Impact of Chemicals on the Age of Menarche: A Literature Review
Abstract
1. Introduction
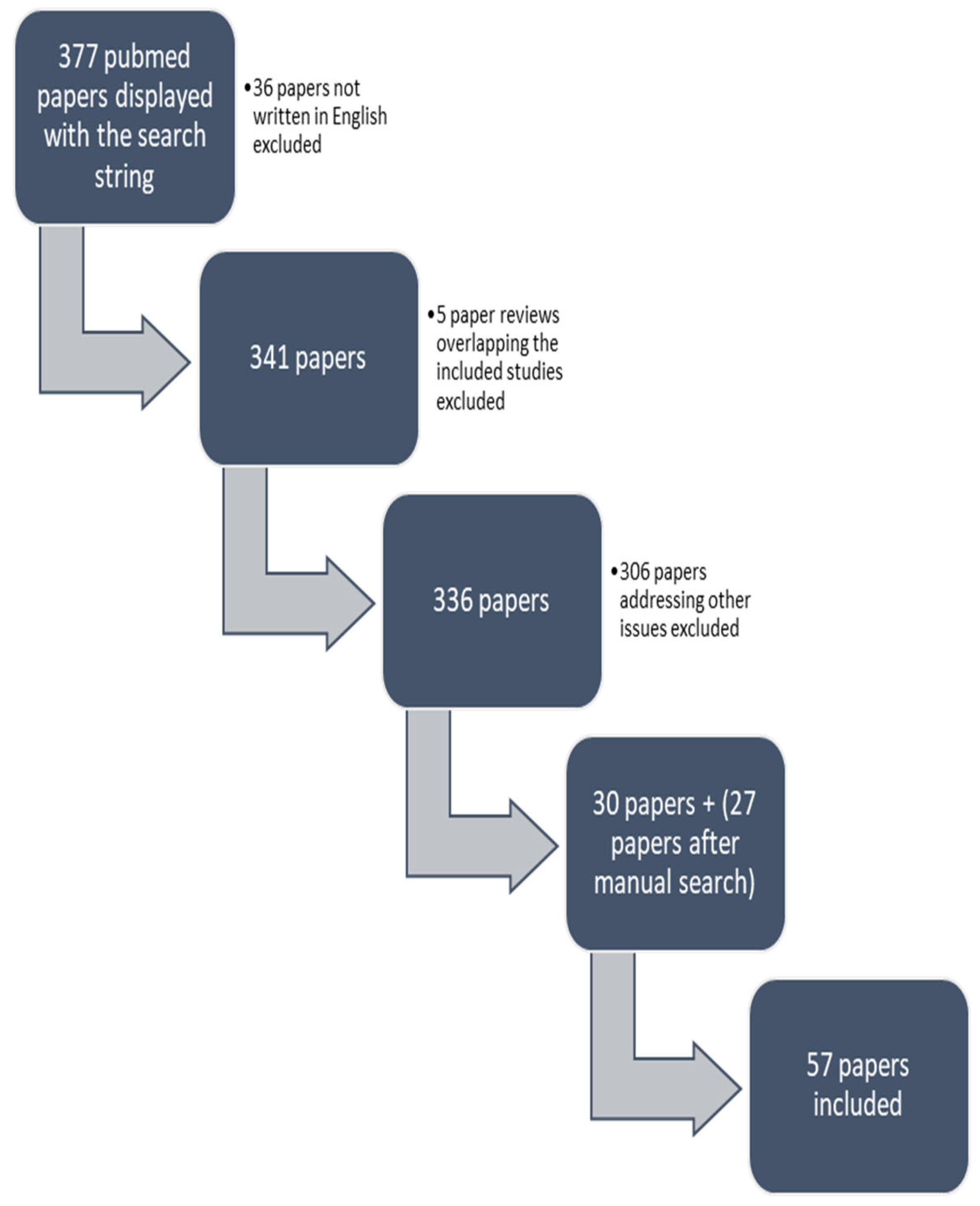
2. Materials and Methods
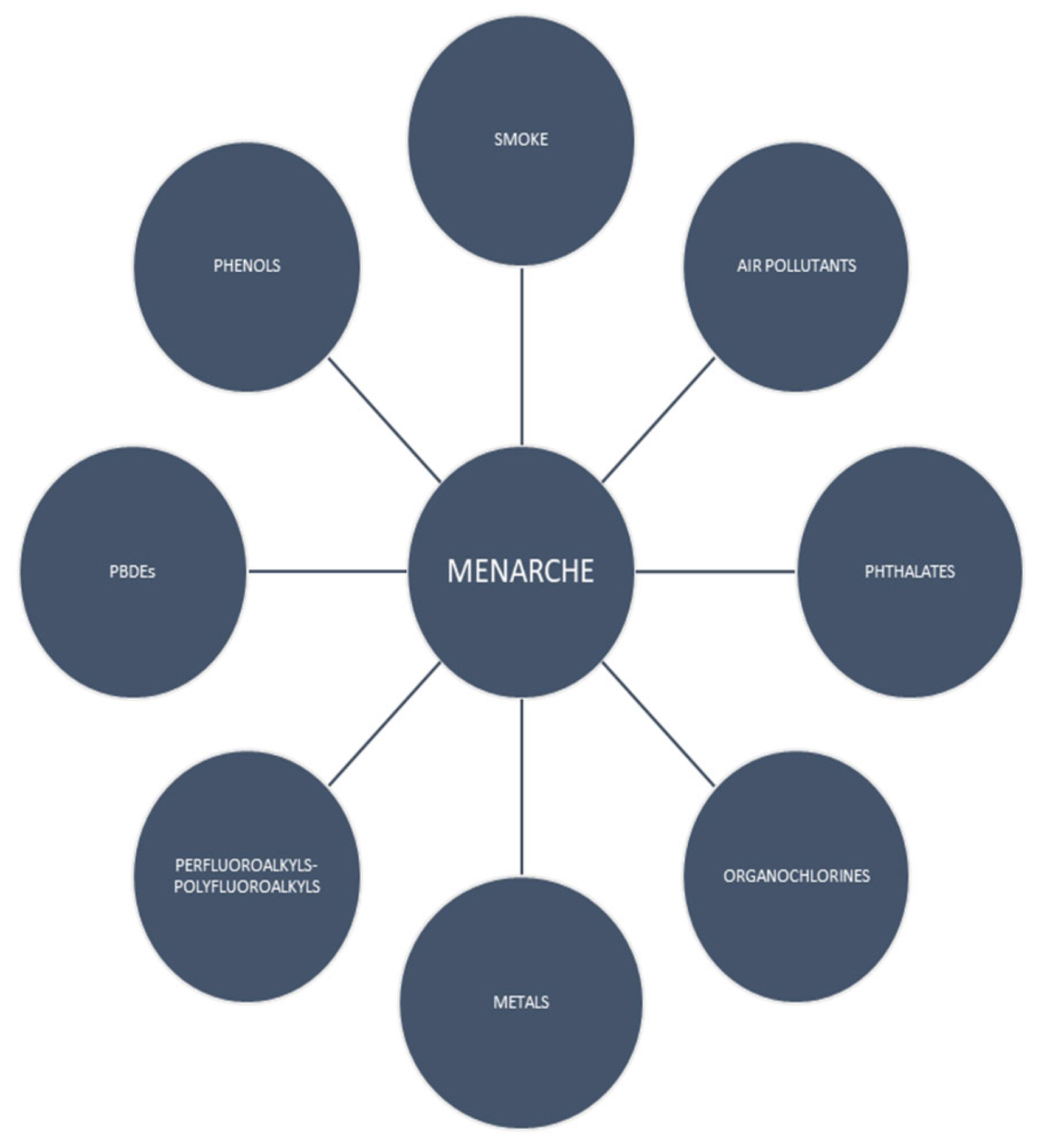
3. Results
3.1. Smoke Exposure
3.2. Phthalates
3.3. Phenols
3.4. Organochlorines
3.5. Perfluoroalkyls and Polyfluoroalkyls
3.6. Metals
3.7. Air Pollutants
3.8. Polybrominated Diphenyl Ethers
3.9. Mixture of Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, G.M. Trends and Patterns in Menarche in the United States: 1995 through 2013–2017. Natl. Health Stat. Rep. 2020, 1–12. [Google Scholar]
- Gaudineau, A.; Ehlinger, V.; Vayssière, C.; Jouret, B.; Arnaud, C.; Godeau, E. Âge à la menarche: Résultats français de l’étude Health Behaviour in School-aged Children. Gynécol. Obstet. Fertil. 2010, 38, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahab, B.; Ardern, C.I.; Hamadeh, M.J.; Tamim, H. Age at menarche in Canada: Results from the National Longitudinal Survey of Children & Youth. BMC Public Health 2010, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Luo, D.; Yan, X.; Zhang, J.; Hu, P.; Ma, J.; Song, Y.; Lau, P.W.C. The mean age of menarche among Chinese schoolgirls declined by 6 months from 2005 to 2014. Acta Paediatr. 2021, 110, 549–555. [Google Scholar] [CrossRef]
- Papadimitriou, A. The Evolution of the Age at Menarche from Prehistorical to Modern Times. J. Pediatr. Adolesc. Gynecol. 2016, 29, 527–530. [Google Scholar] [CrossRef]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.-M.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef]
- Roy, J.R.; Chakraborty, S.; Chakraborty, T.R. Estrogen-like endocrine disrupting chemicals affecting puberty in humans—A review. Experiment 2009, 15, RA137-45. [Google Scholar]
- Zhang, B.; Shi, H.; Wang, Q.; Zhang, Z.; Li, M. Maternal Passive Smoking during Pregnancy and Age of Menarche in Daughters. Asia Pac. J. Public Health 2015, 27, 14S–20S. [Google Scholar] [CrossRef]
- Behie, A.; O’Donnell, M. Prenatal smoking and age at menarche: Influence of the prenatal environment on the timing of puberty. Hum. Reprod. 2015, 30, 957–962. [Google Scholar] [CrossRef]
- Houghton, L.C.; Goldberg, M.; Wei, Y.; Cirillo, P.M.; Cohn, B.A.; Michels, K.B.; Terry, M.B. Why do studies show different associations between intrauterine exposure to maternal smoking and age at menarche? Ann. Epidemiol. 2018, 28, 197–203. [Google Scholar] [CrossRef]
- Windham, G.C.; Bottomley, C.; Birner, C.; Fenster, L. Age at Menarche in Relation to Maternal Use of Tobacco, Alcohol, Coffee, and Tea during Pregnancy. Am. J. Epidemiol. 2004, 159, 862–871. [Google Scholar] [CrossRef]
- Yermachenko, A.; Dvornyk, V. A meta-analysis provides evidence that prenatal smoking exposure decreases age at menarche. Reprod. Toxicol. 2015, 58, 222–228. [Google Scholar] [CrossRef]
