role of Overnight Oximetry. Pediatrics 2004, 113, e19–e25. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Gozal, D.; Ramirez, H.M.; Bandla, H.P.R.; Kheirandish-Gozal, L. Overnight Polysomnography versus Respiratory Polygraphy in the Diagnosis of Pediatric Obstructive Sleep Apnea. Sleep 2014, 37, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, M.; Calori, G.; Castronovo, V.; Ferini-Strambi, L. Respiratory Monitoring by Means of an Unattended Device in Children with Suspected Uncomplicated Obstructive Sleep Apnea: A Validation Study. Chest 2003, 124, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Álvarez, M.L.; Terán-Santos, J.; Ordax Carbajo, E.; Cordero-Guevara, J.A.; Navazo-Egüia, A.I.; Kheirandish-Gozal, L.; Gozal, D. Reliability of Home Respiratory Polygraphy for the Diagnosis of Sleep Apnea in Children. Chest 2015, 147, 1020–1028. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Perez, J.L.; Moya, A. Feasibility of Unattended Home Polysomnography in Children with Sleep-Disordered Breathing. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1960–1964. [Google Scholar] [CrossRef]
- Franco, P.; Bourdin, H.; Braun, F.; Briffod, J.; Pin, I.; Challamel, M.-J. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Arch. Pediatr. 2017, 24 (Suppl. S1), S16–S27. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Keens, T.G.; Ward, S.L. Comparison of Nap and Overnight Polysomnography in Children. Pediatr. Pulmonol. 1992, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.M.; Keens, T.G.; Stabile, M.W.; Bolokowicz, J.; Davidson Ward, S.L. Should Children with Suspected Obstructive Sleep Apnea Syndrome and Normal Nap Sleep Studies Have Overnight Sleep Studies? Chest 2000, 118, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Hudgel, D.; Sateia, M.; Schwab, R. Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar]
- Kirk, V.; Baughn, J.; D’Andrea, L.; Friedman, N.; Galion, A.; Garetz, S.; Hassan, F.; Wrede, J.; Harrod, C.G.; Malhotra, R.K. American Academy of Sleep Medicine Position Paper for the Use of a Home Sleep Apnea Test for the Diagnosis of OSA in Children. J. Clin. Sleep Med. 2017, 13, 1199–1203. [Google Scholar] [CrossRef]
- Pavone, M.; Ullmann, N.; Verrillo, E.; De Vincentiis, G.; Sitzia, E.; Cutrera, R. At-Home Pulse Oximetry in Children Undergoing Adenotonsillectomy for Obstructive Sleep Apnea. Eur. J. Pediatr. 2017, 176, 493–499. [Google Scholar] [CrossRef]
- Pavone, M.; Cutrera, R.; Verrillo, E.; Salerno, T.; Soldini, S.; Brouillette, R.T. Night-to-Night Consistency of at-Home Nocturnal Pulse Oximetry Testing for Obstructive Sleep Apnea in Children. Pediatr. Pulmonol. 2013, 48, 754–760. [Google Scholar] [CrossRef]
- Hoppenbrouwer, X.L.R.; Rollinson, A.U.; Dunsmuir, D.; Ansermino, J.M.; Dumont, G.; Oude Nijeweme-d’Hollosy, W.; Veltink, P.; Garde, A. Night to Night Variability of Pulse Oximetry Features in Children at Home and at the Hospital. Physiol. Meas. 2021, 42, 104003. [Google Scholar] [CrossRef]
- Garde, A.; Dehkordi, P.; Karlen, W.; Wensley, D.; Ansermino, J.M.; Dumont, G.A. Development of a Screening Tool for Sleep Disordered Breathing in Children Using the Phone OximeterTM. PLoS ONE 2014, 9, e112959. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Xu, W.; Han, D. Diagnostic Accuracy of Level IV Portable Sleep Monitors versus Polysomnography for Pediatric Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Med. 2021, 87, 127–137. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, B.; Lee, J.Y.; Kim, H.J. Validating the Watch-PAT for Diagnosing Obstructive Sleep Apnea in Adolescents. J. Clin. Sleep Med. 2018, 14, 1741–1747. [Google Scholar] [CrossRef]
