Symptom Profiles of Children and Young People 12 Months after SARS-CoV-2 Testing: A National Matched Cohort Study (The CLoCk Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Measures
2.2. Statistical Methods
3. Results
3.1. Sample Characteristics
3.2. Symptoms 12 Months Post-Index-Test by Infection Status
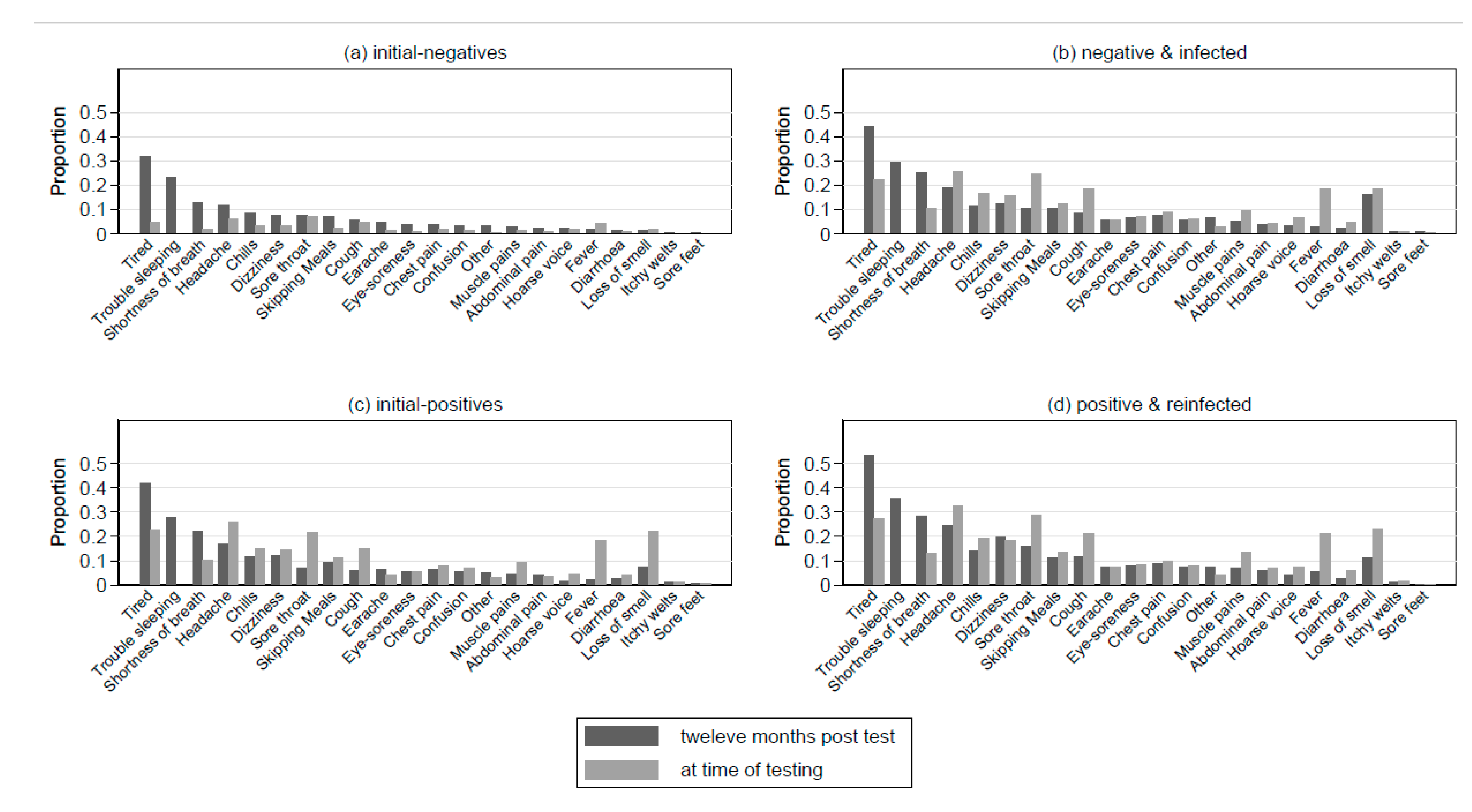
3.3. Specific Symptoms, Quality of Life/Function, and PCC 12-Months Post-Index-Test
3.4. Comparing Symptom Profiles of the PP Group to the NP and PN Groups
3.5. Symptom Profiles by Vaccination Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of Post-Acute Covid-19 in Primary Care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Case Report and Systematic Review Suggest That Children May Experience Similar Long-Term Effects to Adults after Clinical COVID-19. Acta Paediatr. 2021, 110, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness Duration and Symptom Profile in Symptomatic UK School-Aged Children Tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Wilde, H.; Tomlinson, C.; Mateen, B.A.; Selby, D.; Kanthimathinathan, H.K.; Ramnarayan, P.; Du Pre, P.; Johnson, M.; Pathan, N.; Gonzalez-Izquierdo, A.; et al. Hospital Admissions Linked to SARS-CoV-2 Infection in Children and Adolescents: Cohort Study of 3.2 Million First Ascertained Infections in England. BMJ 2023, 382, e073639. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK Patients in Hospital with Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Swann, O.V.; Holden, K.A.; Turtle, L.; Pollock, L.; Fairfield, C.J.; Drake, T.M.; Seth, S.; Egan, C.; Hardwick, H.E.; Halpin, S.; et al. Clinical Characteristics of Children and Young People Admitted to Hospital with Covid-19 in United Kingdom: Prospective Multicentre Observational Cohort Study. BMJ 2020, 370, m3249. [Google Scholar] [CrossRef]