- Kang, S.; Joo, J.; Jang, S.; Park, E. Association of exposure to secondhand smoke at home with early age at menarche in South Korea. Public Health 2020, 185, 144–149. [Google Scholar] [CrossRef]
- Yang, S.; Jin, Y.; He, Y.; Jiang, C.; Cheng, K.K.; Zhang, W.; Lam, T.H. Childhood Passive Smoking Exposure and Age at Menarche in Chinese Women Who Had Never Smoked: The Guangzhou Biobank Cohort Study. PLoS ONE 2015, 10, e0130429. [Google Scholar] [CrossRef]
- Ferris, J.S.; Flom, J.D.; Tehranifar, P.; Mayne, S.T.; Terry, M.B. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr. Périnat. Epidemiol. 2010, 24, 515–523. [Google Scholar] [CrossRef]
- Watkins, D.J.; Téllez-Rojo, M.M.; Ferguson, K.K.; Lee, J.M.; Solano-Gonzalez, M.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ. Res. 2014, 134, 233–241. [Google Scholar] [CrossRef]
- Watkins, D.J.; Sánchez, B.N.; Téllez-Rojo, M.M.; Lee, J.M.; Mercado-García, A.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res. 2017, 159, 143–151. [Google Scholar] [CrossRef]
- Oskar, S.; Wolff, M.S.; Teitelbaum, S.L.; Stingone, J.A. Identifying environmental exposure profiles associated with timing of menarche: A two-step machine learning approach to examine multiple environmental exposures. Environ. Res. 2021, 195, 110524. [Google Scholar] [CrossRef]
- Cathey, A.; Watkins, D.J.; Sánchez, B.N.; Tamayo-Ortiz, M.; Solano-Gonzalez, M.; Torres-Olascoaga, L.; Téllez-Rojo, M.M.; Peterson, K.E.; Meeker, J.D. Onset and tempo of sexual maturation is differentially associated with gestational phthalate exposure between boys and girls in a Mexico City birth cohort. Environ. Int. 2020, 136, 105469. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G.; et al. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 97004. [Google Scholar] [CrossRef]
- Hart, R.; Doherty, D.A.; Frederiksen, H.; Keelan, J.A.; Hickey, M.; Sloboda, D.; Pennell, C.E.; Newnham, J.P.; Skakkebaek, N.E.; Main, K.M. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: A pilot study. Reproduction 2014, 147, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kasper-Sonnenberg, M.; Wittsiepe, J.; Wald, K.; Koch, H.M.; Wilhelm, M. Pre-pubertal exposure with phthalates and bisphenol A and pubertal development. PLoS ONE 2017, 12, e0187922. [Google Scholar] [CrossRef] [PubMed]
- Berman, Y.E.; Doherty, D.A.; Main, K.M.; Frederiksen, H.; Hickey, M.; Keelan, J.A.; Newnham, J.P.; Hart, R.J. Associations between Prenatal Exposure to Phthalates and Timing of Menarche and Growth and Adiposity into Adulthood: A Twenty-Years Birth Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4725. [Google Scholar] [CrossRef] [PubMed]