- Chiner, E.; Cánovas, C.; Molina, V.; Sancho-Chust, J.N.; Vañes, S.; Pastor, E.; Martinez-Garcia, M.A. Home Respiratory Polygraphy Is Useful in the Diagnosis of Childhood Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2020, 9, 2067. [Google Scholar] [CrossRef] [PubMed]
- Oceja, E.; Rodríguez, P.; Jurado, M.J.; Luz Alonso, M.; Del Río, G.; Villar, M.Á.; Mediano, O.; Martínez, M.; Juarros, S.; Merino, M.; et al. Validity and Cost-Effectiveness of Pediatric Home Respiratory Polygraphy for the Diagnosis of Obstructive Sleep Apnea in Children: Rationale, Study Design, and Methodology. Methods Protoc. 2021, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Kheirandish-Gozal, L.; Gozal, D. Pediatric Home Sleep Apnea Testing: Slowly Getting There! Chest 2015, 148, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L.; Kaditis, A.G. Home Sleep Testing for the Diagnosis of Pediatric Obstructive Sleep Apnea: The Times They Are a Changing…! Curr. Opin. Pulm. Med. 2015, 21, 563–568. [Google Scholar] [CrossRef]
- Withers, A.; Maul, J.; Rosenheim, E.; O’Donnell, A.; Wilson, A.; Stick, S. Comparison of Home Ambulatory Type 2 Polysomnography with a Portable Monitoring Device and In-Laboratory Type 1 Polysomnography for the Diagnosis of Obstructive Sleep Apnea in Children. J. Clin. Sleep Med. 2022, 18, 393–402. [Google Scholar] [CrossRef]
- Sivan, Y.; Kornecki, A.; Schonfeld, T. Screening Obstructive Sleep Apnoea Syndrome by Home Videotape Recording in Children. Eur. Respir. J. 1996, 9, 2127–2131. [Google Scholar] [CrossRef]
- Thomas, R.J.; Dalton, S.; Harman, K.; Thacker, J.; Horne, R.S.C.; Davey, M.J.; Nixon, G.M. Smartphone Videos to Predict the Severity of Obstructive Sleep Apnoea. Arch. Dis. Child. 2022, 107, 148–152. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Blunden, S.; Hirte, C.; Parsons, D.W.; Martin, A.J.; Crowe, E.; Williams, D.; Pamula, Y.; Lushington, K. Reduced Neurocognition in Children Who Snore. Pediatr. Pulmonol. 2004, 37, 330–337. [Google Scholar] [CrossRef]
- Kwok, K.L.; Ng, D.K.K.; Cheung, Y.F. BP and Arterial Distensibility in Children with Primary Snoring. Chest 2003, 123, 1561–1566. [Google Scholar] [CrossRef]
- Nixon, G.M.; Brouillette, R.T. Diagnostic Techniques for Obstructive Sleep Apnoea: Is Polysomnography Necessary? Paediatr. Respir. Rev. 2002, 3, 18–24. [Google Scholar] [CrossRef]
- Gozal, D.; Jortani, S.; Snow, A.B.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Kim, J.; Capdevila, O.S. Two-Dimensional Differential in-Gel Electrophoresis Proteomic Approaches Reveal Urine Candidate Biomarkers in Pediatric Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2009, 180, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- De Luca Canto, G.; Pachêco-Pereira, C.; Aydinoz, S.; Major, P.W.; Flores-Mir, C.; Gozal, D. Diagnostic Capability of Biological Markers in Assessment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. J. Clin. Sleep Med. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; McManus, C.J.T.; Kellermann, G.H.; Samiei, A.; Gozal, D. Urinary Neurotransmitters Are Selectively Altered in Children with Obstructive Sleep Apnea and Predict Cognitive Morbidity. Chest 2013, 143, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.T.W.; Chan, R.N.C.; Chan, K.C.C.; Au, C.T.; Li, A.M. Level of Urinary Catecholamine in Children with Sleep Disordered Breathing: A Systematic Review and Meta-Analysis. Sleep Med. 2022, 100, 565–572. [Google Scholar] [CrossRef]