- Zimmermann, P.; Pittet, L.F.; Curtis, N. Long Covid in Children and Adolescents Risk Appears Low, but Many Questions Remain. BMJ 2022, 376, e066809. [Google Scholar] [CrossRef]
- Pinto Pereira, S.M.; Shafran, R.; Nugawela, M.D.; Panagi, L.; Hargreaves, D.; Ladhani, S.N.; Bennett, S.D.; Chalder, T.; Dalrymple, E.; Ford, T.; et al. Natural Course of Health and Well-Being in Non-Hospitalised Children and Young People after Testing for SARS-CoV-2: A Prospective Follow-up Study over 12 Months. Lancet Reg. Health-Eur. 2023, 25, 100554. [Google Scholar] [CrossRef]
- Stephenson, T.; Allin, B.; Nugawela, M.D.; Rojas, N.; Dalrymple, E.; Pinto Pereira, S.; Soni, M.; Knight, M.; Cheung, E.Y.; Heyman, I.; et al. Long COVID (Post-COVID-19 Condition) in Children: A Modified Delphi Process. Arch. Dis. Child 2022, 107, 674–680. [Google Scholar] [CrossRef]
- COVID-19 Schools Infection Survey, England—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyengland/pupilantibodiesandvaccinesentimentmarch2022 (accessed on 19 December 2022).
- Benhood, S.; Newlands, F.; O’Mahoney, L.; Takeda, A.; Haghighat Ghahfarokhi, M.; Bennett, S.; Stephenson, T.; Ladhani, S.N.; Viner, R.M.; Swann, O.; et al. An Updated Systematic Review and Meta-Analysis Conducted by UCL Great Ormond Street Institute of Child in Collaboration with the World Health Organisation; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- Sigfrid, L.; Buonsenso, D.; DunnGalvin, A.; Garralda, E.; Jones, C.; Nicholls, D.E. ISARIC Global COVID-19 Paediatric Follow-Up. Available online: https://isaric.org/research/covid-19-clinical-research-resources/paediatric-follow-up/ (accessed on 8 July 2023).