- Park, O.; Park, J.-T.; Chi, Y.; Kwak, K. Association of phthalates and early menarche in Korean adolescent girls from Korean National Environmental Health Survey (KoNEHS) 2015–2017. Ann. Occup. Environ. Med. 2021, 33, e4. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Shi, H.; Jiang, X.; Zhao, Y.; Fang, X.; Xie, C. Could exposure to phthalates speed up or delay pubertal onset and development? A 1.5-year follow-up of a school-based population. Environ. Int. 2015, 83, 41–49. [Google Scholar] [CrossRef]
- McGuinn, L.A.; Ghazarian, A.A.; Su, L.J.; Ellison, G.L. Urinary bisphenol A and age at menarche among adolescent girls: Evidence from NHANES 2003–2010. Environ. Res. 2015, 136, 381–386. [Google Scholar] [CrossRef]
- Miao, M.; Wang, Z.; Liu, X.; Liang, H.; Zhou, Z.; Tan, H.; Yuan, W.; Li, D.-K. Urinary bisphenol A and pubertal development in Chinese school-aged girls: A cross-sectional study. Environ. Health 2017, 16, 80. [Google Scholar] [CrossRef]
- Wolff, M.S.; Pajak, A.; Pinney, S.M.; Windham, G.C.; Galvez, M.; Rybak, M.; Silva, M.J.; Ye, X.; Calafat, A.M.; Kushi, L.H.; et al. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod. Toxicol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Bigambo, F.M.; Zhang, M.; Zhang, J.; Yang, X.; Yu, Q.; Wu, D.; Wang, X.; Xia, Y. Exposure to a mixture of personal care product and plasticizing chemicals in relation to reproductive hormones and menarche timing among 12–19 years old girls in NHANES 2013–2016. Food Chem. Toxicol. 2022, 170, 113463. [Google Scholar] [CrossRef]
- Binder, A.M.; Corvalan, C.; Calafat, A.M.; Ye, X.; Mericq, V.; Pereira, A.; Michels, K.B. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ. Health 2018, 17, 32. [Google Scholar] [CrossRef]
- Buttke, D.E.; Sircar, K.; Martin, C. Exposures to Endocrine-Disrupting Chemicals and Age of Menarche in Adolescent Girls in NHANES (2003–2008). Environ. Health Perspect. 2012, 120, 1613–1618. [Google Scholar] [CrossRef]
- Harley, K.G.; Berger, K.P.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Calafat, A.M.; Ye, X.; Eskenazi, B. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum. Reprod. 2019, 34, 109–117. [Google Scholar] [CrossRef]
- Denham, M.; Schell, L.M.; Deane, G.; Gallo, M.V.; Ravenscroft, J.; DeCaprio, A.P.; Environment, T.A.T.F.O.T. Relationship of Lead, Mercury, Mirex, Dichlorodiphenyldichloroethylene, Hexachlorobenzene, and Polychlorinated Biphenyls to Timing of Menarche Among Akwesasne Mohawk Girls. Pediatrics 2005, 115, e127–e134. [Google Scholar] [CrossRef]
- Hond, E.D.; Dhooge, W.; Bruckers, L.; Schoeters, G.; Nelen, V.; van de Mieroop, E.; Koppen, G.; Bilau, M.; Schroijen, C.; Keune, H.; et al. Internal exposure to pollutants and sexual maturation in Flemish adolescents. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 224–233. [Google Scholar] [CrossRef]