- Villa, M.P.; Supino, M.C.; Fedeli, S.; Rabasco, J.; Vitelli, O.; Del Pozzo, M.; Gentile, G.; Lionetto, L.; Barreto, M.; Simmaco, M. Urinary Concentration of 8-Isoprostane as Marker of Severity of Pediatric OSAS. Sleep Breath. 2014, 18, 723–729. [Google Scholar] [CrossRef]
- Biltagi, M.A.; Maguid, M.A.; Ghafar, M.A.; Farid, E. Correlation of 8-Isoprostane, Interleukin-6 and Cardiac Functions with Clinical Score in Childhood Obstructive Sleep Apnoea. Acta Paediatr. 2008, 97, 1397–1405. [Google Scholar] [CrossRef]
- Kozanhan, B.; Iyisoy, M.S. Red Cell Distribution Width as a Novel Predictor of Postoperative Respiratory Adverse Events after Adenotonsillectomy. Paediatr. Anaesth. 2017, 27, 609–615. [Google Scholar] [CrossRef]
- Kalogritsas, N.D.; Lachanas, V.A.; Liakos, P.; Alexopoulos, E.I.; Beka, D.; Petinaki, E.; Hajiioannou, J.; Simos, G.; Skoulakis, C.E. Erythropoietin Levels in Children with Obstructive Sleep Apnea. Int. J. Pediatr. Otorhinolaryngol. 2021, 151, 110932. [Google Scholar] [CrossRef]
- Benedek, P.; Lázár, Z.; Bikov, A.; Kunos, L.; Katona, G.; Horváth, I. Exhaled Biomarker Pattern Is Altered in Children with Obstructive Sleep Apnoea Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1244–1247. [Google Scholar] [CrossRef]
- Bhushan, B.; Maddalozzo, J.; Sheldon, S.H.; Haymond, S.; Rychlik, K.; Lales, G.C.; Billings, K.R. Metabolic Alterations in Children with Obstructive Sleep Apnea. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 854–859. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Guleria, R.; Kabra, S.K. Metabolic Alterations and Systemic Inflammation in Overweight/Obese Children with Obstructive Sleep Apnea. PLoS ONE 2021, 16, e0252353. [Google Scholar] [CrossRef] [PubMed]
- Slaats, M.A.; Van Hoorenbeeck, K.; Van Eyck, A.; Vos, W.G.; De Backer, J.W.; Boudewyns, A.; De Backer, W.; Verhulst, S.L. Upper Airway Imaging in Pediatric Obstructive Sleep Apnea Syndrome. Sleep Med. Rev. 2015, 21, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Shott, S.R.; Donnelly, L.F. Cine Magnetic Resonance Imaging: Evaluation of Persistent Airway Obstruction after Tonsil and Adenoidectomy in Children with Down Syndrome. Laryngoscope 2004, 114, 1724–1729. [Google Scholar] [CrossRef]
- Donnelly, L.F.; Shott, S.R.; LaRose, C.R.; Chini, B.A.; Amin, R.S. Causes of Persistent Obstructive Sleep Apnea despite Previous Tonsillectomy and Adenoidectomy in Children with down Syndrome as Depicted on Static and Dynamic Cine MRI. AJR. Am. J. Roentgenol. 2004, 183, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Fregosi, R.F.; Quan, S.F.; Kaemingk, K.L.; Morgan, W.J.; Goodwin, J.L.; Cabrera, R.; Gmitro, A. Sleep-Disordered Breathing, Pharyngeal Size and Soft Tissue Anatomy in Children. J. Appl. Physiol. 2003, 95, 2030–2038. [Google Scholar] [CrossRef]
- Arens, R.; McDonough, J.M.; Costarino, A.T.; Mahboubi, S.; Tayag-Kier, C.E.; Maislin, G.; Schwab, R.J.; Pack, A.I. Magnetic Resonance Imaging of the Upper Airway Structure of Children with Obstructive Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 698–703. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Vos, W.; Van Hoorenbeeck, K.; Boudewyns, A.; Salgado, R.; Verdonck, P.R.; Ramet, J.; De Backer, J.; De Backer, W.; Verhulst, S.L. Functional Respiratory Imaging as a Tool to Assess Upper Airway Patency in Children with Obstructive Sleep Apnea. Sleep Med. 2013, 14, 433–439. [Google Scholar] [CrossRef]