- NHS Digital Mental Health of Children and Young People in England, 2020: Wave 1 Follow Up to the 2017 Survey. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2020-wave-1-follow-up (accessed on 8 July 2023).
- Stephenson, T.; Pinto Pereira, S.M.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and Mental Health 3 Months after SARS-CoV-2 Infection (Long COVID) among Adolescents in England (CLoCk): A National Matched Cohort Study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. Psychometric Properties of the Strengths and Difficulties Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh Mental Well-Being Scale (WEMWBS): Development and UK Validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a Fatigue Scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Wille, N.; Badia, X.; Bonsel, G.; Burström, K.; Cavrini, G.; Devlin, N.; Egmar, A.-C.; Greiner, W.; Gusi, N.; Herdman, M.; et al. Development of the EQ-5D-Y: A Child-Friendly Version of the EQ-5D. Qual. Life Res. 2010, 19, 875–886. [Google Scholar] [CrossRef]
- McKay, M.T.; Andretta, J.R. Evidence for the Psychometric Validity, Internal Consistency and Measurement Invariance of Warwick Edinburgh Mental Well-Being Scale Scores in Scottish and Irish Adolescents. Psychiatry Res. 2017, 255, 382–386. [Google Scholar] [CrossRef]
- Ringdal, R.; Bradley Eilertsen, M.-E.; Bjørnsen, H.N.; Espnes, G.A.; Moksnes, U.K. Validation of Two Versions of the Warwick-Edinburgh Mental Well-Being Scale among Norwegian Adolescents. Scand. J. Public Health 2018, 46, 718–725. [Google Scholar] [CrossRef]
- Child Outcomes Research Consortium Short Warwick-Edinburgh Mental Wellbeing Scale (SWEMWS). Available online: https://www.corc.uk.net/outcome-experience-measures/short-warwick-edinburgh-mental-wellbeing-scale-swemws/ (accessed on 7 July 2023).
- Clarke, A.; Putz, R.; Friede, T.; Ashdown, J.; Adi, Y.; Martin, S.; Flynn, P.; Blake, A.; Stewart-Brown, S.; Platt, S. Warwick-Edinburgh Mental Well-Being Scale (WEMWBS) Acceptability and Validation in English and Scottish Secondary School Students (The WAVES Project); NHS Health Scotland: Edinburgh, UK, 2010. [Google Scholar]
- Morriss, R.; Wearden, A.; Mullis, R. Exploring the Validity of the Chalder Fatigue Scale in Chronic Fatigue Syndrome. J. Psychosom. Res. 1998, 45, 411–417. [Google Scholar] [CrossRef]
- Loge, J.H.; Ekeberg, Ø.; Kaasa, S. Fatigue in the General Norwegian Population. J. Psychosom. Res. 1998, 45, 53–65. [Google Scholar] [CrossRef]
- EuroQol Research Foundation EQ-5D-3L|About. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/ (accessed on 7 July 2023).