- Attfield, K.R.; Pinney, S.M.; Sjödin, A.; Voss, R.W.; Greenspan, L.C.; Biro, F.M.; Hiatt, R.A.; Kushi, L.H.; Windham, G.C. Longitudinal study of age of menarche in association with childhood concentrations of persistent organic pollutants. Environ. Res. 2019, 176, 108551. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Perry, M.J.; Venners, S.A.; Chen, C.; Wang, B.; Yang, F.; Fang, Z.; Zang, T.; Wang, L.; Xu, X.; et al. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup. Environ. Med. 2005, 62, 878–884. [Google Scholar] [CrossRef]
- Cirillo, P.M.; La Merrill, M.A.; Krigbaum, N.Y.; Cohn, B.A. Grandmaternal Perinatal Serum DDT in Relation to Granddaughter Early Menarche and Adult Obesity: Three Generations in the Child Health and Development Studies Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1480–1488. [Google Scholar] [CrossRef]
- Vasiliu, O.; Muttineni, J.; Karmaus, W. In utero exposure to organochlorines and age at menarche. Hum. Reprod. 2004, 19, 1506–1512. [Google Scholar] [CrossRef]
- Gladen, B.C.; Ragan, N.; Rogan, W.J. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J. Pediatr. 2000, 136, 490–496. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ramlau-Hansen, C.H.; Ernst, E.; Olsen, S.F.; Bonde, J.P.; Vested, A.; Halldorsson, T.I.; Rantakokko, P.; Kiviranta, H.; Toft, G. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ. Int. 2016, 92–93, 366–372. [Google Scholar] [CrossRef]
- Namulanda, G.; Maisonet, M.; Taylor, E.; Flanders, W.D.; Olson, D.; Sjodin, A.; Qualters, J.R.; Vena, J.; Northstone, K.; Naeher, L. In utero exposure to organochlorine pesticides and early menarche in the Avon Longitudinal Study of Parents and Children. Environ. Int. 2016, 94, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Axmon, A. Menarche in women with high exposure to persistent organochlorine pollutants in utero and during childhood. Environ. Res. 2006, 102, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Ramlau-Hansen, C.H.; Ernst, E.; Olsen, S.F.; Bonde, J.P.; Vested, A.; Halldorsson, T.I.; Becher, G.; Haug, L.S.; Toft, G. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum. Reprod. 2013, 28, 3337–3348. [Google Scholar] [CrossRef]
- Christensen, K.Y.; Maisonet, M.; Rubin, C.; Holmes, A.; Calafat, A.M.; Kato, K.; Flanders, W.D.; Heron, J.; McGeehin, M.A.; Marcus, M. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ. Int. 2013, 37, 129–135. [Google Scholar] [CrossRef]
- Lopez-Espinosa, M.-J.; Fletcher, T.; Armstrong, B.; Genser, B.; Dhatariya, K.; Mondal, D.; Ducatman, A.; Leonardi, G. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Age of Puberty among Children Living near a Chemical Plant. Environ. Sci. Technol. 2011, 45, 8160–8166. [Google Scholar] [CrossRef]