- Wilcox, L.J.; Bergeron, M.; Reghunathan, S.; Ishman, S.L. An Updated Review of Pediatric Drug-Induced Sleep Endoscopy. Laryngoscope Investig. Otolaryngol. 2017, 2, 423–431. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L. New Approaches to the Diagnosis of Sleep-Disordered Breathing in Children. Sleep Med. 2010, 11, 708–713. [Google Scholar] [CrossRef]
- Goodwin, J.L.; Enright, P.L.; Kaemingk, K.L.; Rosen, G.M.; Morgan, W.J.; Fregosi, R.F.; Quan, S.F. Feasibility of Using Unattended Polysomnography in Children for Research–Report of the Tucson Children’s Assessment of Sleep Apnea Study (TuCASA). Sleep 2001, 24, 937–944. [Google Scholar] [CrossRef]
- Marcus, C.L.; Traylor, J.; Biggs, S.N.; Roberts, R.S.; Nixon, G.M.; Narang, I.; Bhattacharjee, R.; Davey, M.J.; Horne, R.S.C.; Cheshire, M.; et al. Feasibility of Comprehensive, Unattended Ambulatory Polysomnography in School-Aged Children. J. Clin. Sleep Med. 2014, 10, 913–918. [Google Scholar] [CrossRef]
- Kirk, V.G.; Bohn, S.G.; Flemons, W.W.; Remmers, J.E. Comparison of Home Oximetry Monitoring with Laboratory Polysomnography in Children. Chest 2003, 124, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Jonas, C.; Thavagnanam, S.; Blecher, G.; Thambipillay, G.; Teng, A.Y. Comparison of Nocturnal Pulse Oximetry with Polysomnography in Children with Sleep Disordered Breathing. Sleep Breath. 2020, 24, 703–707. [Google Scholar] [CrossRef]
- Villa, M.P.; Pietropaoli, N.; Supino, M.C.; Vitelli, O.; Rabasco, J.; Evangelisti, M.; Del Pozzo, M.; Kaditis, A.G. Diagnosis of Pediatric Obstructive Sleep Apnea Syndrome in Settings with Limited Resources. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 990–996. [Google Scholar] [CrossRef]
- Contencin, P.; Malorgio, E.; Noce, S.; Couloigner, V.; Vigo, A. Questionnaire and Nocturnal Oxymetry in Children with Adenotonsillar Hypertrophy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2010, 127, 137–142. [Google Scholar] [CrossRef][Green Version]
- Mashaqi, S.; Lee-Iannotti, J.; Rangan, P.; Celaya, M.P.; Gozal, D.; Quan, S.F.; Parthasarathy, S. Obstructive Sleep Apnea and COVID-19 Clinical Outcomes during Hospitalization: A Cohort Study. J. Clin. Sleep Med. 2021, 17, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, M.; Corcione, A.; Cimbalo, C.; Annunziata, A.; Basilicata, S.; Fiorentino, G.; Santamaria, F. Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography. Children 2023, 10, 1331. https://doi.org/10.3390/children10081331
Borrelli M, Corcione A, Cimbalo C, Annunziata A, Basilicata S, Fiorentino G, Santamaria F. Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography. Children. 2023; 10(8):1331. https://doi.org/10.3390/children10081331
Chicago/Turabian StyleBorrelli, Melissa, Adele Corcione, Chiara Cimbalo, Anna Annunziata, Simona Basilicata, Giuseppe Fiorentino, and Francesca Santamaria. 2023. "Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography" Children 10, no. 8: 1331. https://doi.org/10.3390/children10081331
APA StyleBorrelli, M., Corcione, A., Cimbalo, C., Annunziata, A., Basilicata, S., Fiorentino, G., & Santamaria, F. (2023). Diagnosis of Paediatric Obstructive Sleep-Disordered Breathing beyond Polysomnography. Children, 10(8), 1331. https://doi.org/10.3390/children10081331