- Ravens-Sieberer, U.; Wille, N.; Badia, X.; Bonsel, G.; Burström, K.; Cavrini, G.; Devlin, N.; Egmar, A.-C.; Gusi, N.; Herdman, M.; et al. Feasibility, Reliability, and Validity of the EQ-5D-Y: Results from a Multinational Study. Qual. Life Res. 2010, 19, 887–897. [Google Scholar] [CrossRef]
- Donnachie, E.; Hapfelmeier, A.; Linde, K.; Tauscher, M.; Gerlach, R.; Greissel, A.; Schneider, A. Incidence of Post-COVID Syndrome and Associated Symptoms in Outpatient Care in Bavaria, Germany: A Retrospective Cohort Study Using Routinely Collected Claims Data. BMJ Open 2022, 12, e064979. [Google Scholar] [CrossRef]
- Roessler, M.; Tesch, F.; Batram, M.; Jacob, J.; Loser, F.; Weidinger, O.; Wende, D.; Vivirito, A.; Toepfner, N.; Seifert, M.; et al. Post COVID-19 in Children, Adolescents, and Adults: Results of a Matched Cohort Study Including More than 150,000 Individuals with COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID Symptoms and Duration in SARS-CoV-2 Positive Children—A Nationwide Cohort Study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef]
- Kikkenborg Berg, S.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID Symptoms in SARS-CoV-2-Positive Children Aged 0–14 Years and Matched Controls in Denmark (LongCOVIDKidsDK): A National, Cross-Sectional Study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Kikkenborg Berg, S.; Dam Nielsen, S.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Vinggaard Christensen, A. Long COVID Symptoms in SARS-CoV-2-Positive Adolescents and Matched Controls (LongCOVIDKidsDK): A National, Cross-Sectional Study. Lancet Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Rojas, N.; De Stavola, L.B.; Norris, T.; Cortina-Borja, M.; Nugawela, D.M.; Hargreaves, D.; Dalrymple, E.; McOwat, K.; Simmons, R.; Stephenson, T.; et al. Developing Survey Weights to Ensure Representativeness in a National, Matched Cohort Study: Results from the Children and Young People with Long Covid (CLoCk) Study. BMC Med. Res. Methodol. 2023. [Google Scholar] [CrossRef]
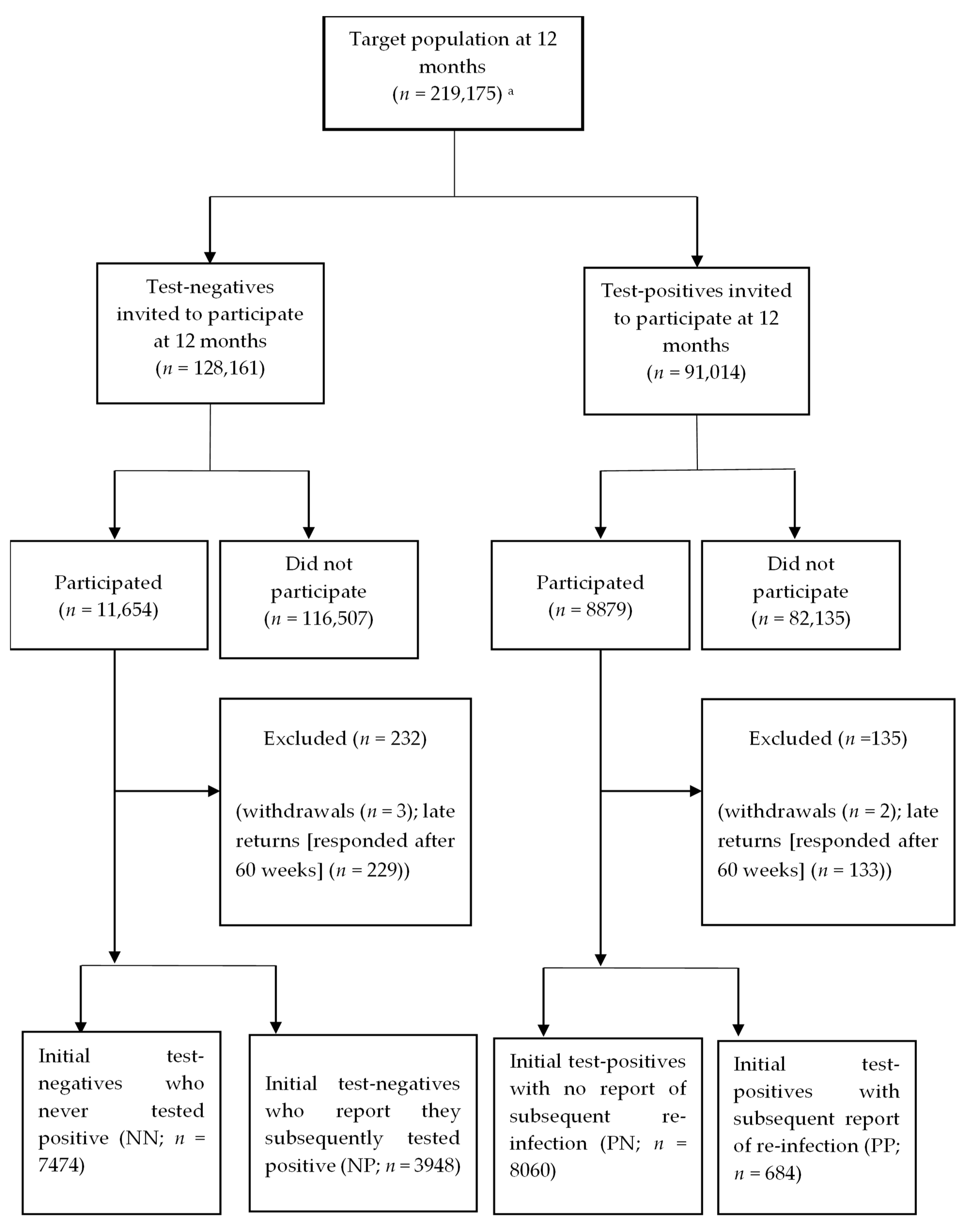