- Ernst, A.; Brix, N.; Lauridsen, L.L.B.; Olsen, J.; Parner, E.T.; Liew, Z.; Olsen, L.H.; Ramlau-Hansen, C.H. Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, 17004. [Google Scholar] [CrossRef]
- Wu, T.; Buck, G.M.; Mendola, P. Blood lead levels and sexual maturation in U.S. girls: The Third National Health and Nutrition Examination Survey, 1988–1994. Environ. Health Perspect. 2003, 111, 737–741. [Google Scholar] [CrossRef]
- Selevan, S.G.; Rice, D.C.; Hogan, K.A.; Euling, S.Y.; Pfahles-Hutchens, A.; Bethel, J. Blood Lead Concentration and Delayed Puberty in Girls. N. Engl. J. Med. 2003, 348, 1527–1536. [Google Scholar] [CrossRef]
- Reynolds, P.; Canchola, A.J.; Duffy, C.N.; Hurley, S.; Neuhausen, S.L.; Horn-Ross, P.L.; Rull, R.P. Urinary cadmium and timing of menarche and pubertal development in girls. Environ. Res. 2020, 183, 109224. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Jin, T. Effects of Cadmium Exposure on Age of Menarche and Menopause. Toxics 2017, 6, 6. [Google Scholar] [CrossRef]
- Igra, A.M.; Rahman, A.; Johansson, A.L.; Pervin, J.; Svefors, P.; El Arifeen, S.; Vahter, M.; Persson, L.; Kippler, M. Early Life Environmental Exposure to Cadmium, Lead, and Arsenic and Age at Menarche: A Longitudinal Mother–Child Cohort Study in Bangladesh. Environ. Health Perspect. 2023, 131, 27003. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Sánchez, B.N.; Téllez-Rojo, M.M.; Basu, N.; Tamayo-Ortiz, M.; Peterson, K.E.; Meeker, J.D.; Watkins, D.J. In utero and peripubertal metals exposure in relation to reproductive hormones and sexual maturation and progression among girls in Mexico City. Environ. Res. 2019, 177, 108630. [Google Scholar] [CrossRef] [PubMed]
- Sen, J.; Chaudhuri, A.B.D. Effect of Arsenic on the Onset of Menarcheal Age. Bull. Environ. Contam. Toxicol. 2007, 79, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Huang, T.-S.; Lin, K.-C.; Kuo, P.; Tsai, P.-C.; Guo, Y.L. Menstrual effects among women exposed to polychlorinated biphenyls and dibenzofurans. Environ. Res. 2011, 111, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hond, E.D.; Roels, H.A.; Hoppenbrouwers, K.; Nawrot, T.; Thijs, L.; Vandermeulen, C.; Winneke, G.; Vanderschueren, D.; Staessen, J.A. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ. Health Perspect. 2002, 110, 771–776. [Google Scholar] [CrossRef]
- Warner, M.; Samuels, S.; Mocarelli, P.; Gerthoux, P.M.; Needham, L.; Patterson, D.G.; Eskenazi, B. Serum Dioxin Concentrations and Age at Menarche. Environ. Health Perspect. 2004, 112, 1289–1292. [Google Scholar] [CrossRef]
- Blanck, H.M.; Marcus, M.; Tolbert, P.E.; Rubin, C.; Henderson, A.K.; Hertzberg, V.S.; Zhang, R.H.; Cameron, L. Age at Menarche and Tanner Stage in Girls Exposed In Utero and Postnatally to Polybrominated Biphenyl. Epidemiology 2000, 11, 641–647. [Google Scholar] [CrossRef]
- Jung, E.M.; Kim, H.S.; Park, H.; Ye, S.; Lee, D.; Ha, E.H. Does exposure to PM10 decrease age at menarche? Environ. Int. 2018, 117, 16–21. [Google Scholar] [CrossRef]
- Wronka, I.; Kliś, K. Effect of air pollution on age at menarche in polish females, born 1993–1998. Sci. Rep. 2022, 12, 4820. [Google Scholar] [CrossRef]
- John, E.M.; Keegan, T.H.; Terry, M.B.; Koo, J.; Ingles, S.A.; Nguyen, J.T.; Thomsen, C.; Santella, R.M.; Nguyen, K.; Yan, B. Urinary biomarkers of polycyclic aromatic hydrocarbons (PAHs) and timing of pubertal development: The California PAH Study. Epidemiology 2022, 33, 777–787. [Google Scholar] [CrossRef]
- Kehm, R.D.; Oskar, S.; Tehranifar, P.; Zeinomar, N.; Rundle, A.G.; Herbstman, J.B.; Perera, F.; Miller, R.L.; Terry, M.B. Associations of prenatal exposure to polycyclic aromatic hydrocarbons with pubertal timing and body composition in adolescent girls: Implications for breast cancer risk. Environ. Res. 2021, 196, 110369. [Google Scholar] [CrossRef]
- Chen, A.; Chung, E.; DeFranco, E.A.; Pinney, S.M.; Dietrich, K.N. Serum PBDEs and age at menarche in adolescent girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ. Res. 2011, 111, 831–837. [Google Scholar] [CrossRef]
- Harley, K.G.; Rauch, S.A.; Chevrier, J.; Kogut, K.; Parra, K.L.; Trujillo, C.; Lustig, R.H.; Greenspan, L.C.; Sjödin, A.; Bradman, A.; et al. Association of prenatal and childhood PBDE exposure with timing of puberty in boys and girls. Environ. Int. 2017, 100, 132–138. [Google Scholar] [CrossRef]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Calafat, A.M.; Sjödin, A.; Marcus, M.; Hartman, T.J. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and early menarche in a population-based cohort of British girls. Environ. Pollut. 2021, 276, 116705. [Google Scholar] [CrossRef]
- Lutterodt, M.; Sorensen, K.; Larsen, K.; Skouby, S.; Andersen, C.Y.; Byskov, A. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum. Reprod. 2009, 24, 2558–2566. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Lee, C.-C.; Wang, J.-Y.; Li, Y.-C.; Chang, H.-W.; Chen, C.-Y.; Bornehag, C.-G.; Wu, P.-C.; Sundell, J.; Su, H.-J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef]
- Janjua, N.R.; Frederiksen, H.; Skakkebæk, N.E.; Wulf, H.C.; Andersson, A.-M. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl. 2008, 31, 118–130. [Google Scholar] [CrossRef]
- Mose, T.; Mortensen, G.K.; Hedegaard, M.; Knudsen, L.E. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol. 2007, 23, 83–91. [Google Scholar] [CrossRef]