| Target Test-Negative Population (n = 128,161) | Initial-Negatives (NN) (n = 7474) | Negatives and Infected (NP) (n = 3948) | Target Test-Positive Population (n = 91,014) | Initial-Positives (PN) (n = 8060) | Positive-Reinfected (PP) (n = 684) | |
|---|---|---|---|---|---|---|
| Response rate: 8.9% | Response rate: 9.6% | |||||
| Sex | ||||||
| Female | 67,194 (52.4%) | 4644 (62.1%) | 2466 (62.5%) | 48,042 (52.8%) | 5023 (62.3%) | 420 (61.4%) |
| Male | 60,967 (47.6%) | 2830 (37.9%) | 1482 (37.5%) | 42,972 (47.2%) | 3037 (37.7) | 264 (38.6%) |
| Age (years) | ||||||
| 11–14 | 65,951 (51.5%) | 3481 (46.6%) | 2271 (57.5%) | 46,106 (50.7%) | 3892 (48.3%) | 369 (54.0%) |
| 15–17 | 62,210 (48.5%) | 3993 (53.4%) | 1677 (42.5%) | 44,908 (49.3%) | 4168 (51.7%) | 315 (46.0%) |
| Ethnicity | Not recorded | Not recorded | ||||
| White | 5529 (74.0%) | 3141 (79.6%) | 6001 (74.5%) | 529 (77.3%) | ||
| Asian, Asian British | 1143 (15.3%) | 420 (10.6%) | 1235 (15.3%) | 90 (13.2%) | ||
| Mixed | 421 (5.6%) | 224 (5.7%) | 422 (5.2%) | 34 (5.0%) | ||
| Black, African, Caribbean | 245 (3.3%) | 92 (2.3%) | 222 (2.8%) | 19 (2.8%) | ||
| Other | 94 (1.3%) | 59 (1.5%) | 140 (1.7%) | 7 (1.0%) | ||
| Unknown | 42 (0.6%) | 12 (0.3%) | 40 (0.5%) | 5 (0.7%) | ||
| Region | ||||||
| East Midlands | 7861 (6.1%) | 401 (5.4%) | 208 (5.3%) | 6248 (6.9%) | 519 (6.4%) | 54 (7.9%) |
| East of England | 24,919 (19.4%) | 1906 (25.5%) | 1232 (31.2%) | 13,982 (15.4%) | 1552 (19.3%) | 127 (18.6%) |
| London | 27,156 (21.2%) | 1832 (24.5%) | 831 (21.1%) | 19,144 (21.0%) | 1782 (22.1%) | 161 (23.5%) |
| North East England | 4825 (3.8%) | 199 (2.7%) | 84 (2.1%) | 3788 (4.2%) | 284 (3.5%) | 28 (4.1%) |
| North West England | 17,950 (14.0%) | 704 (9.4%) | 300 (7.6%) | 13,339 (14.7%) | 872 (10.8%) | 67 (9.8%) |
| South East England | 18,251 (14.2%) | 1236 (16.5%) | 730 (18.5%) | 13,316 (14.6%) | 1431 (17.8%) | 100 (14.6%) |
| South West England | 4563 (3.6%) | 240 (3.2%) | 129 (3.3%) | 3576 (3.9%) | 356 (4.4%) | 44 (6.4%) |
| West Midlands | 12,881 (10.0%) | 600 (8.0%) | 261 (6.6%) | 9800 (10.8%) | 736 (9.1%) | 57 (8.3%) |
| Yorkshire and the Humber | 9755 (7.6%) | 356 (4.8%) | 173 (4.4%) | 7821 (8.6%) | 528 (6.6%) | 46 (6.7%) |
| IMD quintile a | ||||||
| 1 (most deprived) | 31,116 (24.3%) | 1121 (15.0%) | 484 (12.3%) | 22,963 (25.2%) | 1182 (14.7%) | 118 (17.3%) |
| 2 | 25,744 (20.1%) | 1306 (17.5%) | 639 (16.2%) | 19,013 (20.9%) | 1435 (17.8%) | 123 (18.0%) |
| 3 | 23,423 (18.3%) | 1435 (19.2%) | 740 (18.7%) | 16,453 (18.1%) | 1506 (18.7%) | 134 (19.6%) |
| 4 | 23,725 (18.5%) | 1711 (22.9%) | 907 (23.0%) | 16,271 (17.9%) | 1781 (22.1%) | 142 (20.8%) |
| 5 (least deprived) | 24,153 (18.8%) | 1901 (25.4%) | 1178 (29.8%) | 16,314 (17.9%) | 2156 (26.8%) | 167 (24.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto Pereira, S.M.; Nugawela, M.D.; McOwat, K.; Dalrymple, E.; Xu, L.; Ladhani, S.N.; Simmons, R.; Chalder, T.; Swann, O.; Ford, T.; et al. Symptom Profiles of Children and Young People 12 Months after SARS-CoV-2 Testing: A National Matched Cohort Study (The CLoCk Study). Children 2023, 10, 1227. https://doi.org/10.3390/children10071227
Pinto Pereira SM, Nugawela MD, McOwat K, Dalrymple E, Xu L, Ladhani SN, Simmons R, Chalder T, Swann O, Ford T, et al. Symptom Profiles of Children and Young People 12 Months after SARS-CoV-2 Testing: A National Matched Cohort Study (The CLoCk Study). Children. 2023; 10(7):1227. https://doi.org/10.3390/children10071227
Chicago/Turabian StylePinto Pereira, Snehal M., Manjula D. Nugawela, Kelsey McOwat, Emma Dalrymple, Laila Xu, Shamez N. Ladhani, Ruth Simmons, Trudie Chalder, Olivia Swann, Tamsin Ford, and et al. 2023. "Symptom Profiles of Children and Young People 12 Months after SARS-CoV-2 Testing: A National Matched Cohort Study (The CLoCk Study)" Children 10, no. 7: 1227. https://doi.org/10.3390/children10071227
APA StylePinto Pereira, S. M., Nugawela, M. D., McOwat, K., Dalrymple, E., Xu, L., Ladhani, S. N., Simmons, R., Chalder, T., Swann, O., Ford, T., Heyman, I., Segal, T., Semple, M. G., Rojas, N. K., CLoCk Consortium, Shafran, R., & Stephenson, T. (2023). Symptom Profiles of Children and Young People 12 Months after SARS-CoV-2 Testing: A National Matched Cohort Study (The CLoCk Study). Children, 10(7), 1227. https://doi.org/10.3390/children10071227