- Adibi, J.J.; Whyatt, R.M.; Hauser, R.; Bhat, H.K.; Davis, B.J.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Tang, D.; Williams, P.L. Transcriptional Biomarkers of Steroidogenesis and Trophoblast Differentiation in the Placenta in Relation to Prenatal Phthalate Exposure. Environ. Health Perspect. 2010, 118, 291–296. [Google Scholar] [CrossRef]
- Chao, H.-H.; Zhang, X.-F.; Chen, B.; Pan, B.; Zhang, L.-J.; Li, L.; Sun, X.-F.; Shi, Q.-H.; Shen, W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 2012, 137, 249–259. [Google Scholar] [CrossRef]
- Colborn, T.; Saal, F.S.V.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Huber, S.; Becher, G.; Thomsen, C. Characterisation of human exposure pathways to perfluorinated compounds—Comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011, 37, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Gützkow, K.B.; Haug, L.S.; Thomsen, C.; Sabaredzovic, A.; Becher, G.; Brunborg, G. Placental transfer of perfluorinated compounds is selective—A Norwegian Mother and Child sub-cohort study. Int. J. Hyg. Environ. Health 2012, 215, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of Estrogen Receptor-? by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef]
- Lorber, M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 2–19. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Tagliaferri, S.; Caglieri, A.; Mutti, A. Polybrominated diphenyl ether (PBDE) flame retardants: Environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008, 79, 172–183. [Google Scholar]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.A.; Andersson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-Disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadis, X.; Matsas, A.; Panoskaltsis, T.; Bakas, P.; Papadimitriou, D.T.; Christopoulos, P. Impact of Chemicals on the Age of Menarche: A Literature Review. Children 2023, 10, 1234. https://doi.org/10.3390/children10071234
Anastasiadis X, Matsas A, Panoskaltsis T, Bakas P, Papadimitriou DT, Christopoulos P. Impact of Chemicals on the Age of Menarche: A Literature Review. Children. 2023; 10(7):1234. https://doi.org/10.3390/children10071234
Chicago/Turabian StyleAnastasiadis, Xristos, Alkis Matsas, Theodoros Panoskaltsis, Panagiotis Bakas, Dimitrios T. Papadimitriou, and Panagiotis Christopoulos. 2023. "Impact of Chemicals on the Age of Menarche: A Literature Review" Children 10, no. 7: 1234. https://doi.org/10.3390/children10071234
APA StyleAnastasiadis, X., Matsas, A., Panoskaltsis, T., Bakas, P., Papadimitriou, D. T., & Christopoulos, P. (2023). Impact of Chemicals on the Age of Menarche: A Literature Review. Children, 10(7), 1234. https://doi.org/10.3390/children10071234









